Aggression is a normal human behaviour, but the ability to control aggressive impulses may be impaired or lost in a variety of mental disorders or after brain injury. People may also present in the absence of such disorders with repeated, discrete episodes of aggressive acts that do not appear to have been externally provoked (or are out of proportion to any such provocation) and are reportedly because of a failure to resist internally driven aggressive impulses. The DSM–IV 1 attaches a specific diagnostic category of intermittent explosive disorder to such idiopathic aggression, although the ICD–10 2 does not. The DSM–IV approach, which built on the transformation of USA diagnostic behaviour with DSM–III, 3 is still somewhat controversial, Reference Mayes and Horwitz4 not least because it was seen as in part promotional of pharmaceutical interventions.
Anticonvulsants and lithium (as a group known as mood stabilisers) have long been reported to have utility in the treatment of aggression in individuals with epilepsy Reference Ross and Jackson5 and those with intellectual disability, Reference Worrall, Moody and Naylor6,Reference Dale7 but clear evidence for their effectiveness is lacking. Despite the lack of evidence, recent reports suggest that mood stabilisers are frequently used off-license for this indication in hospital settings. A survey of psychiatric in-patients in the UK found that nearly a third were being prescribed at least one mood stabiliser, and in 41% of these cases, the main reason given by the prescriber was to control aggression. Reference Haw and Stubbs8
Previous systematic reviews of mood stabilisers are not optimally informative regarding the treatment of aggression. Several reviews have been conducted that focused on individuals with borderline personality disorder, and even in this fairly homogeneous group, the findings conflict. Reference Mercer, Douglass and Links9,Reference Nose, Cipriani, Biancosino, Grassi and Barbui10 In these reviews, studies were included in which the treatment of aggression was not the primary aim, and therefore they included individuals who did not have high baseline levels of aggression. Another recent systematic review of anticonvulsant drugs, by definition excluding lithium, did not carry out a meta-analysis of their efficacy in reducing aggression due to concerns that the distributions of the data were skewed, and therefore conventional meta-analytic techniques may be inappropriate. Reference Huband, Ferriter, Nathan and Jones11 However, appropriate statistical methods are available for transforming summary data, which can then be used reliably for further analysis.
The aims of this review were to conduct a systematic review and meta-analysis of the effectiveness of mood stabilisers for repeated or excessive aggression: using well-defined criteria for aggression, including but not confined to intermittent explosive disorder; based on randomised placebo-controlled trials of adults recruited specifically for the treatment of that aggression, with any mood stabiliser, including lithium.
Method
Definition of aggression
We adopted the definition used by Berkowitz, that aggression refers to goal-directed motor behaviour that has a deliberate intent to harm or injure another object or person. Reference Berkowitz12 This definition includes verbal and physical acts of aggression, and excludes anger or hostility where there has been no physical act. We required that aggression was measured prospectively (sometimes categorised as ‘state’ aggression), rather than measured retrospectively or globally (or ‘trait’ aggression). We have included only studies in which participants were selected for the trial as having predefined problems with impulsive or repetitive aggression.
Search strategy and selection
We searched for studies, published in English, of randomised placebo-controlled trials of any duration that compared mood stabilisers with placebo for the treatment of impulsive aggression in adults using a comprehensive search strategy (see the online supplement to this paper). We excluded studies of individuals with intellectual disability and neurodegenerative disorders, and studies that did not recruit individuals specifically for the treatment of aggression. We did not exclude studies in which individuals had a personality disorder or substance use disorder, providing they were recruited for the primary purpose of treating repeated, impulsive aggression.
We searched MEDLINE, Embase, PsycINFO and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception (accessed November 2009) using a comprehensive search strategy (online supplement). We also searched the System for Information of Grey Literature (SIGLE), ISI Web of Science conference proceedings, Index to Thesis/Electronic Thesis Online Service and Google Scholar (1989–November 2009). In addition, we searched the citations of all references retrieved. Two authors (J.A. and R.G.) independently reviewed the titles and abstracts obtained from the electronic searches. The full articles were obtained for further scrutiny where indicated. Disagreements were resolved by discussion with a third author (R.M.J.). For the majority of included studies we were able to extract sufficient information from the published findings; for the remainder, we contacted the authors and requested raw data.
