Assessments of prodromal symptoms that identify individuals at high risk of conversion to psychosis – namely 25–35% within 1 year – have been reported (Reference McGorry, Yung and PhillipsMcGorry et al, 2002; Reference McGlashan, Zipursky and PerkinsMcGlashan et al, 2006). Factors prompting conversion to schizophrenia-spectrum disorders within this group remain poorly understood, however.
A speech perception study comparing patients with schizophrenia-spectrum disorder with and without auditory hallucinations and normal controls (Reference Hoffman, Rapaport and MazureHoffman et al, 1999) included a task requesting participants to report any words or phrases ‘heard’ in response to multispeaker babble. This task did not produce differences between those who experienced hallucinations v. those who did not, as predicted. However, more extended, multiword speech illusions emerged in patients with early-phase, schizophreniform psychosis compared with both normal controls and patients with established schizophrenia (further details available from the authors). We consequently developed the hypothesis that extended speech illusions detected in response to multispeaker babble reflect a general tendency to extract spurious, message-like meaning in response to objectively meaningless sensory information, which, over time, can produce a ‘matrix of unreality’, prompting the initial psychotic phase of schizophrenia-spectrum disorders.
METHOD
Our hypothesis was tested prospectively using data collected for the multisite North America Prevention Through Risk Identification, Management and Education (PRIME) clinical trial (Reference McGlashan, Zipursky and PerkinsMcGlashan et al, 2006). This trial enrolled people with operationally defined syndromes prodromal for psychosis consisting of attenuated positive symptoms, or genetic risk plus deterioration (schizotypal personality disorder and/or first-degree relative with psychosis, plus recent loss of social and/or work capacity with a drop of 30 percentage points on the Global Assessment of Functioning sustained for at least 1 month). Participants, who had no prior lifetime history of a psychosis or schizophrenia diagnosis, were randomly allocated to receive either olanzapine (5–15 mg per day) or placebo for 1 year during the double-blind phase of the study. Those whose disorder did not convert to psychosis were invited to remain in the study for a second year with no pharmacological treatment. Those whose disorder converted to sustained psychosis were switched to a 6-month ‘rescue’ arm using open-label olanzapine. All participants provided written informed consent.
We focused on conversion to schizophrenia-spectrum disorder (schizophrenia or schizophreniform disorder) rather than on psychosis broadly defined, given our expectation that specific risk factors would be more likely to cluster within this more uniformly defined and stable diagnostic group (Reference Correll, Lencz and SmithCorrell et al, 2005). Diagnosis was determined using the Comprehensive Assessment of Symptoms and History (CASH; Reference AndreasenAndreasen, 1987). Out of 60 patients with prodromal symptoms enrolled in the trial, 44 were assessed using the ‘babble’ task, because some study sites did not administer the task. This subgroup included one patient exhibiting a psychotic decompensation who was not assessed diagnostically and whose data were consequently dropped from this conversion analysis. Among the remaining 43 patients, 10 experienced conversion to a schizophrenia-spectrum disorder during year 1, and 2 patients’ condition converted during year 2 to a schizophrenia-spectrum disorder following drug or placebo discontinuation.
The babble stimulus derived from overlapping recordings of six speakers (three women, three men) reading neutral texts. Two different speech segments from each speaker were mixed, yielding 12 simultaneous streams of speech heard binaurally using headphones. This verbal stimulus was designed to produce a high density of phonetic information rendering corresponding words virtually undetectable. Stimulus duration was 2 min 33 s. Participants were instructed to repeat any words or phrases that they ‘heard’ while listening to the babble. Only four words (‘increase’, ‘children’, ‘A–OK’, and ‘Republican’) were consistently reproduced across participants in this task. Tape-recordings of responses were transcribed for analysis. The longest phrase generated (counted as the number of words) constituted the length of speech illusion (LSI) score. Interrater reliability for this measure was high (R I=0.98). Examples of responses are given below:
‘another…the children’ (LSI 2; placebo group member, no conversion);
‘bombing…the administration…seem to be having trouble…the ball…the republicans…it's important to…the ball…practice dancing…’ (LSI=5; placebo group member, conversion).
The babble task was administered as part of a neuropsychological battery administered at baseline, 6 months and 1 year. Analyses of other neuropsychological test data were exploratory and hypothesis-generating. Patients also received serial clinical assessments using the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987).
