Communication between the patient and the professional carer lies at the heart of all decisions regarding diagnosis and treatment. There is evidence that professional carers and patients may have divergent views on what constitutes appropriate support and care (Slade et al, Reference Slade, Phelan and Thornicroft1996, Reference Slade, Phelan and Thornicroft1998, Reference Slade, Leese and Taylor1999; Reference Lenert, Ziegler and LeeLenert et al, 2000; Reference Hansson, Vinding and MackeprangHansson et al, 2001), and that bridging these divides is a necessary prelude to any effective professional intervention. Constructive dialogue between patient and professional carer is central to the recovery-oriented service model, with its emphasis on consumercentred outcomes, individualised goals, self-development and choice (Reference DeeganDeegan, 1988; Reference Frese, Stanley and KressFrese et al, 2001; Reference Holloway, Carson and DavisHolloway et al, 2002). The introduction of such recovery-oriented service systems, however, requires constant reinforcement and consumer participation (Reference AnthonyAnthony, 2000), which must begin at the most basic level of communication between patient and professional carer (Reference Eisen, Dickey and SedererEisen et al, 2000).
The Two-Way Communication Checklist (2-COM) is a simple communication tool developed with the aim of improving communication between patient and professional carer in everyday clinical practice. In a previous observational study of 243 patients who completed 2-COM prior to routine appointments, both doctors and patients found the checklist useful in revealing new information. In addition, patients (but not clinicians) considered that the checklist had resulted in a change in treatment. The results indicated that 2-COM was most highly regarded by patients with the greatest number of care needs (Reference van Os, Altamura and Bobesvan Os et al, 2002). However, although encouraging, these results do not in themselves demonstrate that 2-COM changes the behaviour of professional carers, as reflected in changes of treatment and attitude. We therefore set out to examine, in a randomised controlled trial, whether the use of 2-COM as an intervention would result in identifiable changes in clinician behaviour and improved patient–clinician communication, in particular in patients with more severe illness and more need for care.
METHOD
Sample
Patients with a clinical diagnosis of schizophrenia or schizoaffective disorder were recruited at seven European centres: Maastricht (n=10), Oviedo (n=25), Gijónn (n=16), Hamburg (n=4), Copenhagen (n=30), Milan (n=30) and Nice (n=19). Patients were included if they:
-
(a) had a diagnosis of schizophrenia, schizoaffective disorder or schizophreniform disorder according to DSM–IV criteria (American Psychiatric Association, 1994);
-
(b) were over the age of 18 years;
-
(c) were in a stable phase of illness;
-
(d) were in regular out-patient contact with their psychiatric care team (at least once every 6 months);
-
(e) were able to provide informed consent to participate.
Patients were excluded if they:
-
(a) had been discharged from the in-patient unit within the past 2 weeks, or were currently in-patients;
-
(b) were, in the view of the investigator, likely to be admitted for in-patient care within the next 2 weeks;
-
(c) were less than 18 years of age;
-
(d) were unable, by virtue of illness, IQ or any other reason, to provide informed consent to participation in the study.
The great majority of professional carers who participated in the study were clinicians routinely involved in the day-to-day care of patients with schizophrenia.
Power and randomisation
Patients were randomised centrally by an independent, non-investigator agency using a predetermined random sequence. In the power calculation, it was assumed that the intervention would double the probability of any change in management given a 25% baseline chance of treatment change. This required a minimum of 65 patients in each study group.
2-COM
The 2-COM is a simple list of 20 common problems, or areas of perceived need, that might be experienced by patients with severe mental illness. The list includes problems with housing, relationships, money, lack of activities, psychological distress, sexuality, symptoms and treatment side-effects. The basic psychometric properties of the instrument have been described previously (Reference van Os, Altamura and Bobesvan Os et al, 2002; see also http://www.2coms.homestead.com). In summary, 2-COM has shown adequate test–retest reliability and is well accepted by patients as a valued aid to communication with their doctor. Patients are provided with the 2-COM prior to seeing their clinician and given simple instructions to facilitate its completion, guiding patients to indicate which of the 20 problems apply to them and to highlight any that they wish to discuss with their clinician during the subsequent clinic appointment. Field work to date indicates that using a completed checklist to guide discussion during the clinical interview extends the duration of the appointment by an average of 13 min (Reference van Os, Altamura and Bobesvan Os et al, 2002).
