Depression is common in the elderly; 10-15% of elderly persons living in the community manifest clinically relevant symptoms of depression and 1-2% have major depression (Reference Beekman, Copeland and PrinceBeekman et al, 1999). As in younger adults, the course of depression in the elderly shows a high degree of relapse and/or recurrence (Reference Flint and RifatFlint & Rifat, 1999) resulting in a conceptualisation of three phases of antidepressant treatment: acute treatment (resolution of acute depressive symptoms); continuation treatment (4-6 months relapse prevention); and maintenance treatment (recurrence prevention, prophylaxis) (Reference Montgomery, Dufour and BrionMontgomery et al, 1988).
Only a few placebo-controlled studies have investigated the effect of maintenance treatment in the elderly; not all have followed the scheme outlined above. However, the reported studies have shown a beneficial effect of prophylactic treatment (Reference Georgotas, McCue and CooperGeorgotas et al, 1989; Reference Reynolds, Frank and PerelReynolds et al, 1999). This study investigated the efficacy of citalopram in recurrence prevention in elderly patients with major depression, using the three-phase scheme outlined above.
METHOD
This was a single-centre study in out-patients at the Psychiatric Research Clinic, Frederiksberg Hospital. The study consisted of an open-label phase (Periods I-II, acute and continuation treatment periods) and a double-blind, randomised, parallel-group comparison of citalopram (20, 30, or 40 mg/day) and placebo treatment in the prevention of depression recurrence (Period III). The study was conducted according to the Helsinki Declaration (Tokyo, Venice, Hong Kong and Somerset West, amendments 1975, 1983, 1989 and 1996), the amended Draft on Testing Drugs in the Elderly (Note III/536/86-EN, 1988), and the Committee for Proprietary Medicinal Products guidelines (1991) for good clinical practice. Approval by the Ethics Committee and by the Danish Regulatory Authorities was obtained before the study was initiated.
Patient population
Patients were recruited from the Copenhagen and Frederiksberg municipalities in Denmark between March 1996 and December 1997. A letter was sent out to all citizens aged ≥65 years aimed at identifying undiscovered depression in that population. Those who were found to be depressed and fulfilled the entry criteria for the screening procedure at the research clinic were eligible for the study. All patients gave written informed consent before being included in the study.
Patients included had a unipolar major depressive episode (MDE; DSM-IV: 296.2× or 296.3×; American Psychiatric Association, 1993) at a severity corresponding to a total score of ≥22 on the Montgomery—Åsberg Depression Rating Scale (MADRS; Reference Montgomery and ÅsbergMontgomery & Å sberg, 1979).
Patients were excluded from the study if the index episode had lasted more than 12 months; if they had a history of schizophrenia, mania, hypomania, epilepsy, drug or alcohol misuse; or if they had severe somatic disorders. Similarly, patients were excluded if they had received fluoxetine within 5 weeks or other antidepressants within 3 days of the start of the study, lithium, carbamazepine or valproate within 2 weeks of the study, electroconvulsive therapy within 8 weeks of the study or sumatriptan or anticoagulants at study start. Finally, patients were excluded if they had a score of ≥5 on MADRS item 10 (suicidality).
Study design
The study consisted of three periods: Period I was 8 weeks of open, acute treatment with citalopram; Period II was 16 weeks of open continuation treatment with citalopram to consolidate remission; Period III consisted of double-blind treatment with citalopram or placebo for a potential minimum of 48 weeks. The patients continued with double-blind treatment until the last patient had been treated for 48 weeks or had discontinued for any reason (June, 1999).
In Period I, the initial dose of citalopram was 10 mg/day for the first 3 days, then increased to 20 mg/day. After 1 week, the dose could be decreased to 10 mg/day in case of intolerable adverse events; otherwise, the patient continued on 20 mg/day. After 3 weeks, the daily dose was increased to 20 mg for patients on 10 mg. For the remaining patients, the dose was increased by 10 mg after 3 and/or 5 weeks to a maximum of 40 mg, if there was either an increase or no change in Clinical Global Impressions of severity of illness score (CGI-S; Reference GuyGuy, 1976), compared with the score 3 weeks earlier, or if the absolute score was ≥5. Patients with intolerable adverse events at the increased dose were withdrawn.
