Major depressive disorder (MDD) is the most common mental health condition, with more than 264 million people being affected worldwide.1 It has a negative impact on people's quality of life and is very costly for health and care systems.Reference Greenberg, Fournier, Sisitsky, Pike and Kessler2 Cognitive–behavioural therapy (CBT) is an evidence-based psychological intervention that is widely used for treating MDD. There are large variations in terms of the components delivered as part of CBT protocols. The two main elements of CBT for depression are: (a) behavioural activation aiming to understand the potentially reciprocal relationships between negative mood states and behavioursReference Hawley, Padesky, Hollon, Mancuso, Laposa and Brozina3 and (b) cognitive restructuring aiming to increase behaviours associated with positive moods and identify, critically evaluate and challenge maladaptive automatic thoughts.Reference Sudak4 Recent evidence examining the effectiveness of each of these components alone or in combination suggested that they were equally effective in reducing depression in adults compared with treatment as usual or no treatment.Reference Ciharova, Furukawa, Efthimiou, Karyotaki, Miguel and Noma5
Social skills trainingReference Jacobson, Dobson, Truax, Addis, Koerner and Gollan6 (including non-verbal and communication skills as well as assertiveness training), relaxation techniquesReference Jorm, Morgan and Hetrick7 and psychoeducationReference Tursi, Cv, Camacho, Tofoli and Juruena8 may be used to supplement the effectiveness of core CBT. Techniques such as problem-solving skills,Reference Bell and D'Zurilla9 self-management skillsReference Houle, Gascon-Depatie, Bélanger-Dumontier and Cardinal10 and relapse preventionReference Gortner, Gollan, Dobson and Jacobson11 may also be used, most likely because patients with depression may present with multiple comorbidities, including anxieties,Reference Cummings, Caporino and Kendall12 cancerReference Chochinov13 and diabetes.Reference Roy and Lloyd14 It is standard practice for studies focusing on psychological treatments for depression to use core,Reference Collins, Byrne, Hawe and O'Reilly15 complexReference Clarke, Reid and Eubanks16 containing behavioural activation and/or cognitive restructuring with either psychoeducation, skills training modules or relaxation techniques, and/or ultra-complex CBT protocolsReference Leuzinger-Bohleber, Hautzinger and Fiedler17 that include core CBT with at least two or more additional therapeutic components as mentioned above.
To date no study has examined the differential effectiveness of each of these CBT protocols in reducing depression in adult populations. For example, ample is the evidence garnered from several meta-analyses demonstrating the effectiveness of CBT in significantly reducing symptoms of depression in comparison with no intervention or treatment as usual (TAU).Reference Cuijpers, Quero, Noma, Ciharova, Miguel and Karyotaki18–Reference Cuijpers, Berking, Andersson, Quigley, Kleiboer and Dobson20 Despite this, evidence on differences in the effectiveness as well as scope (e.g. subgroups of people with MDD) of the core and multicomponent CBT protocols at post-treatment and in the long term is lacking. There are also uncertainties whether specific characteristics of participants, intervention, therapists or context can influence the effectiveness of core and multicomponent CBT. Understanding whether multicomponent CBT protocols are more effective than core CBT, for which patient groups and how they should be delivered has important practice and policy implications. These include improved access for underserved patient groups and reducing healthcare inequalities, time and training requirements for therapists and overall healthcare service delivery costs.
To address this important gap in the literature, this systematic review and network meta-analysis aimed to (a) comparatively assess the effectiveness of core, complex and ultra-complex CBT protocols in reducing symptoms of depression in adults with depression at post-treatment and in the long term and (b) examine moderators of these protocols, including characteristics of participants, interventions, therapists and context.
Method
Eligibility criteria
Studies involving participants aged 17 years and older with depression were eligible. Depression could have been verified through the use of either validated self-report measures or clinical interviews. Studies that recruited participants with comorbid mental health problems such as anxiety were also included. However, studies focusing on COVID-19-related depression were excluded.