Procedures and assessments
The intervention and assessment took place over three out-patient clinic visits. At visit 1, patients gave informed consent and the clinician recorded a list of all current interventions, including medication and non-medical treatments, together with demographic information and an assessment of current level of functioning, using the Global Assessment of Functioning scale (GAF; Reference Frances, Pincus and FirstFrances et al, 1994). Within 3–14 days of the baseline assessment, the patient attended the clinic for a second clinical interview. Prior to the second visit, patients were randomised to receive either 2-COM (to be completed by the patient immediately before the clinical interview and used as the basis for discussion during the interview), or ‘standard care’–a routine appointment without 2-COM. Immediately after the interview, all patients, whether they had completed 2-COM or not, completed a confidential questionnaire (sealed by the patients themselves) in which they could indicate the perceived quality of communication with their clinician, current views on their relationship with their clinician, and attitudes to their illness and care. Similarly, clinicians completed a repeat of the list of all current interventions, together with an assessment of any changes to the treatment plan implemented after the interview with the patient. Changes in treatment plan were categorised in the questionnaire as:
-
(a) any change in any medication;
-
(b) any change in providing information about treatment;
-
(c) any change in the involvement of other members of the care team (psychiatrist, psychologist, psychotherapist, psychiatric nurse, general nurse, social worker, worker with the homeless, occupational therapist, other health care professional);
-
(d) any change in professional support services (sheltered housing, hospital stay, hostel accommodation, day centre or day hospital, drug or alcohol services, occupational therapy, out-patient clinic services, social services, cognitive–behavioural therapy, family therapy, sheltered work);
-
(e) change in the degree of involvement of informal carers.
Four to six weeks after clinic visit 2, patients attended the clinic for a third, ‘routine’ clinical interview. Both patients and clinicians then completed the same set of post-interview assessments as at visit 2.
Outcomes
The two main outcomes were quality of patient–clinician communication as reported by the patient, and change in clinician behaviour, indicated by any change in management, as reported by the clinician. This dual set of outcomes had been chosen to allow perceived change in communication, as reported by the patient, to be validated alongside changes in behaviour as reported by the clinician. The first outcome of patient–clinician communication was scored by the patient on a four-point scale (higher score indicated better communication), answering the following question: ‘How easy did you find it to discuss the problems and worries you have with your doctor at today's clinic appointment?’ A single dichotomous variable reflecting whether or not clinicians had changed their treatment was calculated for each patient at both visit 2 and visit 3.
Analyses
For each patient, one overall effect size was calculated for the two outcomes–change in treatment, and patient–doctor communication. This statistic incorporated data from the two separate post-intervention study observations (visit 2 and visit 3). In addition, in order to assess the pattern of response over time, effects were calculated separately for visit 2 and visit 3. The data were analysed using regression procedures in STATA version 8 (StataCorp, 2002). For the continuous variable relating to perceived patient–doctor communication, multiple regression analysis was used, whereas for the dichotomous variable relating to doctors’ treatment change, logistic regression was applied. To facilitate interpretation of effect sizes, regression coefficients from the multiple regression analyses were expressed as standard deviations of the response variable. For the dichotomous outcome, effect sizes were expressed in terms of odds ratios and numbers needed to treat (NNT).
As observations at visit 2 and visit 3 were clustered within individuals, the CLUSTER and ROBUST options were used in the STATA regression analyses. This allows for the use of observations that are not wholly independent within clusters (in this case, within individuals) and obtains the Huber–White sandwich estimator of variance instead of the traditional variance estimator. All analyses were adjusted for centre and also for diagnosis, as the randomisation had not been successful for this latter variable (Table 1). Values of P were two-sided with α set at 5%.