At week 8, patients entered Period II if their total MADRS score was ≤11 (Reference MontgomeryMontgomery, 1994); if not, they were withdrawn. However, patients who showed a partial remission were allowed to continue for 4 weeks in Period II before the final assessment about remission was made. Patients who then had a MADRS total score ≤11 continued in the study whereas those with a score >11 were withdrawn. During Period II, the daily dose of citalopram remained fixed at the dose reached in Period I, i.e. 20, 30 or 40 mg/day. Patients experiencing a relapse (MADRS ≥22, confirmed after 3-7 days) in Period II were withdrawn.
Patients completing Period II with a MADRS score ≤11 were randomised on a 1:1 basis, using a block size of 10, to receive double-blind treatment with identical looking tablets of either placebo or citalopram in Period III (same dose as in Period II, randomisation irrespective of dose).
No concomitant psychotropic medication was allowed, except for benzodiazepines and other hypnotics, the dose of which was to remain unchanged after week 8 of Period II. Treatment with benzodiazepines and other hypnotics could not be started during Periods II or III except in case of relapse/recurrence, if the investigator felt that intervention was needed before relapse (Period II) or recurrence (Period III) was confirmed.
Visits and assessments
In Period I, patients were seen for a screening and a baseline visit, and also at weeks 1, 3, 5 and 8. In Period II, patients were seen upon entry (=last visit in Period I), and at weeks 4, 8, 12 and 16. In Period III, patients were seen at entry (=last visit in Period II) and at weeks 2 and 4, and subsequently every 4 weeks until discontinuation or completion. In addition, all patients were seen for a discontinuation or completion visit.
Patients were assessed by the CGI-S, MADRS, the 17-item Hamilton Depression Rating Scale (HDRS; Reference HamiltonHamilton, 1960), and the Melancholia Scale (MES; Reference Bech, Kastrup and RafaelsenBech et al, 1986) at baseline, at all subsequent visits in all periods (except for the visit at week 1 in Period I) and at premature discontinuations. Concomitant medication and adverse events were noted at each visit. Patient history and physical examination were registered at baseline. Laboratory tests were performed at the screening visit in Period I, at entry into Period III and at discontinuation/completion. Vital signs were assessed at entry into Period I, at week 8, at completion of Period II and at the end of each 24-week period in Period III. Height and body weight were recorded at baseline.
Outcome measures and definitions
The primary measure of prophylactic efficacy was the time to recurrence of a depressive episode from the start of Period III. Recurrence of depression was defined as the patient reaching a MADRS total score of ≥22, confirmed after 3-7 days.
The assessment of safety and tolerability of citalopram during prophylactic treatment was based on reporting of adverse events, vital sign measurements and laboratory assessments. Adverse events were either reported spontaneously by the patient, observed by the investigator, or elicited by the investigator using open questioning.
Statistical analysis
In the primary analysis, the log-rank test was used to compare the time to recurrence of depression for patients treated with citalopram or placebo. Survival curves were estimated using the Kaplan—Meier method and overall treatment effect was estimated using the Cox proportional hazard model (Reference Andersen, Borgan and GillAndersen et al, 1993; SAS Institute Inc., 1997).
In order to have a power of at least 90% at a 5% significance level for the primary analysis, a minimum sample size of 150 patients randomised into Period III (75 patients/group) was estimated (Reference Goldman and HillmanGoldman & Hillman, 1992). This was calculated to require inclusion of approximately 300 patients. The actual number of patients included in the study was 230. The power at a 5% significance level was re-estimated to be 60-75%.
RESULTS
Disposition of patients
A total of 230 patients entered Period I (acute treatment; Fig. 1), of whom 172 (74.8%) entered Period II (continuation treatment). After completion of Period II, 121 patients (70.3% of patients entering Period II) were randomised into Period III to receive treatment with either citalopram (20, 30 or 40 mg; 60 patients) or placebo (61 patients). Of the 230 patients, 96 (42%) remitted after 8 weeks of treatment and 149 (65%) patients remitted after 12 weeks of treatment.
