Second-generation antipsychotic medications have emerged as one of the most costly drug classes, with annual spending in the US of £5 billion annually ($10 billion) (Reference RosackRosack, 2006), or £45 ($90) per US household.
Initial randomised trials, primarily sponsored by industry, suggested that these drugs were superior to first-generation antipsychotics in the treatment of schizophrenia – reducing both symptoms and neurological side-effects such as extrapyramidal symptoms and tardive dyskinesia (Reference Tamminga and WoernerTamminga & Woerner, 2002; Reference Davis, Chen and GlickDavis et al, 2003; Reference Tarsy and BaldessariniTarsy & Baldessarini, 2006) as well as reducing healthcare costs (Reference Hamilton, Revicki and EdgellHamilton et al, 1999). However, more recent studies, conducted by independent investigators, cast doubt on these conclusions, finding virtually no substantial difference in health outcomes, quality of life, neurological side-effects or non-drug health costs between these two classes of drugs (Reference Leucht, Wahlbeck and HamannLeucht et al, 2003; Rosenheck et al, Reference Rosenheck, Perlick and Bingham2003, Reference Rosenheck, Leslie and Sindelar2006; Reference Lieberman, Stroup and McEvoyLieberman et al, 2005; Reference Jones, Barnes and DaviesJones et al, 2006; Reference Swartz, Perkins and StroupSwartz et al, 2007). The earlier studies commonly relied on haloperidol as the comparator – a high-potency antipsychotic often used at higher than recommended dosages (Reference Hugenholtz, Heerdink and StolkerHugenholtz et al, 2006) and without prophylactic anticholinergic medication. Using such treatment as a comparator posed an especially high risk of extrapyramidal symptoms, akinetic depression and possibly tardive dyskinesia (Reference RosenheckRosenheck, 2005). One literature review found that in studies that used dosages of 12 mg or less of haloperidol (or the equivalent), no benefit in efficacy or overall tolerability was observed for second-generation antipsychotics, although a reduction in extrapyramidal symptoms was noted (Reference Geddes, Freemantle and HarrisonGeddes et al, 2000). Two reviews have also suggested that industry-sponsored studies of second-generation antipsychotics are especially likely to favour the manufacturers' product (Reference Montgomery, Byerly and CarmodyMontgomery et al, 2004; Reference Heres, Davis and MainoHeres et al, 2006), a phenomenon familiar in other areas of medicine as well (Reference Bekelman, Li and GrossBekelman et al, 2003; Reference Lexchin, Bero and DjulbegovicLexchin et al, 2003).
Cost studies have now demonstrated that the higher costs of second-generation antipsychotics increase annual total health costs by £1200–3000 ($2400–6000) per patient (Rosenheck et al, Reference Rosenheck, Perlick and Bingham2003, Reference Rosenheck, Leslie and Sindelar2006; Reference DugganDuggan, 2005; Reference Jones, Barnes and DaviesJones et al, 2006). A consensus has also emerged that these newer drugs are more likely than first-generation antipsychotics to cause weight gain, diabetes and metabolic syndrome (American Diabetes Association et al, 2004), although short-term cost consequences may be small (Reference Leslie and RosenheckLeslie & Rosenheck, 2005).
The hope remains that second-generation antipsychotics are superior to the earlier drugs on at least one important neurological side-effect: lowering the risk of tardive dyskinesia (Reference KaneKane, 2006; Reference CaseyCasey, 2006; Reference TandonTandon & Constantine, 2006). A recent meta-analysis of eleven 1-year follow-up studies, including four clinical trials, found a lower annual incidence of tardive dyskinesia with second-generation drugs, but its authors acknowledged that their findings might have been biased by the use of high doses of haloperidol in comparison treatments. Like other recent summaries (Reference KaneKane, 2006), the outcome of interest was simple incidence of tardive dyskinesia without consideration of severity of disorder, functional impairment, recovery, relationship to quality of life, or longer-term planning horizons. Clinical decision-making and formulary policy must make use of data on these broader health outcomes.
METHOD
We reviewed published research comparing the risks of tardive dyskinesia in treatments with the two classes of antipsychotic among adults with schizophrenia, noting methodological limitations and biases. Data were then derived from diverse published studies along with some previously unpublished data that addressed the expectable ranges of severity and duration of tardive dyskinesia and its relationship to functional capacity and quality of life. Although no single trial has addressed all of these relevant issues, we combined data from several sources to develop an estimate of the potential cost-effectiveness of second-generation antipsychotics in reducing risk of tardive dyskinesia in adults with schizophrenia. A range of alternative assumptions and planning horizons were considered in a deterministic sensitivity analysis that examined best- and worst-case scenarios. Unfortunately, available data were insufficient to support a full probabilistic sensitivity analysis of the type recommended for regulatory review (Reference BriggsBriggs, 2005; Reference Claxton, Sculpher and McCabeClaxton et al, 2005).
RESULTS
Comparative incidence of tardive dyskinesia with first- and second-generation antipsychotics
The review by Correll et al (Reference Correll, Leucht and Kane2004) summarised data on 1707 patients treated in four randomised trials for an average median duration of 8.8 months, plus 1571 patients treated in seven observational studies. Consistent with older studies (Reference Kane, Woerner and WeinholdKane et al, 1984; Reference Chouinard, Annable and MercierChouinard et al, 1986; Reference Glazer, Morgenstern and DoucetteGlazer et al, 1993); the annual incidence of tardive dyskinesia with first-generation antipsychotics was estimated to be 5.4%. The annual risk with second-generation drugs, in contrast, was estimated at 0.8%, yielding a 4.6% greater attributable risk of this complication with the older drugs. However, further examination of these studies reveals methodological biases that might have led to overestimation of the benefits of the newer antipsychotics.