Outcome measures
We used the frequency and/or severity of aggressive behaviour as the primary outcome measure, the latter being a total or global score derived from both frequency and severity of aggressive behaviour.
Data extraction and assessment of risk of bias
Two authors independently extracted data using a predesigned and piloted template. The same two authors independently assessed risk of bias for each study with respect to randomisation, masking and description of withdrawals, scored according to the Jadad scale, Reference Jadad, Moore, Carroll, Jenkinson, Reynolds and Gavaghan13 as well as for risk of bias because of selective reporting of results or failure to account adequately for individuals dropping out of the study (specifically whether an intention-to-treat analysis was carried out). Studies were categorised as at ‘high’ risk of bias if they had a Jadad score of two or less, or if there was evidence of selective reporting of results, or no intention-to-treat analysis. Otherwise, they were categorised as ‘low’ risk of bias.
Data synthesis and analysis
We calculated standardised mean differences (SMDs) in aggression scores between the intervention and placebo groups at the end of the trial. We analysed cross-over studies as having a parallel design. We carried out a priori subgroup analysis of studies by drug group (phenytoin, carbamazepine/oxcarbazepine, valproate/divalproex, levetiracetam and lithium). For studies that reported outcomes at more than one time point, we selected the final time point for analysis.
Distributions for which the standard deviation divided by the mean was <1 were considered skewed. Reference Altman and Bland14 Visual inspection of the distributions of individual study data made available to us (four studies) showed distributions that were skewed to the left (i.e. approximating a log-normal distribution). As standard meta-analytic methods assume normality we calculated log transformation of the summary data for each study using appropriate methods Reference Higgins, White and Anzures-Cabrera15 and used these data for further analysis. We repeated all analysis using the untransformed summary data for comparison.
We calculated the proportion of statistical heterogeneity for each outcome (i.e. frequency and severity of aggression) using I 2. We undertook a random effect meta-analysis where substantial heterogeneity was found (I 2>50%), and a fixed-effect meta-analysis elsewhere. We investigated factors that potentially contributed to heterogeneity using sensitivity analyses. In these sensitivity analyses, we first excluded each study in turn. We then investigated the effect of excluding studies that we considered had a high risk of bias, and studies that measured only the frequency and not severity of aggression. We used STATA (version 10 for Mac), including the METAN module for all analyses.
Results
Study characteristics
We identified 1726 potentially relevant records, of which only 52 referred to trials of mood stabilisers to treat aggression (Fig. 1). Of these, 29 were excluded as either they were not randomised controlled trials (RCTs, 18 studies) or because the patient group did not meet our inclusion criteria (5 studies were with adults with an intellectual disability and 6 with participants who had an organic illness or neurological injury). A further 13 studies were then excluded (online Table DS1) because the participants were not recruited primarily for the treatment of aggression (9 studies), there was no placebo control (1 study) or there was no measure of aggressive behaviour (3 studies). Categories for exclusion were mutually exclusive, but in practice, most of the excluded studies met more than one exclusion criterion. Ten studies therefore remained for detailed analysis (Table 1; see also online Table DS2 for a more detailed version).