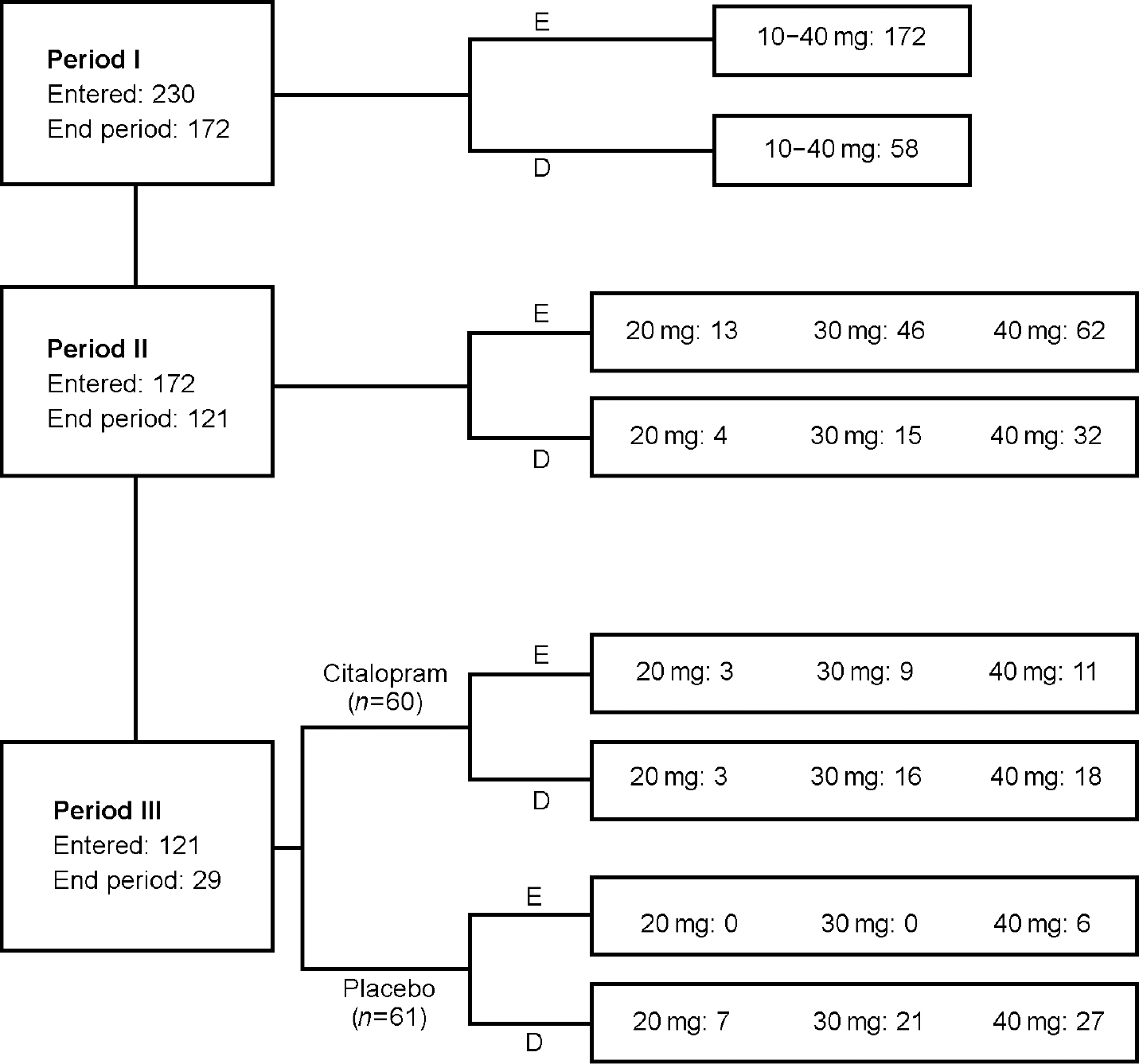
Fig. 1 Disposition of patients. E, end period/study; D, discontinued. Patients who had recurrence of depression are included.
The primary reasons for discontinuation in Period I were adverse events (13%) and withdrawal of consent (10%), whereas in Period II the main reasons were withdrawal of consent (15%), lack of efficacy (8%) and adverse events (5%).
All patients randomised into Period III were included in the intention-to-treat (ITT) population for analysis of efficacy. This population comprised 23% males and 77% females, which is a good representation of a population of elderly patients with depression (Reference Brown, Salive and GuralnikBrown et al, 1995).
Demographics and baseline characteristics of patients
Demographics and baseline characteristics of the patients were similar for the two treatment groups (Table 1). A total of 15% of the randomised patients in both groups had a history of previously diagnosed MDEs, of whom none reported having had more than two. In both treatment groups, the great majority of patients were ‘moderately ill’ or ‘markedly ill’ at baseline (CGI-S ratings), with about half the patients in each of these categories. Approximately 18% of the patients in both groups had previously received pharmacological treatment for depression (except one citalopram patient who had had electroconvulsive therapy). However, none of the patients had been treated with psychotropics within the last 3 months. A family history of major depression was noted for 15% (citalopram) and 11.5% (placebo) of the patients, respectively.