We focused on studies involving core CBT protocols using either behavioural activation, or cognitive restructuring or both as the primary treatment components and based on any type of delivery mode, including face-to-face or online individual/group sessions. Studies that incorporated additional cognitive–behavioural therapeutic components to the two core components above, including problem-solving and self-management techniques, relaxation and social skills training, relapse prevention and psychoeducation, were also included, if they were based on behavioural activation and/or cognitive restructuring modules. CBT protocols were grouped into three discrete categories according to the complexity of the included components: core CBT (comprising behavioural activation and cognitive restructuring), complex CBT (core CBT plus one additional component that included psychoeducation or training in particular skills, including social skills training and relaxation techniques) and ultra-complex CBT (core CBT plus two or more additional components that included problem-solving skills, self-management skills, relaxation techniques, psychoeducation and/or relapse prevention; interventions are defined in Table 1 and therapeutic components of the included studies are described in supplementary Appendix 2, available at https://doi.org/10.1192/bjp.2022.35). Studies comparing the effectiveness of the core components of CBT with each other (e.g. behavioural activation versus cognitive restructuring) or comparing the effectiveness of the different formats of delivery of CBT (e.g. face-to-face CBT versus telephone-delivered CBT or remote online CBT) were excluded.
Table 1 Description of intervention models
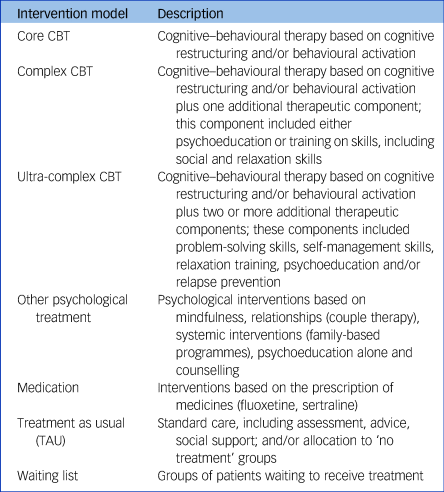
The control groups involved a mixture of individuals on waiting lists, receiving TAU (participants allocated to the ‘no treatment’ condition were also listed in this category because they are prone to seek treatment while a study is being conducted), or any other psychological or pharmacological treatment.
Primary outcomes were validated self-reported measures of depression at baseline, post-treatment and/or additional follow-up points. Studies that did not report their outcomes regarding depressive symptoms at either baseline or post-treatment and/or provided insufficient data for a meta-analysis were excluded.
We included randomised controlled trials (RCTs) evaluating the effectiveness of CBT protocols. We excluded observational, cross-sectional and qualitative studies. Studies that were not in English were also excluded.
Search methods
We searched the bibliographic databases MEDLINE, PsycInfo, Embase, Web of Science and the Cochrane Register of Controlled Trials from 1 January 1990 to 30 November 2021. Two authors (I.A., C.H.) independently screened the titles/abstracts and the full texts of potentially eligible studies and extracted data. Interrater reliability was high (κ = 0.91) for title/abstract screening and high (κ = 0.94) for full-text screening. Disagreements were resolved through discussion. The reference lists of the identified studies and those of previous reviews were examined to ensure that all relevant studies were included. We also contacted experts in the field to enquire about unpublished studies and searched trials registers (ClinicalTrials.gov, ISRCTN, the World Health Organization's International Clinical Trials Registry Platform (ICTRP) and OpenTrials.net) to identify any unpublished or ongoing trials. The full search strategy is available in supplementary Appendix 1.
Data collection and extraction
A data extraction sheet was constructed and pilot tested on six randomly selected papers. Data were extracted on: (a) study/context characteristics: authors, geographic region where the study was conducted and method of measuring depressive symptoms; (b) participant characteristics: age, gender identity and presence of comorbidities; (c) CBT characteristics: number of and/or components of CBT used, number of sessions, length of sessions and delivery format; (d) therapist characteristics: background in mental health services and supervision received; (e) active control/control group characteristics: no treatment, waiting list, TAU, or other psychological and/or pharmacological interventions; and (f) outcomes: measures of depression.
We also extracted arm-level data including information about sample sizes, means and standard deviations for both intervention and control conditions at baseline (when reported), post-treatment and follow-up. Standardised mean difference (s.m.d.) effects and the corresponding standard error were computed using the Comprehensive Meta-Analysis Version 3 (Windows) software.Reference Borenstein, Hedges, Higgins and Rothstein21
Quality assessment and risk of bias
The quality of the RCTs was assessed by two independent raters (I.A., C.H.) using the Cochrane Risk of Bias 2 (RoB 2.0) tool.Reference Higgins and Green22 Additionally, we applied the confidence in network meta-analysis (CINeMA) frameworkReference Nikolakopoulou, Higgins, Papakonstantinou, Chaimani, Del Giovane and Egger23 to assess the certainty of evidence covering the six key domains: within-study bias, reporting bias, indirectness, imprecision, heterogeneity and incoherence.