Table 1 Summary of included randomised placebo-controlled trials of mood stabilisers for the treatment of aggressive behaviour (see online Table DS2 for a more detailed version of this table)
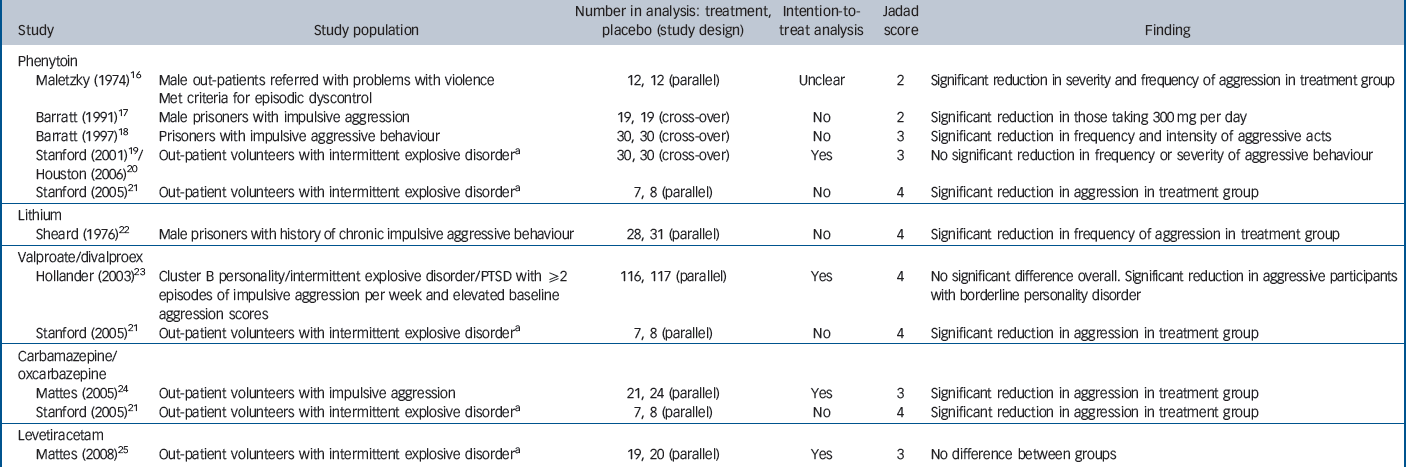
| Study | Study population | Number in analysis: treatment, placebo (study design) | Intention-to-treat analysis | Jadad score | Finding |
|---|---|---|---|---|---|
| Phenytoin | |||||
| Maletzky (1974) Reference Maletzky and Klotter16 | Male out-patients referred with problems with violence Met criteria for episodic dyscontrol | 12, 12 (parallel) | Unclear | 2 | Significant reduction in severity and frequency of aggression in treatment group |
| Barratt (1991) Reference Barratt, Stanford, Felthous and Kent17 | Male prisoners with impulsive aggression | 19, 19 (cross-over) | No | 2 | Significant reduction in those taking 300 mg per day |
| Barratt (1997) Reference Barratt, Stanford, Kent and Felthous18 | Prisoners with impulsive aggressive behaviour | 30, 30 (cross-over) | No | 3 | Significant reduction in frequency and intensity of aggressive acts |
| Stanford (2001) Reference Stanford, Houston, Mathias, Greve, Villemarette-Pittman and Adams19 /Houston (2006) Reference Houston and Standford20 | Out-patient volunteers with intermittent explosive disordera | 30, 30 (cross-over) | Yes | 3 | No significant reduction in frequency or severity of aggressive behaviour |
| Stanford (2005) Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21 | Out-patient volunteers with intermittent explosive disordera | 7, 8 (parallel) | No | 4 | Significant reduction in aggression in treatment group |
| Lithium | |||||
| Sheard (1976) Reference Sheard, Marini, Bridges and Wagner22 | Male prisoners with history of chronic impulsive aggressive behaviour | 28, 31 (parallel) | No | 4 | Significant reduction in frequency of aggression in treatment group |
| Valproate/divalproex | |||||
| Hollander (2003) Reference Hollander, Tracy, Swann, Coccaro, McElroy and Wozniak23 | Cluster B personality/intermittent explosive disorder/PTSD with ≥2 episodes of impulsive aggression per week and elevated baseline aggression scores | 116, 117 (parallel) | Yes | 4 | No significant difference overall. Significant reduction in aggressive participants with borderline personality disorder |
| Stanford (2005) Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21 | Out-patient volunteers with intermittent explosive disordera | 7, 8 (parallel) | No | 4 | Significant reduction in aggression in treatment group |
| Carbamazepine/oxcarbazepine | |||||
| Mattes (2005) Reference Mattes24 | Out-patient volunteers with impulsive aggression | 21, 24 (parallel) | Yes | 3 | Significant reduction in aggression in treatment group |
| Stanford (2005) Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21 | Out-patient volunteers with intermittent explosive disordera | 7, 8 (parallel) | No | 4 | Significant reduction in aggression in treatment group |
| Levetiracetam | |||||