RESULTS
In order to undertake a time-dependent assessment of conversion risk, data for the year 1 placebo and year 2 drug discontinuation phases of the study were analysed together as a single ‘no drug’ condition that was compared with the olanzapine condition using a Cox regression analysis. Overall, the association between LSI and subsequent conversion risk was nonsignificant (hazard ratio (HR) 1.28, 95% CI 0.93–1.75, P=0.13). However, the interaction between LSI and condition was statistically significant (HR=0.51, 95% CI 0.27–0.95, P=0.035). For the no-drug condition alone, LSI was robustly associated with subsequent conversion (HR=1.78, 95% CI 1.26–2.53, P=0.0011), whereas for the olanzapine condition this association was absent (HR=0.92, 95% CI 0.55–1.55, P=0.75). An identical time-dependence analysis of concurrent composite PANSS positive, negative and general psychopathology symptoms revealed no significant association with subsequent conversion (HR range 1.05–1.12, P range 0.09–0.38), including when analysing data for the no-drug condition alone (HR range 1.03–1.07, P range 0.30–0.51).
The capacity of LSI scores to predict subsequent conversion to schizophrenia-spectrum disorder during the no-drug condition was considered. Optimal classification accuracy was obtained using a cut-off of 4 or above for the maximum LSI score observed at the onset of and during no-drug periods to predict subsequent conversion. Overall classification accuracy was high (Fig. 1; Fisher's exact test, P=0.0001; positive predictive value 0.80, negative predictive value 0.94).
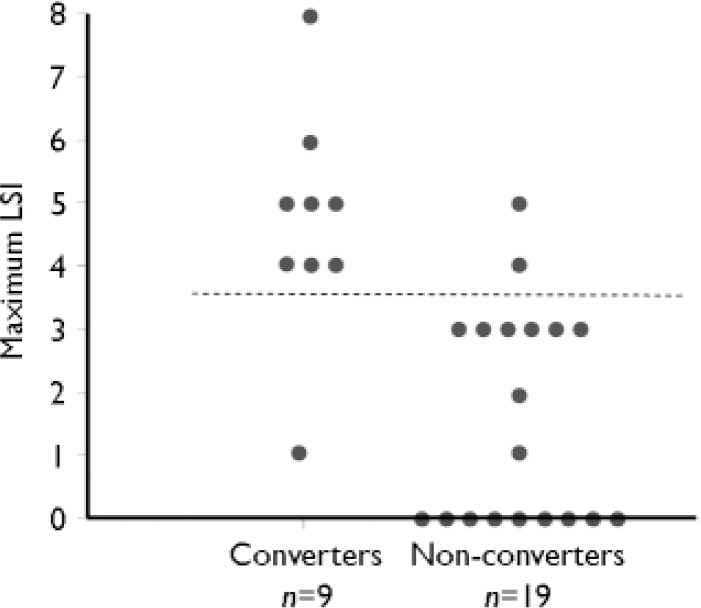
Fig. 1 Maximum length of speech illusion (LSI) during ‘no drug’ intervals (placebo in year 1 or no pharmacological treatment in year 2) grouped according to those whose condition converted to a schizophrenia-spectrum disorder during these intervals and those who did not. The dashed line shows the optimum cut-off point for predicting conversion.
DISCUSSION
Elevated LSI scores signalled subsequent increased risk of schizophrenia-spectrum disorders when participants were not receiving olanzapine. In this condition, each unit increase in LSI score predicted an amplification of conversion risk of 78%. In contrast, concurrent composite symptoms measures did not signal subsequent risk of conversion when examined using the same analysis. These data suggest that elevated LSI scores during the prodrome signalled a covert conversion risk unexpressed by concurrent symptoms. Elevated LSI scores observed in this study might have been caused by excessive top-down processing of phonetic inputs, distorted perceptual processing or misinterpretation of percepts. Extracting spurious messages from meaningless input by patients at risk may extend beyond speech processing per se, as suggested by the Nobel prizewinner John Nash, whose schizophrenic illness emerged subsequent to his detecting ‘encrypted messages’ embedded in letter patterns appearing in the New York Times, which he attributed to space aliens or foreign governments (Reference NasarNasar, 1998).
The significant interaction between condition and LSI scores reflected in the Cox regression analysis of conversion suggests that early administration of olanzapine reduced risk associated with elevated LSI scores.
The relatively small pool of prodromal patients reported here underscores the need for further studies of spurious message-like meaning induced by babble as a predictor of conversion to schizophrenia-spectrum disorders. Confirmation that LSI predicts risk of conversion – and that antipsychotic drugs reduce this risk – would be an important advance insofar as serial LSI assessments might then be used to identify the patients with prodromal symptoms most likely to benefit from preventive drug therapy.




eLetters
No eLetters have been published for this article.