Table 1 Distribution of demographic and clinical variables in each treatment group at baseline assessment
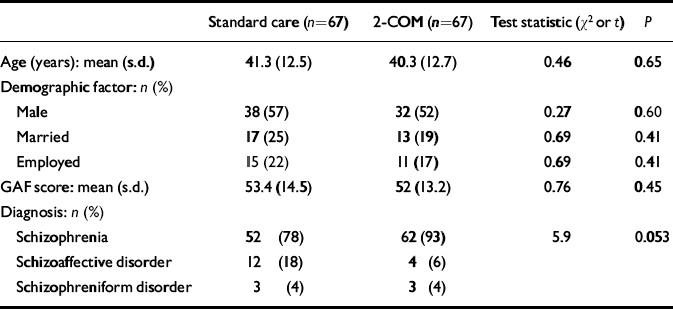
| Standard care (n=67) | 2-COM (n=67) | Test statistic (χ2 or t) | P | |
|---|---|---|---|---|
| Age (years): mean (s.d.) | 41.3 (12.5) | 40.3 (12.7) | 0.46 | 0.65 |
| Demographic factor: n (%) | ||||
| Male | 38 (57) | 32 (52) | 0.27 | 0.60 |
| Married | 17 (25) | 13 (19) | 0.69 | 0.41 |
| Employed | 15 (22) | 11 (17) | 0.69 | 0.41 |
| GAF score: mean (s.d.) | 53.4 (14.5) | 52 (13.2) | 0.76 | 0.45 |
| Diagnosis: n (%) | ||||
| Schizophrenia | 52 (78) | 62 (93) | 5.9 | 0.053 |
| Schizoaffective disorder | 12 (18) | 4 (6) | ||
| Schizophreniform disorder | 3 (4) | 3 (4) |
Illness severity effect modification
Interactions with illness severity were explored by introducing a term for the interaction of continuous baseline GAF score and the 2-COM intervention in the models of the outcomes.
Relating 2-COM needs to change in treatment
The likelihood of treatment change and the quality of patient–doctor communication within the intervention group were analysed as a function of total number of needs scored on 2-COM by the patient. In addition, in order to be able to describe the needs that were most associated with treatment change, post hoc analytic and descriptive analyses were carried out using individual needs as predictors of treatment change. For needs that were most strongly associated with treatment change, the odds of non-medication treatment changes v. the odds of medication treatment changes associated with these needs were numerically compared.
RESULTS
Sample
The sample included 134 participants, 67 in each treatment arm. The mean age was 40.8 years (s.d.=12.5), and the mean GAF score at baseline was 52.9 (s.d.=13.8). Sixty-one (45%) of the participants were women, 30 (22%) were (living as) married and 26 (20%) were employed. The study group was thus representative of a typical out-patient population. There was no large or statistically significant difference between groups in each treatment arm in terms of these variables, but there were significant diagnostic differences (Table 1).
Outcomes
Patients using 2-COM rated communication with their doctor as significantly better than patients on ‘standard care’ (2-COM group mean score 3.4, standard care group mean score 3.2; adjusted B 0.33, P=0.031). This effect size was approximately similar for the second visit (adjusted B 0.29, P=0.10) and the third visit (adjusted B 0.37, P=0.046). There was no interaction between scores on quality of patient–doctor communication and baseline illness severity, as measured by the continuous GAF score (adjusted B interaction –0.014, P=0.253). Similarly, within the intervention group, there was no interaction between ratings for quality of communication and the number of needs identified by the patient (adjusted B –0.021, P=0.25).
Patients in the 2-COM group were more likely to have had their treatment changed, as reported by the doctor, than were those in the standard care group (2-COM 74%, standard care 61%; adjusted OR=2.2, 95% CI 1.02–4.7; NNT=8). This effect size was much larger for the second visit (adjusted OR=3.7, 95% CI 1.4–9.6, P=0.009; NNT=6) than for the third visit (adjusted OR=1.5, 95% CI 0.6–3.3, P=0.39; NNT=15). No interaction with severity of illness as expressed by continuous GAF score was apparent (adjusted OR interaction 0.98, P=0.59). However, within the intervention group, the larger the number of needs reported, the greater was the likelihood of treatment change (adjusted OR per increase in need 1.16, 95% CI 1.07–1.25, P<0.0001).
Analyses at level of individual needs
Within the 2-COM intervention group, some needs were more likely than others to induce changes in treatment at the second visit (Table 2). Reported needs associated with the strongest likelihood of treatment change at the second visit were problems with sleep, not being able to enjoy oneself, feeling tense, being easily upset, having unpleasant thoughts, problems with medication, problems with family or other persons, problems with money, problems finding things to do, problems going out, and wanting more information about illness and treatment. Of these, the perceived need for information about illness and treatment had by far the greatest effect size. For all these items, the odds for non-medication changes in treatment were numerically greater than the odds for a change in medication, even when the provision of information as a treatment change was excluded (data not shown).