Of the four randomised trials – the studies that provide the highest quality of evidence – the one that showed the greatest advantage for second-generation antipsychotics compared ziprasidone, which had an annualised incidence of tardive dyskinesia of 6.8%, with placebo, which was reported to have an annualised incidence of this side-effect of 35.7% (Reference Arato, O'Connor and MeitzerArato et al, 2002). Although tardive dyskinesia occasionally emerges in schizophrenia in the absence of antipsychotic medication (Reference Chakos, Alvir and WoernerChakos et al, 1996; Reference FentonFenton, 2000), the 35.7% annualised incidence rate reported with placebo most probably reflects withdrawal dyskinesia due to residual effects from previous antipsychotic medications, coupled with discontinuation of anticholinergics (Reference WoodsWoods, 1999), rather than a true association of placebo and tardive dyskinesia.
The other three randomised trials used haloperidol as the comparator at relatively high doses (13–15 mg), without prophylactic anticholinergics. Using data from the trial with the largest number of participants (Reference Beasley, Dellva and TamuraBeasley et al, 1999), Correll et al (Reference Correll, Leucht and Kane2004) included only those patients with 6 weeks or more of treatment which left only 36% of the original 1714 participants, a notable attrition from the original randomised sample. In the original publication that included the entire sample (Reference Beasley, Dellva and TamuraBeasley et al, 1999) 35 of 48 (73%) cases of tardive dyskinesia occurred during the first 6 weeks, further suggesting that withdrawal dyskinesia or extrapyramidal side-effects were not well differentiated from tardive dyskinesia. In a third study, incidence of tardive dyskinesia was based on simple adverse events reporting, an imprecise and unstandardised measure of this complex syndrome (Reference Csernansky, Mahmoud and BrennerCsernansky et al, 2002).
Although the experimental data supporting reduced risk of this side-effect with second-generation antipsychotics are thus flawed, the conviction that these drugs lower the risk of tardive dyskinesia is not entirely based on such direct evidence. Many have inferred that less frequent occurrence of extrapyramidal symptoms with these drugs results in a lower risk of tardive dyskinesia (Reference Tamminga and WoernerTamminga & Woerner, 2002; Tarsey & Baldessarini, 2006; Reference Tenback, van Harten and SlooffTenback et al, 2006). However, a meta-analysis of 31 randomised controlled trials that included 2320 patients found no difference in risk of extrapyramidal symptoms between low-potency first-generation drugs and second-generation drugs other than clozapine (Reference Leucht, Wahlbeck and HamannLeucht et al, 2003). Nor has the hypothesis been sustained that anticholinergics, prescribed to prevent extrapyramidal symptoms, themselves increase risk of tardive dyskinesia (Reference Gardos and ColeGardos & Cole, 1983). It is thus likely that the reduction in risk of extrapyramidal symptoms as well as of tardive dyskinesia with second-generation antipsychotics has been overstated.
Perhaps because larger numbers of patients have now been exposed to the newer antipsychotics, recent observational studies (Reference Halliday, Farrington and MacdonaldHalliday et al, 2002; Reference Lee, Sykora and GillLee et al, 2005; Reference Rochon, Stukel and SykoraRochon et al, 2005; Woods, personal communication, 2007) as well as randomised trials (Reference Lieberman, Stroup and McEvoyLieberman et al, 2005; Reference Jones, Barnes and DaviesJones et al, 2006) have found rates of extrapyramidal side-effects and tardive dyskinesia that were no greater with first-generation than second-generation drugs. Since evidence for the superiority of the newer antipsychotic (other than the infrequently used drug clozapine) is weak, and contrary evidence is increasing, the 4.6% attributable risk advantage that we use in our analysis is likely to be an optimistic, upper-bound estimate for adults with schizophrenia.
Severity
Severity of illness and functional impairment are central to health outcome assessment but have been largely neglected in research on tardive dyskinesia. The most widely used measure of this disorder, the 10-item Abnormal Involuntary Movements Scale (AIMS; Reference GuyGuy, 1976), rates involuntary movements in seven topographic body zones (e.g. mouth, arms, trunk) and along three global dimensions (objective severity, subjective experience and functional impairment). The definition of tardive dyskinesia used in most studies is a dichotomous measure representing onset of either moderately severe movements in one of the seven body zones or mild movements in two (Reference Schooler and KaneSchooler & Kane, 1982). Only a few published studies have reported data from the global severity ratings that are included in the AIMS, although severity should clearly be considered in the evaluation of any health outcome. One study of out-patients with first-episode schizophrenia reported that of the 16% of patients who developed tardive dyskinesia, 89% had mild symptoms, 11% moderate symptoms and none severe symptoms of this side-effect (Reference Chakos, Alvir and WoernerChakos et al, 1996). A recent European study of 8739 chronically ill out-patients found that of 9.4% with evidence of tardive dyskinesia, 80% had no significant interference in functioning or quality of life from it (Reference Tenback, van Harten and SlooffTenback et al, 2006). Thus 80–90% of out-patients with schizophrenia diagnosed with tardive dyskinesia appear to have mild symptoms.