Data synthesis
We first did the pairwise meta-analyses using DerSimonian–Laird random effects. We calculated standardised mean differences using Hedges’ g and interpreted them according to Cohen's criteria.Reference Hedges24,Reference Durlak25 A negative s.m.d. indicated that the reduction in depression scores was in favour of the CBT protocols. We presented pooled effect results with 95% confidence intervals and used forest plots with I 2 (with test-based 95% confidence intervals) to display statistical heterogeneity.Reference Higgins, Thompson, Deeks and Altman26 Because the s.m.d. is based on standardised means and not a specific scale (i.e. is unit-less), we back-transformed the s.m.d. pooled effects to the mean difference using the method explained in the Cochrane Handbook.Reference Higgins, Thompson, Deeks and Altman27
We then synthesised the study effect sizes by using a network meta-analysis which allowed for the simultaneous evaluation of our seven interventions while preserving the within-study randomisation.Reference Dias, Welton and Sutton28 To ensure transitivity within the network, we compared the distribution of the clinical variables (i.e. age, gender and baseline depression score) by grouping the different CBT protocols, other psychological treatments, medication, TAU and ‘no treatment’ groups into nodes.Reference Bafeta, Trinquart, Seror and Ravaud29 A Bayesian random-effects network meta-analysis model was used with a normal likelihood for the post-treatment outcome analysis. The 95% credible interval (CrI) displayed uncertainty in the posterior effects and multivariate distributions were used to account for the correlations induced by multigroup studies. We considered the I 2 statistic and the (heterogeneity) variance in the random effects distribution (τ2) to measure the extent of the influence of variability across and within studies on treatment effects. The surface under the cumulative ranking curve (SUCRA)Reference Salanti, Ades and Ioannidis30 was used to rank the treatments’ performance, as well as the P-score, a frequentist analogue to SUCRA. We statistically evaluated consistency by separating out direct evidence from indirect evidence using node splitting.Reference Higgins, Jackson, Barrett, Lu, Ades and White31 Cochrane's Q statistic was used to calculate consistency throughout the entire network.Reference Krahn, Binder and König32 The CINeMA judgements were included in the league table of results and forest plots.
Meta-regression analyses were conducted on the post-treatment outcomes only because the exact same studies were included in the long-term analysis, meaning that the variables would be the same. The study and participant characteristics that were included in the analyses were: geographical continent where the study was conducted (1, North and South America; 2, Europe; 3, Africa; 4, Asia; 5, Oceania); socioeconomic status (high versus low); age (≤30 years, 31–59 years, ≥60 years); gender (males versus females); diagnosis (self-reported versus formal/interview); recruitment (community versus in-patient); and comorbidities (none, mental health, physical). The intervention characteristics included intensity of the treatment (low: 1–8 sessions; medium: 9–15 sessions; high: 16+ sessions); delivery by a mental health specialist (yes, no, not applicable); CBT format (individual face-to-face/telephone, face-to-face group, online/face-to-face self-help with some therapist support, online self-help with no therapist support); and measure of depression used (Patient Health Questionnaire (PHQ-9); Beck Depression Inventory (BDI); Hamilton Rating Scale for Depression (HRSD); Center for Epidemiology Depression (CES-D); other). The influence of the quality appraisal scores (low, medium, high) on the effects of different CBT protocols in reducing depressive symptoms was also examined. We assessed goodness of fit for each model by comparing total residual deviance and deviance information criterion.
The models of the post-treatment outcome analysis were fitted in OpenBUGS (version 3.2.3 for Windows)33 using uninformative prior distributions for the treatment effects and a minimally informative prior distribution for common heterogeneity standard deviation. Uninformative priors (that is, N(0,1000)) were assumed for all meta-regression coefficients. Model convergence was established by visual inspection of three Monte Carlo Markov chains after considering the Brooks–Gelman–Rubin diagnostic. Statistical evaluation of inconsistency and production of network graphs and results figures was done using the ‘netmeta’ package in R version 4.0.5 (Windows) (R Foundation for Statistical Computing).Reference Rücker, Krahn, König, Efthimiou, Davies and Papakonstantinou34 Network meta-analysis of the post-treatment outcome was duplicated in a frequentist environment by using the same package in R.
A time adjusted analysis of the network involving the outcome data from the 54 studies replacing the post-treatment data was done at 26 weeks to assess the long-term effectiveness of the interventions. This analysis was done using the frequentist approach in ‘netmeta’. Our definition of the long-term effects of CBT interventions as being 6 months (26 weeks) follows the assumptions made from a previous study that this was when the effects appeared to start to wane.Reference Cuijpers, Quero, Noma, Ciharova, Miguel and Karyotaki18
To examine the presence of bias due to small-study effects, we used a comparison-adjusted network funnel plot to visually scrutinise the criterion of symmetry.Reference Chaimani and Salanti35 We also statistically compared the effect sizes between short- and long-term outcomes (i.e. <26 weeks versus ≥26 weeks) using the ratio of means (ROM) formula.Reference Altman and Bland36 All statistical codes used to perform the network models are available in supplementary Appendix 13.