Table 1 Demographic and baseline characteristics of the intention to treat patients entering Period III
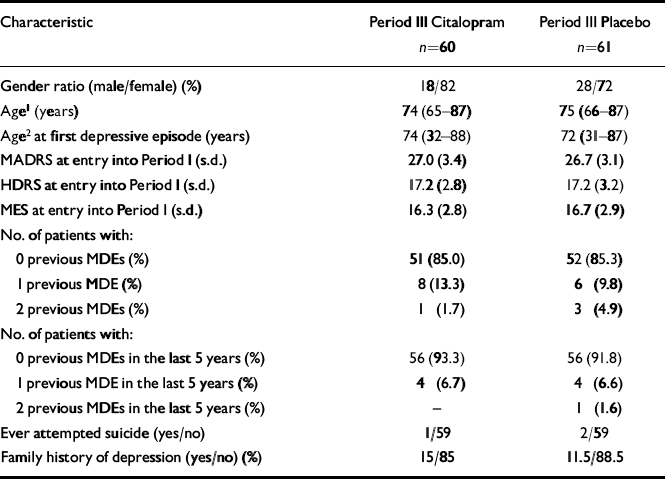
| Characteristic | Period III Citalopram n=60 | Period III Placebo n=61 |
|---|---|---|
| Gender ratio (male/female) (%) | 18/82 | 28/72 |
| Age1(years) | 74 (65-87) | 75 (66-87) |
| Age2at first depressive episode (years) | 74 (32-88) | 72 (31-87) |
| MADRS at entry into Period I (s.d.) | 27.0 (3.4) | 26.7 (3.1) |
| HDRS at entry into Period I (s.d.) | 17.2 (2.8) | 17.2 (3.2) |
| MES at entry into Period I (s.d.) | 16.3 (2.8) | 16.7 (2.9) |
| No. of patients with: | ||
| 0 previous MDEs (%) | 51 (85.0) | 52 (85.3) |
| 1 previous MDE (%) | 8 (13.3) | 6 (9.8) |
| 2 previous MDEs (%) | 1 (1.7) | 3 (4.9) |
| No. of patients with: | ||
| 0 previous MDEs in the last 5 years (%) | 56 (93.3) | 56 (91.8) |
| 1 previous MDE in the last 5 years (%) | 4 (6.7) | 4 (6.6) |
| 2 previous MDEs in the last 5 years (%) | — | 1 (1.6) |
| Ever attempted suicide (yes/no) | 1/59 | 2/59 |
| Family history of depression (yes/no) (%) | 15/85 | 11.5/88.5 |
Concurrent medical conditions and medication
There was no significant difference between treatment groups in respect of concurrent diseases and concomitant drug therapy. Approximately 72% of the patients in both treatment groups had an ongoing medical condition at baseline, and about 78% of the patients in both groups were continuing with at least one kind of medication upon entry into Period III.
MADRS, HDRS and MES scores
For the ITT population, the baseline total MADRS scores (s.d.) at entry into Periods I and III were 27.0 (3.4) and 4.7 (3.7), respectively, in the citalopram group and 26.7 (3.1) and 3.9 (3.5), respectively, in the placebo group. The total HDRS scores were 17.2 (2.8) and 3.3 (2.7) (citalopram) and 17.2 (3.2) and 2.9 (2.6) (placebo), respectively, and the total MES scores were 16.3 (2.8) and 3.6 (2.8) (citalopram) and 16.7 (2.9) and 3.0 (2.7) (placebo), respectively.
Recurrence of depression in the double-blind Period III
In the 60 patients randomised to continue on citalopram, there were 19 recurrences (32%) in contrast to 41 recurrences in the 61 patients randomised to placebo (67%). The total observation time from randomisation into Period III until recurrence, completion or discontinuation for other reasons than recurrence, was 53.8 (citalopram) and 30.3 (placebo) person-years, respectively. At week 48, 18 patients in the citalopram group and 38 patients in the placebo group had experienced a recurrence of depression (Table 2). The estimated probability of no recurrence within 48 weeks was 0.67 for patients treated with citalopram and 0.27 for placebo-treated patients. The time to recurrence in Period III differed significantly between the treatment groups, in favour of citalopram (log-rank test, χ2 value 18.45, d.f.=1, P<0.0001; Fig. 2). The hazard ratio (citalopram v. placebo) was estimated at 0.32 (95% CI 0.19-0.56) using a Cox regression model with treatment as the only predictive factor.