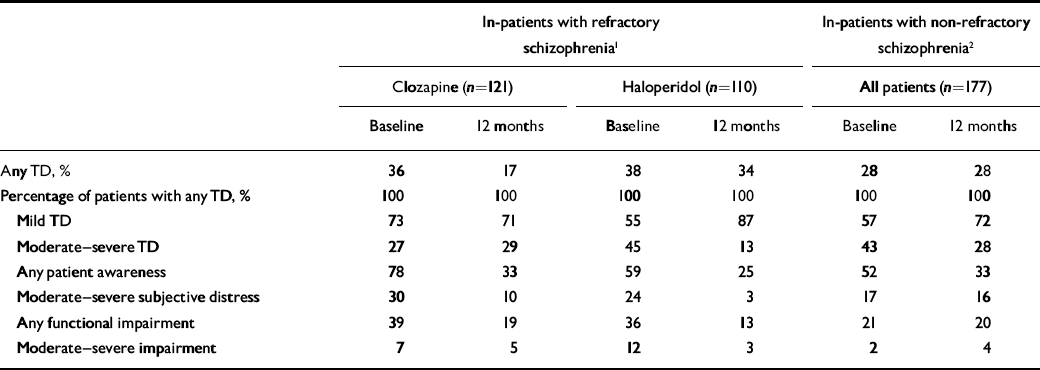
For contrast, we present new data from participants in two large US Department of Veterans Affairs (VA) trials of psychiatric in-patients – patients whose illness is presumably more severe – for whom both baseline and 1-year follow-up data were available. The first trial involved participants with refractory schizophrenia who were receiving in-patient treatment with clozapine or haloperidol (Reference Rosenheck, Cramer and XuRosenheck et al, 1997); and the second, in-patients who were assigned to olanzapine or haloperidol (Reference Rosenheck, Perlick and BinghamRosenheck et al, 2003). Since there were robust differences in tardive dyskinesia between treatment groups in the first study, we present data separately for each treatment group. Data from the second study are pooled (Table 1). Over the 1-year follow-up, the prevalence of any tardive dyskinesia (mild to severe) was more than halved among patients taking clozapine, declined only slightly among those taking haloperidol in the same trial and was unchanged in the study of patients treated with olanzapine and haloperidol (Table 1, row 1).
Table 1 Distribution of severity of tardive dyskinesia at baseline and at 1-year follow-up in three samples
| In-patients with refractory schizophrenia 1 | In-patients with non-refractory schizophrenia 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clozapine (n=121) | Haloperidol (n=110) | All patients (n=177) | ||||||||
| Baseline | 12 months | Baseline | 12 months | Baseline | 12 months | |||||
| Any TD, % | 36 | 17 | 38 | 34 | 28 | 28 | ||||
| Percentage of patients with any TD, % | 100 | 100 | 100 | 100 | 100 | 100 | ||||
| Mild TD | 73 | 71 | 55 | 87 | 57 | 72 | ||||
| Moderate-severe TD | 27 | 29 | 45 | 13 | 43 | 28 | ||||
| Any patient awareness | 78 | 33 | 59 | 25 | 52 | 33 | ||||
| Moderate-severe subjective distress | 30 | 10 | 24 | 3 | 17 | 16 | ||||
| Any functional impairment | 39 | 19 | 36 | 13 | 21 | 20 | ||||
| Moderate-severe impairment | 7 | 5 | 12 | 3 | 2 | 4 | ||||
TD, tardive dyskinesia
1. Rosenheck et al (Reference Rosenheck, Cramer and Xu1997)
2. Rosenheck et al (Reference Rosenheck, Perlick and Bingham2003)
Of patients with any tardive dyskinesia, a half to a quarter had moderate–severe ratings initially (Table 1, row 4), and samples that had no change in the overall rate of this condition showed sharp declines in rates of moderate–severe symptoms – by well over a third in the clozapine–haloperidol study and by almost half in the olanzapine–haloperidol study – so that at 12 months 71–87% of those with tardive dyskinesia had mild illness (Table 1). Even smaller proportions of these patients reported moderate to severe subjective distress about their tardive dyskinesia – 17% to 30% across the three samples at baseline – and only 3–16% reported moderate to severe distress 1 year later (Table 1, row 6). Ratings of moderate to severe functional impairment due to tardive dyskinesia affected about 10% of those with this condition at baseline and declined to substantially less than 5% at 1 year (Table 1; row 7). Thus published data, although limited, suggest that only 10–20% of identified tardive dyskinesia among out-patients is rated as more than mild (Reference Chakos, Alvir and WoernerChakos et al, 1996; Reference Tenback, van Harten and SlooffTenback et al, 2006), and data from hospitalised patients show only a third to a half of those with the disorder to have moderate or more severe ratings, dropping to about a quarter 1 year later. Prevalence of moderate subjective distress or dysfunction was even less frequent, affecting no more than 10%. The fact that tardive dyskinesia can be severe and debilitating in infrequent cases must not be minimised, but a health outcomes perspective must recognise that most tardive dyskinesia is mild and causes limited distress or impairment.
Changing incidence and recovery
In a long-term study of 971 patients with schizophrenia, initiated during the 1970s, the incidence of tardive dyskinesia with first-generation antipsychotic therapy declined with time, from 6.1% in the first year of illness to 2.1% by year 20 (Reference Tamminga and WoernerTamminga & Woerner, 2002). Unfortunately, similar longitudinal data are not available concerning treatment with second-generation antipsychotics.