This study was conducted in accordance with the Cochrane HandbookReference Higgins and Green22 and was registered with PROSPERO (registration number CRD42021237846). Reporting was consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for network meta-analysis.Reference Hutton, Salanti and Caldwell37 See supplementary Appendix 14 for the completed checklist.
Patient and public involvement
This study was guided by two patients with lived experience of depression who had received CBT in the past. They contributed to the refinement of the research questions, classifications of the treatment protocols and interpretation of the results. They will also support the dissemination of the findings of the study.
Results
A total of 3975 articles were retrieved. After full-text screening of 511 studies, 107 RCTs (involving 15 248 participants) met our inclusion criteria (Fig. 1). Of those, 54 (50%) studies (involving 6383 participants) provided follow-up data. Supplementary Appendix 2 lists the included studies, and supplementary Appendix 4 summarises their characteristics.
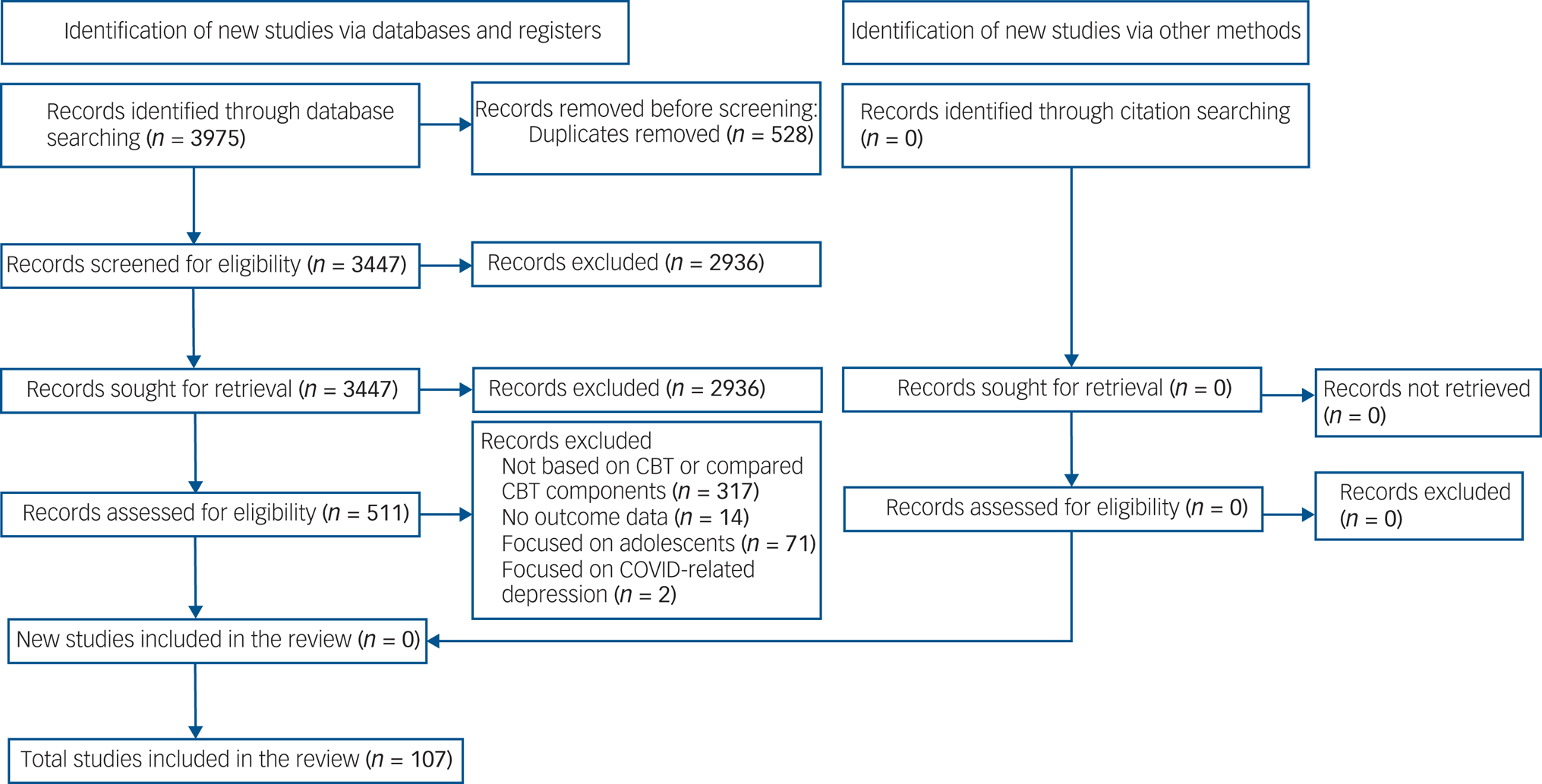
Fig. 1 PRISMA 2020 flow diagram for the entire review.