| Mattes (2008) Reference Mattes25 | Out-patient volunteers with intermittent explosive disordera | 19, 20 (parallel) | Yes | 3 | No difference between groups |
Of the included studies, one study Reference Houston and Standford20 reported results of an extension of a previous one, Reference Stanford, Houston, Mathias, Greve, Villemarette-Pittman and Adams19 and here we obtained individual patient data from the authors and calculated relevant summary outcome values on the basis of intention to treat. We obtained individual patient data from another study, Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21 and additional relevant summary values for two further studies. Reference Mattes24,Reference Mattes25 One trial Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21 had three treatment arms, which we have reported as separate studies in our results.
We were able to extract sufficient data from eight papers (a total of ten trials) to be used in the meta-analysis, which included 489 participants, 247 of whom received the active treatment. Four of the studies used phenytoin (all using a dose of 300 mg per day), and one study each used lithium (serum levels 0.6–1 mEq/l), divalproex (serum levels maintained between 80–120 μ g/ml); valproate (dose 750 mg/day), carbamazepine (dose 450 mg/day); oxcarbazepine (dose 1200–2400 mg/day) and levetiracetam (dose 1000–3000 mg/day). All but two studies Reference Maletzky and Klotter16,Reference Barratt, Stanford, Felthous and Kent17 had a Jadad score of three or above.
Only three studies Reference Hollander, Tracy, Swann, Coccaro, McElroy and Wozniak23–Reference Mattes25 clearly reported results using intention-to-treat analysis, and we were able to calculate values using intention-to-treat analysis from one further study reported across two articles. Reference Stanford, Houston, Mathias, Greve, Villemarette-Pittman and Adams19,Reference Houston and Standford20 All studies were carried out in North America. They all reported gaining informed consent from participants, although explicit reporting of institutional review was rare. In three of the studies, participants were diagnosed as having intermittent explosive disorder or intermittent explosive disorder – revised (Table 1 and online Table DS2). The difference between intermittent explosive disorder and intermittent explosive disorder – revised is that the latter does not exclude those who have a comorbid substance use disorder. The other studies selected participants on the basis of frequent impulsive aggressive behaviour without necessarily making a diagnosis. Two studies recruited prisoners, the remainder were out-patients, the majority of whom were recruited by advertisement and were not necessarily diagnosed as having a psychiatric disorder prior to the study. All studies excluded people who had a diagnosis of a psychotic disorder or bipolar affective disorder or who were already prescribed psychoactive medication. All trials were between 6 and 12 weeks duration.
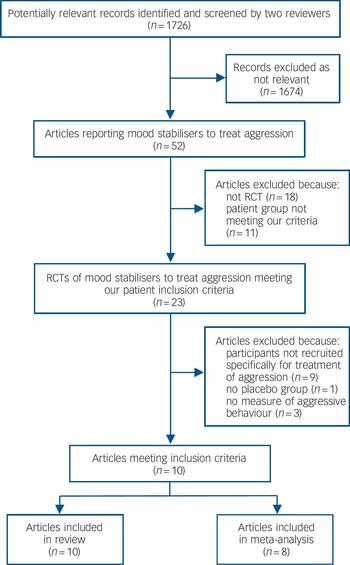
Fig. 1 Flow chart of study selection.
RCT, randomised controlled trial.