A recent 1-year clinical trial that involved first-episode schizophrenia found that among those treated with haloperidol, 9% had persistent tardive dyskinesia compared with 5% of those treated with risperidone (P=0.28) (Reference Schooler, Rabinowitz and DavidsonSchooler et al, 2005). However, these persistent cases represented only 19% of all diagnosed tardive dyskinesia, 81% of which lasted less than 3 months, with similar recovery rates for the two drugs (80% v. 82%). In an earlier era, Chakos et al (Reference Chakos, Alvir and Woerner1996) reported that in first-episode schizophrenia 24% of people experiencing episodes of tardive dyskinesia showed recovery within 3 months, rising to 35% of those with onset in year 2, and then falling to 11% of those with onset in year 4. In the previously cited long-term study of first-generation antipsychotics (Reference Tamminga and WoernerTamminga & Woerner, 2002), 40% of those tardive dyskinesia with onset during the first year of illness recovered within 3 months; this figure dropped to 26% of incident cases in year 5 and to 19% in year 15.
Although estimates of onset and recovery from tardive dyskinesia thus vary widely across studies, it appears that its annual incidence in patients taking conventional antipsychotics drops from 6% to 2% over 20 years, whereas recovery can be as high as 80% in the first year but also declines, to as little as 15%, in later years. Although comparable data are not available for second-generation drugs, evaluation of the health consequences of tardive dyskinesia should consider recovery rates as well as incidence rates.
Treatment of tardive dyskinesia with second-generation drugs
There have been reports that tardive dyskinesia can be effectively treated with second-generation antipsychotics. Although it has long been claimed that, paradoxically, either lowering or increasing the dosage of first-generation drugs can sometimes reduce symptoms of this disorder (Reference Tamminga and WoernerTamminga & Woerner, 2002), recent studies involving clozapine (Reference Tamminga, Thaker and MoranTamminga et al, 1994) and olanzapine (Reference Kinon, Jeste and Kollack-WalkerKinon et al, 2004) suggest more complete recovery with the newer drugs. After one sample of patients with tardive dyskinesia were treated for several months with olanzapine, they showed little evidence of recurrence when dosages were subsequently reduced. The 4.6% reduction in risk estimated for second-generation antipsychotics in the comprehensive review (Reference Correll, Leucht and KaneCorrell et al, 2004), does not address the potential benefit of switching to these drugs that may be available to patients who develop tardive dyskinesia while taking a conventional antipsychotic.
It is notable in this respect that the cost estimates we use below for first-generation antipsychotics from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) (Reference Rosenheck, Leslie and SindelarRosenheck et al, 2006), incorporate the cost of the transition of about half of the patients receiving these drugs to treatment with second-generation antipsychotics, as occurred during the trial. The cost data used in our descriptive cost-effectiveness analysis thus favour the newer drugs because they incorporate the naturalistic cost impact of patients' switch in treatment. Thus, even though tardive dyskinesia was not the reason for most of the treatment switches in CATIE, our cost estimates include the costs that would be incurred through switching patients from conventional to atypical antipsychotics – switching that would further lower the risk of tardive dyskinesia in real-world practice and that might foster recovery from cases of this side-effect that had occurred. The 4.5% benefit in tardive dyskinesia reduction with second-generation antipsychotics would thus be reduced in real-world practice.
Tardive dyskinesia and quality of life
Although assessment of quality of life is an essential feature of health outcomes research, only one small study (n=60) has examined the association of tardive dyskinesia and reduced quality of life. This study found modestly lower quality of life in patients with this condition, but the sample was too small to allow statistical adjustment for the greater severity of schizophrenia symptoms that was also found in these patients and could have been the cause of their lower quality of life (Reference Browne, Roe and LaneBrowne et al, 1996).
To examine this issue further we present more data from the two VA clinical trials referenced above (Rosenheck et al, Reference Rosenheck, Cramer and Xu1997, Reference Rosenheck, Perlick and Bingham2003). In these analyses average scores on the Heinrichs–Carpenter Quality of Life Interview (QoLI; Reference Heinrichs, Hanlon and CarpenterHeinrichs et al, 1984) were compared across different levels of tardive dyskinesia, as defined by the overall global severity scale of the AIMS. The QoLI is a 20-item, schizophrenia-specific scale that assesses social and instrumental functioning, community activities and intrapsychic (subjective) well-being. Items are scored from 0 to 6 (total score range 0–120), with higher scores indicating better quality of life. Because (as noted above) symptoms of schizophrenia are likely to have a potentially confounding impact on analysis of the relationship between tardive dyskinesia and quality of life, we also analysed these data with multivariate adjustment for the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987).