Descriptive characteristics of studies, population, intervention and outcomes
Combined, 43% (n = 46) of the studies were conducted in North and South America, 31.8% (n = 34) in Europe and the UK, 11.2% (n = 12) in Oceania, 13% (n = 14) across Asia, including such countries as China, Japan, Pakistan, Iran and Thailand, and 1% (n = 1) in Nigeria, Africa.
The age of the participants ranged between 22.6 and 74.7 years (mean 41.5; s.d. = 13.5) with 5627 (36.3%) of the overall sample identifying as male. Fifty-eight (54.2%) studies diagnosed depression using formal diagnoses, whereas the remaining 49 (45.8%) used self-report measures. Of those studies in which participants reported additional physical (n = 26; 70.3%) or mental (n = 11; 29.7%) health problems, the most common were HIV, cancer, multiple sclerosis, cardiovascular problems and diabetes, whereas anxiety disorders (n = 8; 72.7%) were among the most common comorbid mental health problem.
Of the total 127 comparisons included in this review, 67 (52.8%) were based on ultra-complex CBT, 36 (28.3%) on complex CBT and 24 (18.9%) on core CBT protocols. All CBT protocols were compared with multiple comparators including TAU (57; 44.8%), no intervention and/or waiting list conditions (36; 28.3%), another psychological therapy, including interpersonal and psychodynamic psychotherapy (22; 17.3%), and medication alone (13; 10.2%). Regarding the mode of delivery of the psychological interventions, 46 (36.2%) studies used a face-to-face individual format, 40 (31.5%) used group sessions, 37 (29.1%) used an online format with and/or without a therapist's support, 3 (2.4%) used either a face-to-face or online teaching-based format and 1 (0.8%) used self-help. The average number of sessions was 9.8 (s.d. = 4.7), with a mean length of 72.7 min (s.d. = 27.5).
Assessment of risk of bias
The overall bias appraisal revealed that 79 studies (73.8%) showed moderate risk, 16 (15%) studies demonstrated low risk and 12 (11.2%) showed high risk of bias. An area of bias that was potentially problematic was selective reporting bias: 91 (85%) of studies showed moderate or high risk of bias. Results of the full risk of bias assessment are reported in supplementary Appendix 5.
Network meta-analysis for main outcomes
Figure 2 shows the network of eligible comparisons for all core CBT packages for the post-treatment outcomes from the 107 studies. The network of evidence included 7 interventions, 15 248 participants, 90 two-arm studies and 17 multi-arm studies.

Fig. 2 Network graph and forest plot of network meta-analysis for main outcomes.
CBT, cognitive–behavioural therapy; TAU, treatment as usual; a, low confidence of evidence; b, moderate confidence of evidence.
Inconsistency analysis
We found evidence of statistical inconsistency through node splitting analysis owing to comparisons of complex CBT (z = −4.67, P < 0.0001) with no treatment, complex CBT with TAU (z = 2.94, P = 0.003) and core CBT with no treatment (z = 2.19, P = 0.029) (supplementary Appendix 6). The inconsistency for complex CBT compared with TAU was due to one study,Reference Bowers, Stuart, Macfarlane and Gorman38 which showed a high overall risk of bias, a large effect size and large standard error. The inconsistency for complex CBT compared with no treatment was owing to one study,Reference Hegerl, Hautzinger and Mergl39 which revealed high risk of bias because of missing data, concerns due to the unknown randomisation procedure used and the extremely wide confidence interval. Finally, the inconsistency for core CBT compared with no treatment was due to one study,Reference Collins, Byrne, Hawe and O'Reilly15 which had a high risk of bias for the randomisation process used and concerns of measurement outcome and outcome reporting bias. Because consistency (transitivity) is a central assumption of network meta-analysis, we removed all three trials, leaving 104 RCTs in the network.