Fig. 2 Kaplan—Meier estimates of the time to recurrence of depression in the intention-to-treat population; citalopram (n=60, unbroken lines), placebo (n=61, dashed lines). The 95% confidence intervals are shown as thinner lines (omitted for the first 12 weeks for clarity). The difference in time to recurrence was statistically significant (log-rank test (d.f.=1), χ 2 18.45, P<0.0001). Nineteen patients treated with citalopram versus 41 patients treated with placebo discontinued due to recurrence of depression. For number of patients at risk please refer to Table 2.
Table 2 Number of patients still under observation, the cumulative number of recurrences and the estimated cumulative proportion of recurrence-free patients (using Kaplan—Meier method) at selected time points
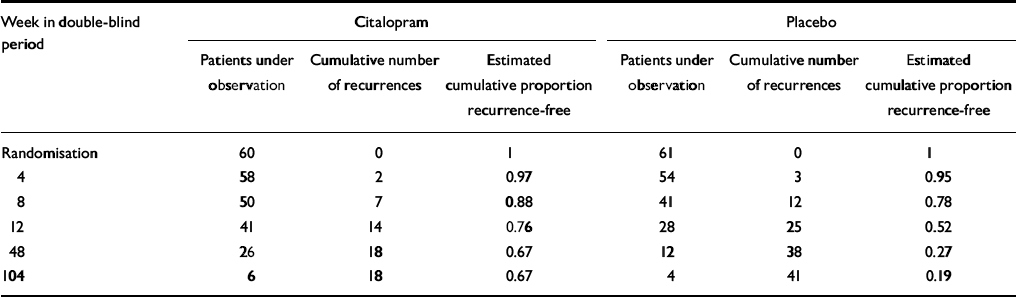
| Week in double-blind period | Citalopram | Placebo | ||||
|---|---|---|---|---|---|---|
| Patients under observation | Cumulative number of recurrences | Estimated cumulative proportion recurrence-free | Patients under observation | Cumulative number of recurrences | Estimated cumulative proportion recurrence-free | |
| Randomisation | 60 | 0 | 1 | 61 | 0 | 1 |
| 4 | 58 | 2 | 0.97 | 54 | 3 | 0.95 |
| 8 | 50 | 7 | 0.88 | 41 | 12 | 0.78 |
| 12 | 41 | 14 | 0.76 | 28 | 25 | 0.52 |
| 48 | 26 | 18 | 0.67 | 12 | 38 | 0.27 |
| 104 | 6 | 18 | 0.67 | 4 | 41 | 0.19 |
Although the study was not powered for subgroup analysis, and despite the fact that a limited number of patients received 20 mg/day, the difference in time to recurrence between citalopram— and placebo-treated patients was statistically significant at all three dose levels (log-rank test, 20 mg/day, P=0.0009; 30 mg/day, P=0.0227; 40 mg/day, P=0.0188). In the 20 mg/day group, none of the patients continuing on citalopram experienced a recurrence whereas all the patients switched to placebo did.
Discontinuation in the double-blind Period III
The majority of discontinuations in Period III occurred in the placebo group (55 (90%), compared with 37 (62%) in the citalopram group). The most frequent reason was recurrence of depression (citalopram 19 (32%); placebo 41 (67%)) followed by withdrawal of consent (about 24% in both groups). A few patients discontinued because of adverse events (citalopram 10%; placebo 13%) with no difference in time to withdrawal between the two groups (log-rank test, P=0.1842).
Safety and tolerability
In Period I, the most frequent adverse events (≥5%) were nausea, diarrhoea, headache, increased sweating, tremor, dizziness and fatigue (Table 3). Similar symptoms, but with a reduced frequency, were seen in Period II. Sexual side-effects (spontaneous reporting) were rare and weight gain/loss was reported by only two patients.