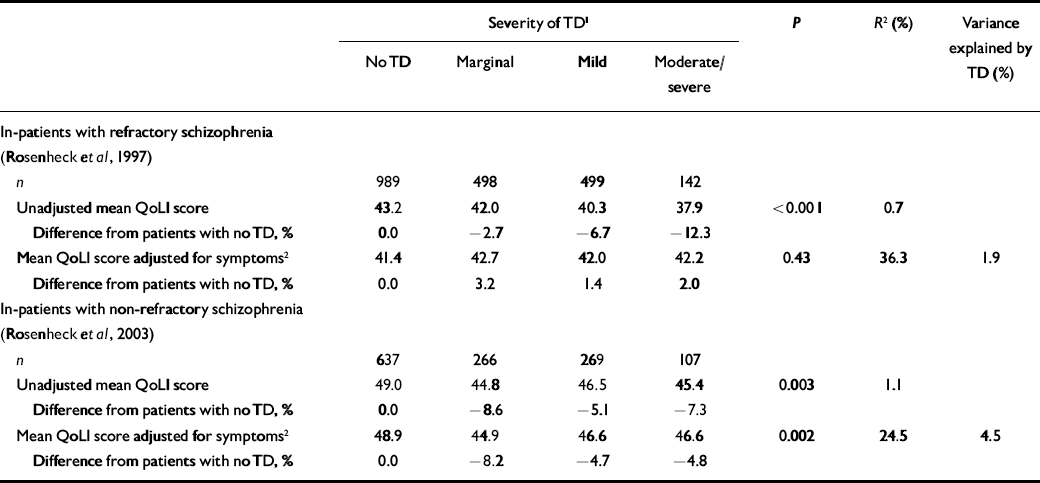
Data from the clozapine–haloperidol study (Reference Rosenheck, Cramer and XuRosenheck et al, 1997) showed a small but progressive decline in quality of life with more severe tardive dyskinesia (Table 2). Patients with moderate or severe tardive dyskinesia had 12.3% lower QoLI scores than those without this side-effect, and severity of dyskinesia explained less than 1% of QoLI variance. After covarying for schizophrenia symptoms there was no significant difference in QoLI scores across tardive dyskinesia severity levels (P=0.43) (Table 2), but the model explained 36% of the variance. Differences in symptom severity in this sample thus entirely accounted for the modest differences in quality of life associated with tardive dyskinesia.
Table 2 Quality of Life Interview scores (least square means) by severity of tardive dyskinesia (analysis of covariance based on all available observations from 423 and 309 unique patients respectively in each sample)
| Severity of TD 1 | P | R 2 (%) | Variance explained by TD (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No TD | Marginal | Mild | Moderate/severe | |||||||
| In-patients with refractory schizophrenia (Reference Rosenheck, Cramer and XuRosenheck et al, 1997) | ||||||||||
| n | 989 | 498 | 499 | 142 | ||||||
| Unadjusted mean QoLI score | 43.2 | 42.0 | 40.3 | 37.9 | <0.001 | 0.7 | ||||
| Difference from patients with no TD, % | 0.0 | -2.7 | -6.7 | -12.3 | ||||||
| Mean QoLI score adjusted for symptoms 2 | 41.4 | 42.7 | 42.0 | 42.2 | 0.43 | 36.3 | 1.9 | |||
| Difference from patients with no TD, % | 0.0 | 3.2 | 1.4 | 2.0 | ||||||
| In-patients with non-refractory schizophrenia (Reference Rosenheck, Perlick and BinghamRosenheck et al, 2003) | ||||||||||
| n | 637 | 266 | 269 | 107 | ||||||
| Unadjusted mean QoLI score | 49.0 | 44.8 | 46.5 | 45.4 | 0.003 | 1.1 | ||||
| Difference from patients with no TD, % | 0.0 | -8.6 | -5.1 | -7.3 | ||||||
| Mean QoLI score adjusted for symptoms 2 | 48.9 | 44.9 | 46.6 | 46.6 | 0.002 | 24.5 | 4.5 | |||
| Difference from patients with no TD, % | 0.0 | -8.2 | -4.7 | -4.8 | ||||||
QoLI, Quality of Life Interview; TD, tardive dyskinesia
1. Global scores on the Abnormal Involuntary Movements Scale
2. Rated on the Positive and Negative Syndrome Scale
Data from the VA study of patients with non-refractory disorder (Reference Rosenheck, Perlick and BinghamRosenheck et al, 2003) also showed significantly lower quality-of-life scores with tardive dyskinesia (by 5–9%; Table 2), again explaining only 1% of the variance. After adjusting for symptom severity, differences in QoLI scores with tardive dyskinesia remained statistically significant (P=0.002), but the variance explained by the model increased to 24%. Severity of tardive dyskinesia thus explained only 4.5% of total explained variance in quality of life when symptoms of schizophrenia were also considered.
Although these analyses demonstrate a significant relationship between tardive dyskinesia and a disease-specific measure of quality of life the effect of this disorder was small, with a 5–12% reduction in magnitude of QoLI scores and an r 2 value of about 1%, suggesting that it explained only 1% of the variance in quality of life, a far smaller proportion than symptoms of schizophrenia.
Tardive dyskinesia and quality of life in cost-effectiveness analysis
Cost-effectiveness analysis is based on the use of a common metric for health outcomes that is not specific to any particular illness. The standard measure of quality of life for such analyses, as recommended by the 1996 US Public Health Service Task Force (Reference Gold, Siegel and RussellGold et al, 1996), is the quality-adjusted life-year (QALY), a year of life rated on a scale from 0 (worst possible health) to 1 (perfect health). These units are assessed with methods in which disease-specific health states are evaluated with universal health equivalents such as the risk of death or years of life lost, and with procedures such as the ‘standard gamble’ or the ‘time trade-off’.