Table 2 Post hoc analysis of likelihood of treatment change for each individual item of the Two-Way Communication Checklist in the intervention group at the second visit
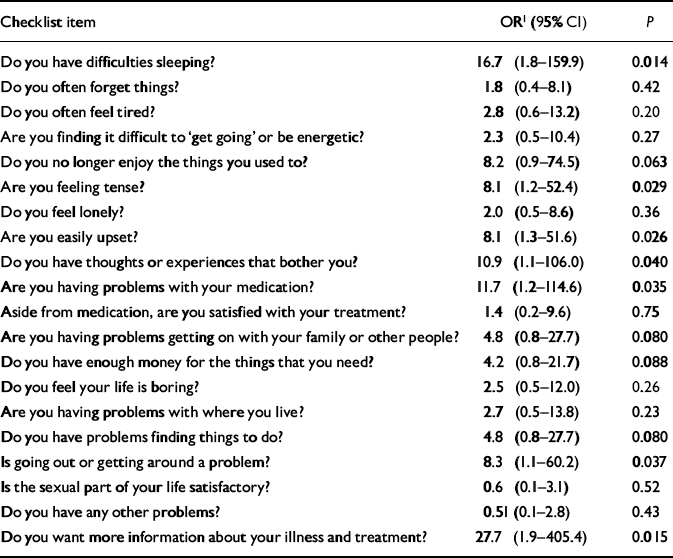
| Checklist item | OR1 (95% CI) | P |
|---|---|---|
| Do you have difficulties sleeping? | 16.7 (1.8-159.9) | 0.014 |
| Do you often forget things? | 1.8 (0.4-8.1) | 0.42 |
| Do you often feel tired? | 2.8 (0.6-13.2) | 0.20 |
| Are you finding it difficult to ‘get going’ or be energetic? | 2.3 (0.5-10.4) | 0.27 |
| Do you no longer enjoy the things you used to? | 8.2 (0.9-74.5) | 0.063 |
| Are you feeling tense? | 8.1 (1.2-52.4) | 0.029 |
| Do you feel lonely? | 2.0 (0.5-8.6) | 0.36 |
| Are you easily upset? | 8.1 (1.3-51.6) | 0.026 |
| Do you have thoughts or experiences that bother you? | 10.9 (1.1-106.0) | 0.040 |
| Are you having problems with your medication? | 11.7 (1.2-114.6) | 0.035 |
| Aside from medication, are you satisfied with your treatment? | 1.4 (0.2-9.6) | 0.75 |
| Are you having problems getting on with your family or other people? | 4.8 (0.8-27.7) | 0.080 |
| Do you have enough money for the things that you need? | 4.2 (0.8-21.7) | 0.088 |
| Do you feel your life is boring? | 2.5 (0.5-12.0) | 0.26 |
| Are you having problems with where you live? | 2.7 (0.5-13.8) | 0.23 |
| Do you have problems finding things to do? | 4.8 (0.8-27.7) | 0.080 |
| Is going out or getting around a problem? | 8.3 (1.1-60.2) | 0.037 |
| Is the sexual part of your life satisfactory? | 0.6 (0.1-3.1) | 0.52 |
| Do you have any other problems? | 0.51 (0.1-2.8) | 0.43 |
| Do you want more information about your illness and treatment? | 27.7 (1.9-405.4) | 0.015 |
DISCUSSION
The study showed that 2-COM, a simple intervention providing patients with a means to aid discussion of their needs during their routine visit to the clinician, resulted in improved patient–clinician communication, which persisted over time, when compared with standard care. The effect size of this improvement (s.d.=0.3) was small to moderate. This improvement in communication was mirrored by a change in the behaviour of the clinician, as demonstrated by a greater likelihood of change in management in the period immediately after the 2-COM intervention. Although reported improvements in quality of communication were not influenced by the number or type of need identified, treatment change was more likely to occur in patients with higher levels of perceived need. Furthermore, some needs were more likely to induce treatment change than others, in particular the need for information on illness and treatment. Non-medication changes displayed stronger association with these needs than did changes in medication, even when information provision as a treatment change was excluded, indicating that clinicians did not respond to patients’ needs solely with medication changes or the provision of more information. Thus, these findings suggest that 2-COM produces needs-related changes in treatment immediately after the intervention, followed by a stable and durable improvement in quality of communication as perceived by the patient, regardless of the number of reported needs.