Main outcomes
Figure 2 shows the results of the network meta-analysis for the main outcomes of all eligible trials after performing the inconsistency analysis. All active interventions, including core CBT (s.m.d. = −1.14, 95% CrI −1.72 to −0.55 [m.d. = −8.44, 95% CrI −12.73 to −4.07], n = 6 studies), complex CBT (s.m.d. = −1.24, 95% CrI −1.85 to −0.64 [m.d. = −9.18, 95% CrI −13.69 to −4.74], n = 9), ultra-complex CBT (s.m.d. = −1.45, 95% CrI −1.88 to −1.02 [m.d. = −10.73, 95% CrI −13.91 to −7.55], n = 21), other psychological psychotherapies (s.m.d. = −0.76, 95% CrI −1.35 to −0.16 [m.d. = −5.62, 95% CrI −9.99 to −1.18]; n = 3), medication (s.m.d. = −0.80, 95% CrI −1.58 to −0.01 [m.d. = −5.92, 95% CrI −11.69 to −0.07]; n = 2) and TAU (s.m.d. = −0.74, 95% CrI −1.24 to −0.23 [m.d. = −5.48, 95% CrI −9.18 to −1.70]; n = 2) showed statistically significant benefits compared with no treatment. Large heterogeneity was present in the network meta-analysis, with I 2 = 91.5% (95% CI 90.3–92.6%) (supplementary Appendix 7). These results were consistent when analysed in a frequentist framework. The pairwise meta-analysis results for the main outcomes were also consistent for core and multicomponent CBT when compared with either TAU or no treatment (supplementary Appendix 8).
The SUCRA also supported the network meta-analysis showing the best performing intervention as ultra-complex CBT (SUCRA = 93.9%) followed by complex CBT (SUCRA = 77.7%) (supplementary Appendix 9).
The league table showing the results of the network meta-analysis comparing the effects of all interventions (Fig. 3) showed that both ultra-complex (s.m.d. = −0.71, 95% CrI −1.05 to −0.38 [m.d. = −5.25, 95% CrI −7.77 to −2.81]) and complex CBT protocols (s.m.d. = −0.50, 95% CrI −0.95 to −0.06 [m.d. = −3.70, 95% CrI −7.03 to −0.44]) were the only interventions that maintained a significant effect when compared with TAU. Ultra-complex CBT was also significantly more effective than the use of other psychological treatments (s.m.d. = −0.69, 95% CrI −1.19 to −0.20 [m.d. = −5.11, −8.81 to −1.48]). To ensure the certainty of evidence, we incorporated the CINeMA judgements into Fig. 3. The evidence according to CINeMA varied from low (n = 6 head-to-head comparisons), to moderate (n = 5) to high (n = 7) confidence overall (supplementary Appendix 10). Funnel plots and Egger's test for assessing asymmetry indicated strong evidence for publication bias (P < 0.0001) (supplementary Appendix 11).

Fig. 3 Head-to-head comparisons of all intervention groups for the main outcome network analysis.
The interventions are described in Table 1. CBT, cognitive–behavioural therapy; TAU, treatment as usual. Data are shown as s.m.d. (95% CrI); –, no direct treatment comparisons. Darker blue cells (bottom) show network meta-analysis estimates; lighter blue cells (top) show direct pairwise meta-analysis estimates. The certainty of the evidence (according to the confidence in network meta-analysis (CINeMA) framework) is: a, very low confidence; b, low confidence; c, moderate confidence; d, high confidence; e, very high confidence. Full results from CINeMA are provided in supplementary Appendix 8.
Covariate adjusted network at 26 weeks (6 months) including follow-up data
The long-term effectiveness of each active intervention, including various CBT protocols, other psychological treatment, medication and TAU, was assessed in the covariate-adjusted network model for 26 weeks or more use (see Fig. 4 for forest plot and Fig. 5 for league table of comparisons). Ultra-complex CBT (s.m.d. = −1.09, 95% CI −1.61 to −0.56 [m.d. = −8.07, 95% CI −8.58 to −4.14]), complex CBT (s.m.d. = −0.73, 95% CI −1.36 to −0.11 [m.d. = −5.40, 95% CI −10.06 to −0.81]), other psychological treatments (s.m.d. = −0.71, 95% CI −1.37 to −0.04 [m.d. = −5.25, 95% CI −10.14 to −0.30]) and TAU (s.m.d. = −0.48, 95% CI −0.86 to −0.10 [m.d. = −3.55, 95% CI −6.36 to −0.74]) maintained significance after 26 weeks post-treatment when compared with no treatment.