Overall change in aggression
A pooled analysis of the ten studies showed an overall significant reduction in the severity of aggressive behaviour (Fig. 2; SMD = –1.02, 95% CI –1.54 to –0.50), although heterogeneity was high (I 2 = 84.7%). We repeated the analysis on the untransformed data and found that the reduction in the severity of aggressive behaviour remained significant (overall SMD = –0.82, 95% CI –1.29 to –0.36, I 2 = 81.7%). When analysed by drug type, significant effects were found in the pooled analysis of the three phenytoin trials (SMD = –1.34, 95% CI –2.16 to –0.52), in the one lithium trial (SMD = –0.81, 95% CI –1.35 to –0.28), and the two oxcarbazepine/carbamazepine trials (SMD = –1.20, 95% CI –1.83 to –0.56). No reduction in aggression was found for the pooled results of the two valproate studies or for the one levetiracetam study.

Fig. 2 Forrest plot of studies of randomised placebo-controlled studies of mood stabilisers for the treatment of aggression.
SMD, standardised mean difference.
Sensitivity analysis
We removed each study in turn and found little change in heterogeneity in pooled analyses. We then analysed only studies that reported frequency of aggression as the outcome measure. The pooled results of these studies Reference Maletzky and Klotter16,Reference Barratt, Stanford, Kent and Felthous18–Reference Houston and Standford20,Reference Sheard, Marini, Bridges and Wagner22 showed a significant effect of treatment over placebo on aggression (SMD = – 1.1, 95% CI –1.75 to –0.46, I 2 = 76.6%). Similarly, a significant relationship was also found between mood stabiliser medication and aggression in the six studies that reported global or total severity of aggression Reference Maletzky and Klotter16,Reference Barratt, Stanford, Kent and Felthous18,Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21,Reference Hollander, Tracy, Swann, Coccaro, McElroy and Wozniak23–Reference Mattes25 (pooled estimate; SMD = –1.20, 95% CI –1.90 to –0.49, I 2 = 87.8%).
We then analysed only those studies in which we considered the risk of bias to be low (i.e. studies that we determined had a Jadad score of three or more, and had used intention-to-treat analysis). There was one study each that had investigated phenytoin, Reference Stanford, Houston, Mathias, Greve, Villemarette-Pittman and Adams19,Reference Houston and Standford20 valproate, Reference Hollander, Tracy, Swann, Coccaro, McElroy and Wozniak23 oxcarbazepine Reference Mattes24 and levitaracetam. Reference Mattes25 Of these, only the study into oxcarbazepine alone showed a significant reduction in aggression (SMD = –1.02, 95% CI –1.64 to –0.39). The pooled results of these four studies (that included a total of 347 participants) showed no significant reduction in aggression (SMD = – 0.28, 95% CI –0.73 to 0.17, I 2 = 71.4%;Fig. 3).
Adverse events
Two studies reported headache Reference Houston and Standford20,Reference Stanford, Helfritz, Conklin, Villemarette-Pittman, Greve and Adams21,Reference Mattes25 as a side-effect, causing discontinuation in one participant. One study reported high rates of sedation (65%) and dizziness (20%). Reference Mattes25 Another study reported nausea, asthenia, increased appetite, depression, tremor and nervousness as side-effects. Reference Hollander, Tracy, Swann, Coccaro, McElroy and Wozniak23 Side-effects were reported to be minor but were not detailed in two studies, Reference Mattes24,Reference Barratt, Stanford, Kent and Felthous18 and were not mentioned in three other studies.
Discussion
Summary of evidence
The current study reviewed randomised placebo-controlled trials that used a mood stabiliser to treat aggressive behaviour. We found evidence that treatment reduced either the frequency or severity of aggressive behaviour. There was evidence for significant reductions in aggression for those taking phenytoin, lithium and carbamazepine/oxcarbazepine, but not valproate or levetiracetam. Almost all of the studies reported adequate methods of randomisation and concealment, but many suffered from a risk of bias as a result of inadequate reporting of individuals who had dropped out, particularly with lack of intention-to-treat analysis. When we included only those studies in which there was a low risk of bias, we found no pooled evidence of any significant benefit. When studies were analysed by drug category, only one category – oxcarbazepine – showed any significant effect, but this finding was based on only one study. Side-effects, where reported, tended to be mild; there were few cases of participants discontinuing the trial as a result of them.