Table 3 All adverse events appearing in ≥ 3 patients in at least 1 of the treatment periods
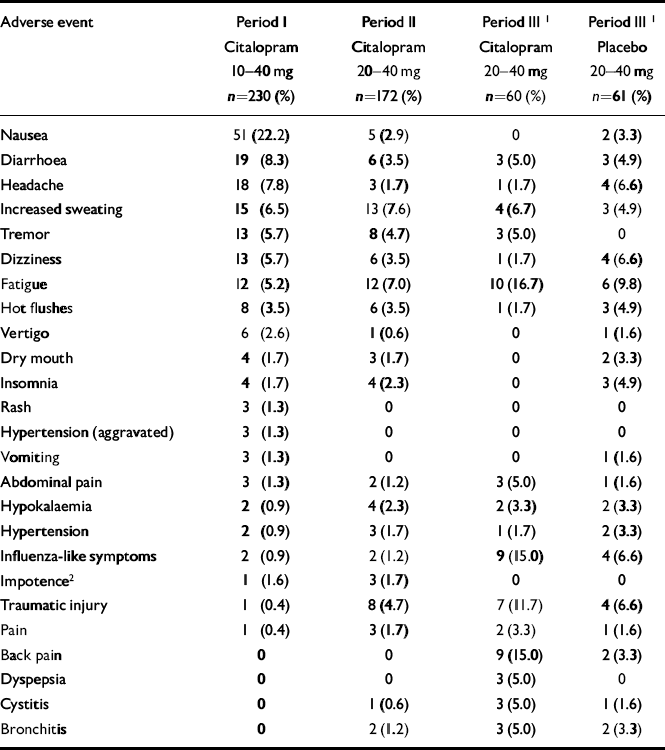
| Adverse event | Period I Citalopram 10-40 mg n=230 (%) | Period II Citalopram 20-40 mg n=172 (%) | Period III1Citalopram 20-40 mg n=60 (%) | Period III1Placebo 20-40 mg n=61 (%) |
|---|---|---|---|---|
| Nausea | 51 (22.2) | 5 (2.9) | 0 | 2 (3.3) |
| Diarrhoea | 19 (8.3) | 6 (3.5) | 3 (5.0) | 3 (4.9) |
| Headache | 18 (7.8) | 3 (1.7) | 1 (1.7) | 4 (6.6) |
| Increased sweating | 15 (6.5) | 13 (7.6) | 4 (6.7) | 3 (4.9) |
| Tremor | 13 (5.7) | 8 (4.7) | 3 (5.0) | 0 |
| Dizziness | 13 (5.7) | 6 (3.5) | 1 (1.7) | 4 (6.6) |
| Fatigue | 12 (5.2) | 12 (7.0) | 10 (16.7) | 6 (9.8) |
| Hot flushes | 8 (3.5) | 6 (3.5) | 1 (1.7) | 3 (4.9) |
| Vertigo | 6 (2.6) | 1 (0.6) | 0 | 1 (1.6) |
| Dry mouth | 4 (1.7) | 3 (1.7) | 0 | 2 (3.3) |
| Insomnia | 4 (1.7) | 4 (2.3) | 0 | 3 (4.9) |
| Rash | 3 (1.3) | 0 | 0 | 0 |
| Hypertension (aggravated) | 3 (1.3) | 0 | 0 | 0 |
| Vomiting | 3 (1.3) | 0 | 0 | 1 (1.6) |
| Abdominal pain | 3 (1.3) | 2 (1.2) | 3 (5.0) | 1 (1.6) |
| Hypokalaemia | 2 (0.9) | 4 (2.3) | 2 (3.3) | 2 (3.3) |
| Hypertension | 2 (0.9) | 3 (1.7) | 1 (1.7) | 2 (3.3) |
| Influenza-like symptoms | 2 (0.9) | 2 (1.2) | 9 (15.0) | 4 (6.6) |
| Impotence2 | 1 (1.6) | 3 (1.7) | 0 | 0 |
| Traumatic injury | 1 (0.4) | 8 (4.7) | 7 (11.7) | 4 (6.6) |
| Pain | 1 (0.4) | 3 (1.7) | 2 (3.3) | 1 (1.6) |
| Back pain | 0 | 0 | 9 (15.0) | 2 (3.3) |
| Dyspepsia | 0 | 0 | 3 (5.0) | 0 |
| Cystitis | 0 | 1 (0.6) | 3 (5.0) | 1 (1.6) |
| Bronchitis | 0 | 2 (1.2) | 3 (5.0) | 2 (3.3) |
Although data should be interpreted with caution, given the low number of events and the longer exposure time (about twice as long) for patients treated with citalopram than for patients on placebo, the pattern of adverse events for those who continued in Period III was similar in the two treatment groups (Table 3). The only events occurring in Period III at a frequency >5% and at least twice as frequently in the citalopram group as in the placebo group, were back pain and influenza-like symptoms. None of these events were considered to be related to treatment. The events most consistently rated as treatment— related were nausea, diarrhoea, headache, increased sweating, tremor, dizziness and fatigue, consistent with the known adverse event profile of citalopram (Reference Noble and BenfieldNoble & Benfield, 1997). Of these, only increased sweating, tremor and fatigue were seen with a statistically higher frequency in the citalopram group compared with the placebo group in Period III.