Fig. 4 Network graph and forest plot of network meta-analysis for time-adjusted analysis.
CBT, cognitive–behavioural therapy; TAU, treatment as usual; wks, weeks.

Fig. 5 League table of head-to-head comparisons of all interventions assessed at 26 weeks (6 months) of long-term use.
Cognitive–behavioural therapy (CBT) interventions are ranked in order of P-scores and are as described in Table 1 but with the time adjustment of 26 weeks. wks, weeks; psy, psychological; TAU, treatment as usual. Data are shown as s.m.d. (95% CI); –, no direct evidence available. Darker blue cells (bottom) show network meta-analysis estimates; lighter blue cells (top) show direct pairwise meta-analysis estimates.
Comparisons between short- and long-term outcomes
Our analyses showed that the effect sizes of ultra-complex CBT (ROM = −0.36, 95% CI 0.31 to −1.04), complex CBT (ROM = −0.51, 95% CI 0.36 to −1.38), core CBT (ROM = −0.79, 95% CI −1.87 to 0.29), medication (ROM = −0.10, 95% CI −1.89 to 1.69), other psychological treatments (ROM = −0.05, 95% CI 0.84 to −0.94) and TAU (ROM = −0.26, 95% CI 0.37 to 0.89) when matched and compared between the two time periods (<26 wks versus ≥26 wks) did not significantly differ in terms of reduction in depression scores.
Meta-regressions
The results of the meta-regression analyses for all the separate CBT and combined CBT protocols are presented in supplementary Appendix 12. Meta-regressions revealed that individuals presenting comorbid mental health problems could benefit more from ultra-complex CBT (P-value ranging between 0.04 and 0.03), and coupled with this, depression reductions were greater when symptoms were assessed with the use of formal interviews (P = 0.037). Measuring depression using any scale excluding the PHQ-9,Reference Kroenke, Spitzer and Williams40 the BDI,Reference Beck, Steer and Brown41 the HRSDReference Hamilton42 and the CES-DReference Lewinsohn, Seeley, Roberts and Allen43 contributed to significant reductions in effect sizes (P ranging between 0.03 and 0.02) for combined CBT compared with no treatment. Younger patients (≤30 years) appeared to contribute better outcomes for those receiving ultra-complex CBT (P ranging between 0.001 and 0.01). There was an indication that participants from lower socioeconomic backgrounds responded better to both ultra-complex (P = 0.05) and complex CBT (P = 0.03). Furthermore, ultra-complex CBT was more effective when delivered by a non-mental health specialist (e.g. nurse, graduate student, other; P = 0.04). Group format did not appear to benefit those receiving core CBT, but these analyses were based on only five studies and should be interpreted with caution. Last, the strength of the analyses was not affected by the overall quality appraisal scores of the studies.
Discussion
Summary of main findings
This network meta-analysis compared the effectiveness of core, complex and ultra-complex CBT protocols in reducing depression among adults at post-treatment and 26-week follow-up. Core, complex and ultra-complex CBT protocols were equally effective in reducing depression at post-treatment when compared with no treatment. However, only the ultra-complex and complex CBT protocols sustained these positive effects beyond 26 weeks when compared with no treatment. Both ultra-complex and complex CBT protocols were effective when compared with TAU post-treatment, but only ultra-complex CBT sustained its significance when compared with other psychological treatments for depression. Individuals presenting with comorbid mental health problems and receiving formal interviews also benefited more from the ultra-complex multicomponent CBT. Furthermore, ultra-complex CBT was more effective when delivered by non-mental health specialists (e.g. nurses). This finding may be explained by the fact that nurses providing ultra-complex CBT may also treat these patients for additional conditions and play the role of the main care co-ordinator for a wider range of health problems. However, additional research is needed to verify this. Age appeared to affect the effectiveness of CBT, with participants younger than 30 and those that were 31–59 years responding better to ultra-complex and complex CBT respectively. Meta-regression analyses also demonstrated that participants of lower socioeconomic status responded better to both ultra-complex and complex CBT. Group therapy did not benefit participants receiving core CBT.