Limitations
Studies involving patient–doctor communication pose particular challenges with regard to masking. There is the possibility that the behaviour of clinicians changed because they wanted to show that 2-COM worked, and that patients, for similar reasons, reported positive experiences about their interactions with the clinician. However, none of the participating clinicians had previously been involved in the development of 2-COM. Moreover, our previous work indicated that clinicians tend to be only moderately positive about 2-COM in terms of its perceived impact on their practice and their appreciation of patient needs. Interestingly, despite the views expressed by clinicians in the earlier 2-COM evaluation, the results of this study indicate that 2-COM does indeed have an impact on clinicians’ management of patients.
Interpretation of findings
The positive experiences of patients in this study accord with the findings of our earlier observational study, and have face validity given the fact that 2-COM actually lengthens the visit to the clinician (Reference van Os, Altamura and Bobesvan Os et al, 2002). Treatment change was more likely in patients with higher levels of reported needs, but not in patients with lower GAF scores as rated by the clinician. However, although the interactions with GAF score were not significant, both were suggestive of a greater effect for patients with lower GAF scores (i.e. patients with more impairments). Therefore, it is likely to be the patients with more perceived needs and greater levels of impairment who benefit most from 2-COM. This replicates our previous finding that 2-COM is considered most useful by patients with the greatest level of need (Reference van Os, Altamura and Bobesvan Os et al, 2002). There are a number of possible explanations for this relationship. Patients with fewer problems might experience less difficulty in unaided communication with their clinician; an alternative explanation would be that those with more problems might have insufficient time during the routine visit to discuss all their needs. Another explanation is that patients with schizophrenia, in particular those with more severe illness, might have difficulties initiating the goal-directed actions that are necessary to expose, discuss and resolve issues that make up care needs (Reference FrithFrith, 1987). The results suggest that providing patients with an opportunity to set the agenda for the visit actually engenders and facilitates a discussion about care needs, resulting in real changes in management that might otherwise not have occurred. As these changes occurred in the context of discussion of patients’ needs, it is tempting to speculate that these, in turn, might positively influence patient outcomes. However, the goal of this study was to assess whether 2-COM would result in any actual change of behaviour, rather than to investigate the extent to which these behaviours might eventually produce better patient outcomes, other than patient perception of quality of communication. Longer follow-up would be required to assess the effects on longer-term clinical outcomes. It is likely, however, that in order for 2-COM to influence clinical outcome, constant reinforcement and continued patient participation would be required (Reference AnthonyAnthony, 2000).
Implications for practice
Although further work needs to be conducted, the results of this simple randomised, controlled trial confirm that not just the implementation of the treatment plan, but also the communication leading up to decisions on treatment and care, should be specific focuses of attention. Use of 2-COM prolongs the clinical interview by approximately 13 min on average. The instrument is well regarded by patients; the improvement in communication brought about by its use may have considerable benefits in the treatment of schizophrenia. Helping the patient to become vocal in the decision process may be highly rewarding for clinical practice.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Structured communication about perceived needs induces changes in the clinical management of patients with schizophrenia.
-
▪ Having the patient set the agenda in discussing treatment needs improves the quality of patient–doctor communication.
-
▪ Focusing on simple, non-pharmacological interventions may have considerable value in the treatment of patients with multiple needs.
LIMITATIONS
-
▪ The study did not evaluate whether the changes in clinical management brought about by the intervention resulted in long-term effects on clinical outcome.
-
▪ Masking cannot be implemented in some non-pharmacological trials, with a consequent risk of bias.
-
▪ The effect of the intervention was investigated over a period of 6 weeks; no conclusions can be drawn beyond that period.
Acknowledgement
The study was supported by an unrestricted grant from AstraZeneca. AstraZeneca had no input into the design, conduct, analysis or reporting of. the study.





eLetters
No eLetters have been published for this article.