Fig. 3 Forrest plot of studies of randomised placebo-controlled studies of mood stabilisers for the treatment of aggression including only those studies with a low risk of bias.
SMD, standardised mean difference.
Most of the studies were conducted with out-patients, and it is not clear if similar results would be found in other settings. The studies did not exclude individuals with a history of comorbid substance use disorders or personality disorders. This may have contributed towards heterogeneity, but has the benefit of being more representative of people with problem aggression.
Limitations
Heterogeneity between studies was high, and therefore it may be considered inappropriate to pool the results. We carried out subgroup analysis to mitigate the possible differential effect of different drug categories. With the exception of carbamazepine/oxcarbazepine, drug categories showed high within-group heterogeneity (where there was more than one study in each category). We used random-effects meta-analysis, and performed sensitivity analyses that did not reveal any factors that contributed significantly towards heterogeneity. Our finding of a high level of heterogeneity is in itself useful as it emphasises that: evidence from studies to date is inconsistent; not all mood stabilisers are likely to have the same effect; and there may be important differences in patient characteristics or in the measurement of aggression between studies, which should be taken into account in future studies.
We included two studies in the analysis that had used a cross-over design and analysed them as though they were derived from a parallel study, as data were not available to undertake a paired analysis. This is a conservative approach as it means that the cross-over studies would be relatively underweighted in the pooled analysis. Although methods are available for imputing values, we consider that our approach is robust and unlikely to significantly affect the results.
Implications for practice
Some mood stabilisers (oxcarbazepine, phenytoin and lithium) may well have a place in the treatment of aggressive behaviour for some individuals. The magnitude of the effect is difficult to determine, although any reduction in aggression may be considered a success in some patients. We used the standardised mean difference of log-transformed data as the principal summary measure. Although this is statistically appropriate, the interpretation of such a value in terms of the amount of change in aggression that would be expected in an individual is not straightforward. Moreover, group effects are likely to obscure considerable individual differences in response. This also has to be balanced against the potential side-effects of the medication, and the likelihood that treatment would need to be long term. We make no recommendation as to particular type of mood stabiliser, but to date, the best evidence of efficacy is available for carbamazepine/oxcarbazepine, phenytoin and possibly lithium. Given the limitations in the evidence currently available that prohibit conclusive findings of clear superiority of any of these mood stabilisers, we would recommend following general guidelines for psychopharmacological treatment, Reference Tyrer and Bateman26 particularly to agree the length of time of trial of medication in advance, to titrate the dose, to objectively measure the response and to discontinue if there is no observed benefit.
Recommendations for research
These results highlight the need for further RCTs to investigate mood stabilisers for the treatment of aggression. We excluded all studies in which the primary aim was not the treatment of aggression, and as such, excluded several studies that reported good effect for other anti-epileptic or mood-stabilising drugs, especially in those with borderline personality disorder. These drugs include topiramate and lamotrigine, and we recommend that further studies are undertaken in other patient groups with these medications with the specific aim of targeting aggression. We also recommend that future studies use and report clear definitions of the inclusion criteria as well as the measure of aggression used. Several studies that we excluded measured anger as opposed to aggression. Although aggressive feelings and aggressive acts are likely to be correlated, it is the inability to control the aggressive feelings and to inhibit aggressive impulses that is impaired and this should be the focus of further study.
In conclusion, we found that there was an overall reduction in aggression in people treated with a mood stabiliser; however, many of the studies on which this review was based were at risk of bias, and therefore further RCTs are needed.
Funding
R.M.J. is supported by a Joint MRC/Welsh Assembly Clinical Research Training Fellowship ().







eLetters
No eLetters have been published for this article.