Comparison with similar research
These findings are in accord with the treatment guidelines of the National Institute for Health and Clinical Excellence (NICE) and current meta-analyses suggesting that CBT protocols delivered in any format (e.g. individual, group, telephone, guided self-help) are effective treatments for depression in adults.Reference Cuijpers, Noma, Karyotaki, Cipriani and Furukawa19 However, this meta-analysis is unique as it is the first to provide evidence on the effectiveness of core, complex and ultra-complex CBT protocols at post-treatment and long-term. For instance, in a recent network meta-analysis,Reference Cuijpers, Quero, Noma, Ciharova, Miguel and Karyotaki18 211 of the 331 included trials (64%) were classified as CBT but were defined only as a single intervention. Thus, most of the direct evidence contributing to the network was heavily supported from the CBT intervention and little is known about the effects that additional components might have on the clinical efficacy of CBT. Moreover, behavioural activation, a core CBT component for depression, and problem-solving, which is usually used in conjunction with core CBT protocols, were presented as two distinct interventions that were different from CBT. This categorisation may cause confusion regarding the effective CBT components that should be chosen in practice and for varying patient subgroups. A key finding of our study is that all the CBT protocols independent of complexity are significant in reducing depression at post-treatment, with both ultra-complex and complex CBT protocols remaining most effective in longer term.
Strengths and weaknesses
This network meta-analysis is the first to look at the effects of CBT protocols involving multifaceted components over time and has tried to identify the point at which these effects start to wane. We also increased the methodological rigour of our analyses by applying the CINeMA assessment criteria,Reference Nikolakopoulou, Higgins, Papakonstantinou, Chaimani, Del Giovane and Egger23 and assessed heterogeneity through network meta-regression analyses at post-treatment with the inclusion of 11 variables for exploring patient, intervention and study effects.
Five limitations warrant discussion. First, our searches included studies that were published after January 1990. This decision was made because most of the studies published before this date had scored low in the methodological appraisal exercises of previous reviews.Reference Cuijpers, Berking, Andersson, Quigley, Kleiboer and Dobson20 Second, although complex multicomponent CBT protocols were more effective when compared with other psychological treatments for depression, these psychological treatments included a large variety of multifaceted components based on mindfulness alone, couple therapy, systemic interventions, psychoeducation and counselling. Future studies should compare those studies using CBT and each of these psychological treatments separately. Third, our analyses did not include any studies involving individuals diagnosed with depression and comorbid personality disorders. Therefore, it should be noted that the effectiveness of the ultra-complex CBT protocols to treat depression in those with additional mental health problems may not be experienced by individuals with comorbid personality disorders.Reference Goddard, Wingrove and Moran44 These individuals may gain the most benefit from an amalgamation of psychological and pharmacological treatments.Reference Driessen and Hollon45,Reference Matusiewicz, Hopwood, Banducci and Lejuez46 Fourth, little information was provided regarding the concurrent use of medication in the studies included in this review. To overcome this limitation, a more detailed reporting of concurrent medication use is needed that would allow for meta-regression analyses to better determine its clinical benefits. Fifth, although the therapeutic components of the included studies were evaluated based on the published study manuals or the information as reported in the actual papers, it is still possible that there were differences between these reports and the components used. Therefore, these results should be interpreted with caution.
Implications for clinicians and policy makers
We found that core, complex and ultra-complex CBT protocols were equally effective in reducing symptoms of depression at post-treatment. Our back-transformed estimates showed that all CBT protocols reduced depressive symptoms by up to at least 8.44 points on the BDI scale, which was substantial. Ultra-complex and complex CBT sustained these effects in the long-term and reduced depression by at least 8 and 5 points respectively. In people with mental health comorbidities and hence patients with more severe depression, the additional clinical benefits of the ultra-complex CBT over core or complex CBT protocols were significant, and ultra-complex CBT might therefore be the preferred option. However, for the rest of people with depression, the decision to recommend core, complex or ultra-complex CBT protocols should be based not only on expected clinical benefits (in the form of reductions in depressive symptoms, because these were equivalent) but also on other factors such as accessibility, staff availability and costs.
Supplementary material
Supplementary material is available online at http://doi.org/10.1192/bjp.2022.35.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgement
We thank Matilda Angelaki for her generous support and guidance.
Author contributions
I.A. had the initial research idea,formulated the research questions and designed the study. I.A., C.H. and A.H. searched for published work, selected articles, extracted and analysed data. I.A., A.H. and P.G. drafted the protocol and manuscript. A.H. and M.P. helped with searching for articles and data selection and extraction. A.H., M.P. and I.A. substantially contributed to designing the searches and the statistical analysis plan, writing the manuscript and interpreting the findings. A.H. and I.A. performed the statistical analysis. P.G. and C.H. contributed to the manuscript by providing review comments and edits. All authors have read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. I.A. is the guarantor.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.










eLetters
No eLetters have been published for this article.