Changes in laboratory or vital sign parameters were seen occasionally but were generally all single-parameter changes, unlikely to be related to the use of citalopram. A total of 8 of the 230 patients had alanine aminotransferase values out of normal range after baseline. However, three of these patients already had abnormal values at baseline, and all values were only slightly increased. One patient discontinued prematurely because of an increase in alanine aminotransferase, which the investigator considered probably to be related to treatment. The parameter had normalised 4 months later.
The abrupt discontinuation of citalopram in patients randomised to the placebo group neither induced new adverse events nor resulted in an increased intensity of adverse events present at randomisation.
A total of 34 serious adverse events were reported in 28 patients during the study, of which 18 were reported during the open treatment periods and 16 (11 in the citalopram group and 5 in the placebo group) during the double-blind treatment period. There were two serious adverse events with outcome death: one from oesophageal carcinoma, occurring during the open treatment period; and one for unknown reasons, occurring in a placebo patient. Neither of these deaths nor the other serious adverse events were judged by the investigator to be related to treatment.
DISCUSSION
This study clearly demonstrates the advantage of citalopram over placebo in the prevention of recurrence of depression in elderly patients with unipolar major depression. It is the first placebo-controlled, double-blind study evaluating the effect of a selective serotonin reuptake inhibitor (SSRI) against recurrent depression in elderly patients using the generally accepted three-phase prophylactic design (Reference Montgomery, Dufour and BrionMontgomery et al, 1988). The hazard ratio of recurrence in citalopram-treated patients versus those on placebo was estimated at 0.32. Thus, the risk of recurrence was three times higher for patients on placebo than for patients on citalopram. The efficacy of citalopram was seen at all dose levels tested (20, 30 and 40 mg/day).
Comparison with studies in 18— to 65-year-olds
Essentially similar results were obtained in a recent placebo-controlled citalopram study of the same design in 18— to 65-year-old patients with recurrent depression (Reference Hochstrasser, Isaksen and KoponenHochstrasser et al, 2001). Reynolds et al (Reference Reynolds, Frank and Perel1994) also found that outcomes of maintenance therapy were remarkably similar in elderly and younger patients with depression. Compared with the 18— to 65-year-olds (Reference Bougerol, Scotto and PatrisBougerol et al, 1997), elderly patients tended to need a longer time to respond to acute treatment and a shorter time to recurrence. Both a longer time to remission (Reference Reynolds, Frank and KupferReynolds et al, 1996) and a higher and more rapid rate of recurrence with older age have been reported previously (Reference Reynolds, Frank and PerelReynolds et al, 1999).
Only 15% of the randomised elderly patients in this study had a history of previously diagnosed depression. Furthermore, a family history of depression was about half as frequent in this study as in a comparable study in 18— to 65-year-olds (Reference Hochstrasser, Isaksen and KoponenHochstrasser et al, 2001) consistent with the known profile in late-onset depression (Reference SmallSmall, 1998). In spite of the absence of these known risk factors for recurrence of depression, the results of the present study demonstrate that the population it included was at high risk of recurrence. The results confirm that the risk of recurrence in geriatric patients is high, even after first-episode depression (Reference Flint and RifatFlint & Rifat, 1999; Reference Reynolds, Frank and PerelReynolds et al, 1999).
Study design
This study was designed to allow sufficient time to resolve symptoms of an episode of unipolar major depression and to distinguish relapse of an episode from recurrence of a new episode (Reference Montgomery, Dufour and BrionMontgomery et al, 1988). Period I was designed to optimise response to citalopram (flexible dose and treatment extension up to 12 weeks), possibly explaining why only 3% of the patients discontinued because of lack of efficacy, reflecting the fact that a long time for resolution of symptoms could be required in some patients (Reference QuitkinQuitkin, 1992). Period II was designed to consolidate remission from the present depressive episode (Reference Montgomery, Dufour and BrionMontgomery et al, 1988). The following 48-week minimum duration of prophylactic therapy was chosen on the basis of the current knowledge of the time to recurrence seen in most depressive patients (about 24 weeks), but allowing enough time to obtain knowledge of recurrences occurring at later time-points; and of statistical power calculations.