A recent series of studies has estimated QALYs in schizophrenia using a multistep procedure (Lenert et al, Reference Lenert, Sturly and Rupnow2003, Reference Lenert, Sturley and Rapaport2004; Reference Mohr, Cheng and ClaxtonMohr et al, 2004). First, on the basis of cluster analysis of a large PANSS data-set, eight empirically derived schizophrenia health states were defined. Next, a group of experts working with professional actors developed video scripts to convey these health states to raters, along with five common drug side-effects, including severe tardive dyskinesia. Using these video presentations, the health states were rated by 620 members of the general public using the standard gamble, the favoured method for measuring QALYs (Reference Gold, Siegel and RussellGold et al, 1996). In the standard gamble people are queried about the risk of death that they would accept to be cured of each health state, e.g. ‘If you were in this health state, how great a risk of dying would you accept to be fully cured?’ In particular, one video clip, determined by the panel of experts to represent a case of severe tardive dyskinesia, showed a man with relentless jaw and arm movements, who had difficulty speaking and with whom his doctor expresses sympathy but no hope of cure. People imagining that they were in this health state were willing to accept a 14.3% chance of death on average if they could be cured of this problem, a modest effect consistent with the analysis of QoLI data reported above. The resultant QALY rating, determined by subtracting the accepted risk of death from 1.0 (perfect health) would be 0.857. The QALYs derived through this series of videotaped vignettes using this method ranged from 0.44 to 0.88 for the eight schizophrenia health states, whereas QALYs for side-effects ranged from a low of 0.857 for severe tardive dyskinesia to 0.959 for weight gain.
Cost-effectiveness
In summarising the diverse array of information presented above, we again follow the method of calculation recommended by the US Public Health Service Task Force (Reference Gold, Siegel and RussellGold et al, 1996) for comparing the cost-effectiveness of treatments: the incremental cost-effectiveness ratio (ICER), the ratio of differences in the cost of treatments to differences in their benefits. This ratio reflects how much one would have to pay, on average, for a certain benefit. We apply a range of estimated ICERs in a sensitivity analysis of best-case and worst-case scenarios, to evaluate the consistency of the results across different measures and assumptions based on the diverse data reviewed above.
Cost differences
For the incremental cost of second-generation antipsychotics we use three annualised estimates derived from the CATIE study (Reference Rosenheck, Leslie and SindelarRosenheck et al, 2006) (Table 3), a randomised trial of over 1400 patients assigned to receive one first-generation antipsychotic (perphenazine) or one of four second-generation drugs (olanzapine, risperidone, quetiapine or ziprasidone) and followed for 18 months. The three cost estimates include:
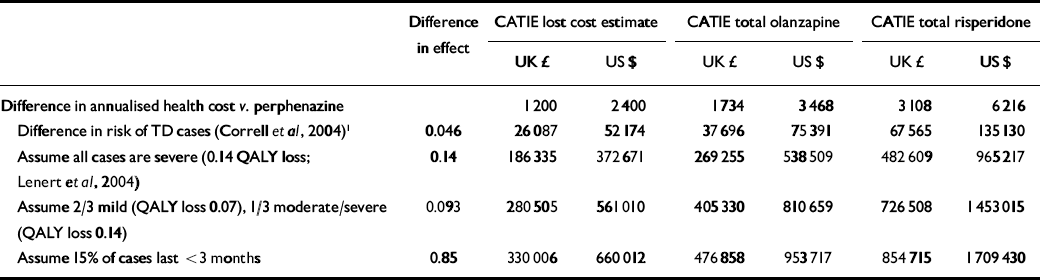
Table 3 Cost-effectiveness ratio - second-generation v. first-generation antipsychotics: sensitivity analysis
| Difference in effect | CATIE lost cost estimate | CATIE total olanzapine | CATIE total risperidone | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UK £ | US $ | UK £ | US $ | UK £ | US $ | |||||
| Difference in annualised health cost v. perphenazine | 1 200 | 2 400 | 1 734 | 3 468 | 3 108 | 6 216 | ||||
| Difference in risk of TD cases (Reference Correll, Leucht and KaneCorrell et al, 2004) 1 | 0.046 | 26 087 | 52 174 | 37 696 | 75 391 | 67 565 | 135 130 | |||
| Assume all cases are severe (0.14 QALY loss; Reference Lenert, Sturley and RapaportLenert et al, 2004) | 0.14 | 186 335 | 372 671 | 269 255 | 538 509 | 482 609 | 965 217 | |||
| Assume 2/3 mild (QALY loss 0.07), 1/3 moderate/severe (QALY loss 0.14) | 0.093 | 280 505 | 561 010 | 405 330 | 810 659 | 726 508 | 1 453 015 | |||
| Assume 15% of cases last <3 months | 0.85 | 330 006 | 660 012 | 476 858 | 953 717 | 854 715 | 1 709 430 | |||
CATIE, Clinical Antipsychotic Trials of Intervention Effectiveness; QALY, quality-adjusted life-year; TD, tardive dyskinesia
1. These estimates represent the cost per QALY assuming each case of TD is poor health equivalent to death (QALY loss due to TD=1.0)
-
(a) a lower-bound estimate of £1200 ($2400) per year based on stable drug cost differences of £100 ($200) per month between perphenazine and second-generation antipsychotics during the last 9 months of the trial, when many patients assigned to perphenazine were taking the newer drugs (Reference Rosenheck, Leslie and SindelarRosenheck et al, 2006);
-
(b) an intermediate cost estimate of £1700 ($3500) per year (the annualised difference in monthly total health costs between perphenazine and olanzapine, the next least costly treatment, over the entire trial);
-
(c) an upper-bound cost difference of £3100 ($6200) per year, representing the annualised total health cost difference between perphenazine and quetiapine, the most expensive treatment in the trial.