Treatment
The doses of 20, 30 or 40 mg/day citalopram were chosen on the basis of previous 12-week studies of citalopram in elderly patients with depression (Reference Kyle, Petersen and OverøKyle et al, 1998) and on the well-established long-term efficacy and safety of doses of 20-40 mg/day citalopram in 18— to 65-year-old patients (Reference Robert and MontgomeryRobert & Montgomery, 1995). The dose to which patients responded during acute treatment, and remained well on during continuation treatment, was maintained during prophylactic treatment, as studies have indicated that a full dose of anti-depressants is more effective than a reduced dose during prophylactic treatment (Reference Franchini, Gasperini and PerezFranchini et al, 1998).
Relapse versus recurrence
It might be questioned whether the depressive episodes recorded in Period III were relapses rather than recurrences. However, because the design of the study followed the recommendations given by the experts in this field (Reference Montgomery, Dufour and BrionMontgomery et al, 1988), new depressive episodes occurring in Period III were regarded as recurrences. This is further substantiated by the fact that the duration of an untreated depressive episode is usually considered to be 6-8 months (American Psychiatric Association, 1993). When patients entered Period III in this study, their index episode had started at least 6.5 months earlier. Furthermore, if the patients, although symptom free, were still in the depressive phase of their index episode when they entered Period III, it is most likely that the patients randomised to placebo would relapse very early. Thus, the difference in treatment effect of citalopram and placebo should be greatest at the time immediately following randomisation. The fact is, however, that a substantial part of the recurrences occurred in weeks 8-12 after randomisation, and citalopram was superior to placebo in preventing recurrence throughout the observation period.
Safety and tolerability of prophylactic treatment
Prophylactic treatment with citalopram was well tolerated in the elderly patients in this study. This is an important finding, given the particular vulnerability of elderly patients to adverse drug reactions and the high frequency of concurrent diseases and concomitant drug therapy in these patients (Reference PollockPollock, 1999).
The pattern of adverse events was in agreement with other results regarding long-term safety of SSRIs (Reference Zajecka, Amsterdam and QuitkinZajecka et al, 1999) and was comparable to the adverse events seen in placebo-treated patients. Notably, sexual symptoms, a common and bothersome reaction to SSRIs (Reference Kennedy, Eisfeld and DickensKennedy et al, 2000), were reported with very low frequency. However, the frequency could have been underestimated because specific enquiries were not made about these events. Weight gain was only reported by one patient (citalopram) in Period III in agreement with a previous long-term study (Reference Hochstrasser, Isaksen and KoponenHochstrasser et al, 2001).
Premature discontinuation
Apart from recurrence, the most common primary reasons for premature discontinuations in Period III were withdrawal of consent and adverse events, which is to be expected in a long-term study in an elderly population. It is of major importance that the abrupt discontinuation of citalopram in patients randomised to the placebo group caused no worsening of the adverse event profile. Although the sample size was small and the study was not designed to investigate this, it indicates that abrupt discontinuation of citalopram in the elderly is not associated with discontinuation symptoms.
In conclusion, citalopram effectively reduces the rate of recurrence of depression in elderly patients. Moreover, long-term treatment with citalopram is well tolerated. This study confirms available data indicating that maintenance treatment in patients with depression is efficacious in reducing recurrence rates and is beneficial even after first-episode depression.
CLINICAL IMPLICATIONS
-
• Continued citalopram treatment beyond the first 6 months of a depressive episode significantly reduces the risk of recurrence.
-
• The risk of recurrence in a group of elderly patients with depression is three times higher following switch to placebo than following continuation on the active drug.
-
• Long-term treatment in the elderly (average 75 years of age) is well tolerated.
LIMITATIONS
-
• The study does not permit evaluation of reduced dose effectiveness in maintenance treatment.
-
• Because fewer patients were included in the study than originally planned, the power of the study was decreased.
-
• Although conclusive and concordant with data in adults, confirmation of study findings is required as this is the first study of its kind with a selective serotonin reuptake inhibitor.








eLetters
No eLetters have been published for this article.