These annualised cost estimates are similar to those suggested in two sets of published cost estimates presented for application to the CATIE data (Reference Basil, Mathews and AdetunjiBasil et al, 2006; Reference DavisDavis, 2006) as well as to the results of two other clinical trials that compared the costs of first- and second-generation antipsychotics (Reference Rosenheck, Perlick and BinghamRosenheck et al, 2003; Reference Jones, Barnes and DaviesJones et al, 2006). None of the specific trials that evaluated risk of tardive dyskinesia included cost estimates, but we believe these estimated cost differences represent an appropriate range and are consistent with many studies.
Cost-effectiveness
In our first estimate of cost-effectiveness we used the best-case scenario for second-generation antipsychotics, i.e. the smallest estimate of increased costs and the greatest estimate of health gain. The lowest cost difference from CATIE (£1200 or $2400) and greatest benefit for second-generation drugs (4.6% fewer cases) as suggested by (Correll et al (Reference Correll, Leucht and Kane2004) yielded an ICER of £26 000 ($52 000) per case of tardive dyskinesia avoided, increasing to £68 000 ($135 000) per case avoided with the higher cost estimates (Table 3, row 2). In terms of cost per QALY, this analysis is the equivalent of assuming that each case of tardive dyskinesia prevented represents a 1.0 QALY gain, i.e. the equivalent of avoiding death and maintaining perfect health for 4.6% of those treated. In our second approximation we used the more realistic published estimate of a 0.143 reduction in QALYs per case of severe tardive dyskinesia (Reference Lenert, Sturley and RapaportLenert et al, 2004) rather than the 1.0 QALY estimate used in the first analysis. In this second analysis we assumed that all cases of tardive dyskinesia were severe (in spite of the evidence presented above that this is not the case). In this set of analyses ICERs ranged from £186 000 ($373 000) per QALY to £483 000 ($965 000) per QALY (Table 3, row 3). Since most tardive dyskinesia is, as we have shown, mild in severity, the third set of analyses estimated – again conservatively – that two-thirds of cases were mild and a third moderate to severe. We further assumed that those with mild disorder lose half as many QALYs (0.7 QALYs) as those with moderate to severe symptoms (the 0.143 QALY estimate presented above). With two-thirds of cases losing 0.7 QALYs and one-third losing 0.143 QALY, the weighted average loss across the entire population of patients with tardive dyskinesia would be 0.093 QALYs gained per case avoided. Using this severity-adjusted estimate of QALY benefits with second-generation antipsychotics, ICERs were found to increase further, ranging from £280 000 ($561 000) per QALY to £727 000 ($1 453 000) per QALY (Table 3, row 4). Finally, we incorporated the assumption that at least 15% of cases recover and thus included only 85% of second-generation antipsychotic benefits. Estimated ICERs under these assumptions increased further to a range of £330 000 ($660 000) per QALY to £855 000 ($1 700 000) per QALY (Table 3, row 5).
Extending the planning horizon
The analysis we have presented represents a 1-year time horizon occurring roughly at the mid-point of potentially lifelong schizophrenia. Because longitudinal data are not available on the long-term risk of tardive dyskinesia with second-generation antipsychotic medication, it is not possible to estimate precisely the reduction in risk with these drugs over a lifetime. It is appropriate, however, to focus on a 5-year projection at present, because between 2007 and 2012 patents on both risperidone and olanzapine are likely to expire, bringing substantially lower prices for these drugs.
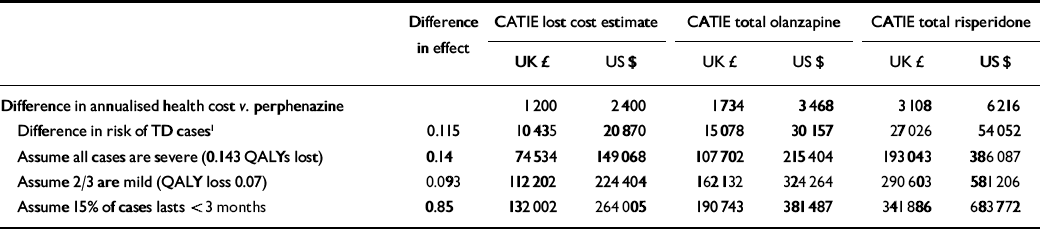
In a best-case clinical scenario for second-generation antipsychotics the annual attributable risk of 4.6% would accumulate linearly over 5 years. The incremental risk for first-generation drugs is thus 5 × 0.046=23% at the end of 5 years, with an average annualised attributable risk of half this magnitude (11.5%). Since costs and risks accrue simultaneously, discounting is not necessary. Assuming that cost differences remain the same over the 5 years, we estimate cost-effectiveness ratios of £75 000 ($149 000) per QALY to £193 000 ($386 000) per QALY, assuming all cases of tardive dyskinesia are severe (Table 4); £112 000 ($224 000) per QALY to £291 000 ($581 000) per QALY, assuming two-thirds of cases are mild; and £132 000 ($264 000) per QALY to £342 000 ($684 000) per QALY assuming 15% of cases recover. This projection is conservative because rates of tardive dyskinesia will be lower than projected among the group taking first-generation drugs because the new-case incidence of this side-effect declines with age, and because we have built into the cost estimates the assumption that half the sample taking first-generation drugs switches to the newer antipsychotics, which will both prevent some cases of tardive dyskinesia and facilitate recovery in some incident cases.
Table 4 Cost-effectiveness ratio - second-generation v. first-generation antipsychotics: 5-year projection
| Difference in effect | CATIE lost cost estimate | CATIE total olanzapine | CATIE total risperidone | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UK £ | US $ | UK £ | US $ | UK £ | US $ | |||||
| Difference in annualised health cost v. perphenazine | 1 200 | 2 400 | 1 734 | 3 468 | 3 108 | 6 216 | ||||
| Difference in risk of TD cases 1 | 0.115 | 10 435 | 20 870 | 15 078 | 30 157 | 27 026 | 54 052 | |||
| Assume all cases are severe (0.143 QALYs lost) | 0.14 | 74 534 | 149 068 | 107 702 | 215 404 | 193 043 | 386 087 | |||
| Assume 2/3 are mild (QALY loss 0.07) | 0.093 | 112 202 | 224 404 | 162 132 | 324 264 | 290 603 | 581 206 | |||
| Assume 15% of cases lasts <3 months | 0.85 | 132 002 | 264 005 | 190 743 | 381 487 | 341 886 | 683 772 | |||
CATIE, Clinical Antipsychotic Trials of Intervention Effectiveness; QALY, quality-adjusted life-year; TD, tardive dyskinesia
1. These estimates represent the cost per QALY assuming each case of TD is poor health equivalent to death (QALY loss due to TD=1.0)
DISCUSSION
With recent evidence that second-generation antipsychotics may be no more effective and pose no greater risk of Parkinsonian side-effects than first-generation drugs, reduced risk of tardive dyskinesia may be their principal remaining advantage. Although several recent independent trials as well as several observational studies have not found substantial advantages for the newer antipsychotics in reducing the risk of tardive dyskinesia, we based our evaluation on a meta-analysis of older studies that found an annual incidence of this side-effect that was lower by 4.6% with second-generation drugs. We considered important components of health outcome neglected in previous studies of tardive dyskinesia, including severity, duration and quality of life attributable to this condition, and approximated a 5-year planning horizon.
Because available data are limited we used estimates from a variety of sources, in each instance presenting both best-case and worst-case scenarios. Even under the best-case clinical 5-year scenario, the cost per QALY for second-generation antipsychotics based on reducing risk of tardive dyskinesia ranged from £75 000 ($150 000) per QALY to £190 000 ($380 000) per QALY.
The approximate cut-off for reimbursement of drugs in the UK, Australia and Canada has been reported to range from £20 000 ($40 000) per QALY to £25 000 ($50 000) per QALY (Reference NeumannNeumann, 2005: pp. 99, 102–3, 105). Thus, reduction of risk of tardive dyskinesia with second-generation antipsychotics as estimated here, even for the best-case scenario, does not seem likely to meet conventional standards for cost-effective treatments. These findings could be an argument for lowering payments for these products to a level at which tardive dyskinesia benefits would be worth the price.
Our study is limited by the small number of randomised trials that have compared the risk of tardive dyskinesia between the two classes of antipsychotics. Data on severity, duration and QALYs of states of this side-effect are also quite limited. The applicability of US cost data to the UK situation is unclear, but cost differences in the US CATIE trial (Reference Rosenheck, Leslie and SindelarRosenheck et al, 2006) were very similar to those of the UK (Cost Utility of the Latest Antipsychotics in Schizophrenia Study (CUtLASS; Reference Jones, Barnes and DaviesJones et al, 2006). We also did not account for diabetes or other metabolic risks, which would result in poorer health and increased costs, further shifting the cost-effectiveness balance against the second-generation drugs.
Our estimates also do not apply to elderly people, in whom studies have shown greater risks of tardive dyskinesia with first-generation antipsychotics (Reference Jeste, Caliguri and PaulsenJeste et al, 1995; Reference Correll, Leucht and KaneCorrell et al, 2004), although most older patients do not receive antipsychotic treatment for schizophrenia, the clinical focus of this review. One recent study of older American patients found no net benefit of second-generation drugs even compared with placebo in Alzheimer's disease (Reference Schneider, Tariot and DagermanSchneider et al, 2006).
It must be acknowledged that owing to the limited data available and the necessity of estimating outcomes and costs from different studies, this presentation is based on a bounding argument in which we found that even in the best-case scenario for second-generation antipsychotics benefits were unlikely to justify the increased costs by conventional standards. Since we lack information on the probability distribution of the various outcomes, our data are insufficient to support a full probabilistic sensitivity analysis (Reference BriggsBriggs, 2005), the standard of cost-effectiveness analysis for policy making (Reference Claxton, Sculpher and McCabeClaxton et al, 2005). However, such analysis may not be as critical in this case as in other situations, because even when we examined the best-case deterministic scenario for the newer antipsychotics, costs per QALY exceeded the generally acceptable threshold. Probabilistic sensitivity analysis, which tests whether our results could have resulted from chance alone, would not be likely to change this conclusion to one favouring these drugs.
There has been considerably more controversy in the USA than in the UK about whether cost-effectiveness analysis should influence clinical practice or healthcare policy (Reference UbelUbel, 2000; Reference NeumannNeumann, 2005). The analyses presented here suggest that, in view of recent studies showing little or no advantage of second-generation antipsychotics for symptoms or extrapyramidal side-effects, reduced risk of tardive dyskinesia does not appear likely to provide sufficient health benefit by itself to justify the predominant use of these agents in treatment of schizophrenia. However, in view of the many limitations noted above, the implications of these findings for either policy or practice must be applied with considerable caution and must ultimately be determined by public debate among relevant stake-holders and governmental agencies (Reference Daniels and SabinDaniels & Sabin, 2002).
Acknowledgements
David Paltiel, PhD, provided helpful suggestions on cost-effectiveness methodology.







eLetters
No eLetters have been published for this article.