Despite much progress in the treatment of mood disorders, depression is a leading cause of disease burden and disability for older adults. Furthermore, even with antidepressant treatment, older people often experience residual symptoms and impaired quality of life. Thus, prevention of late-life depression is a clinical and public health priority. Reference Reynolds, Cuijpers, Patel, Cohen, Dias and Chowdhary1 Biological and observational data support protective and/or ameliorative influences of folate and other homocysteine-lowering or one-carbon metabolism nutritional factors in depression Reference Beydoun, Shroff, Beydoun and Zonderman2–Reference Ng, Feng, Niti, Kua and Yap7 including among older adults. However, potential roles of folate and B vitamins as tools for late-life depression prevention would ideally be investigated with the scrutiny of randomised, double-blind, placebo-controlled trials. Yet, the experimental evidence is limited, particularly in large-scale settings. Existing randomised controlled trials (RCTs) Reference Ford, Flicker, Thomas, Norman, Jamrozik and Almeida8,Reference Walker, Mackinnon, Batterham, Jorm, Hickie and McCarthy9 addressing B vitamins and depression risk among generally healthy community-dwelling older adults have reported null associations. By contrast, one study Reference Almeida, Marsh, Alfonso, Flicker and Davis10 involving older adults at particularly high risk for depression (recent history of cerebrovascular incident) revealed significant reductions in depression risk among those randomised to long-term folic acid and B vitamins. Yet, in a larger study Reference Andreeva, Galan, Torrès, Julia, Hercberg and Kesse-Guyot11 that included participants with a key medical risk factor (cardiovascular disease (CVD) survivors), there were no differences in depression risk for folate/B vitamins v. placebo.
However, the optimal approach to the question of whether B vitamins/folate can prevent depression in older adults would likely involve a large-scale, long-term trial of supplements at high doses; indeed, the average study period for prior large-scale trials Reference Walker, Mackinnon, Batterham, Jorm, Hickie and McCarthy9,Reference Andreeva, Galan, Torrès, Julia, Hercberg and Kesse-Guyot11 was <5 years, and B vitamin doses were notably lower than those utilised elsewhere. Reference Almeida, Marsh, Alfonso, Flicker and Davis10,Reference Armitage, Bowman, Clarke, Wallendszus and Bulbulia12,Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 In addition, the sample would ideally involve sufficiently large numbers of people who are generally healthy as well as those with high-risk factors. However, a de novo investigation of this kind would be prohibitively expensive and resource intensive. Therefore, we conducted an analysis of whether folic acid and B-vitamin supplementation can prevent incident depression in the setting of a large-scale RCT of primary and secondary CVD prevention – the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS). Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 Notably, the trial consisted of 5442 women (mean age 63 years) who were treated for an average of 7 years with combined daily supplements of folic acid (2.5 mg), vitamin B6 (50 mg) and vitamin B12 (1 mg) v. placebo; thus, WAFACS featured a study period that was years longer, and supplement doses 5- to 10-fold higher, than in prior large-sample trials. Reference Walker, Mackinnon, Batterham, Jorm, Hickie and McCarthy9,Reference Andreeva, Galan, Torrès, Julia, Hercberg and Kesse-Guyot11 Objectives of this study were: to evaluate whether long-term B-vitamin/folate supplementation reduces overall risk of incident depression in WAFACS, and specifically, to address effects on late-life depression risk (i.e. among people aged ≥65 years). Further, we examined whether effects of folic acid and B-vitamin supplementation on depression risk would vary according to baseline factors: dietary intakes of folate, vitamin B6 and vitamin B12; alcohol consumption; and medical comorbidity, a key risk factor for late-life depression. Reference Schoevers, Smit, Deeg, Cuijpers, Dekker and van Tilburg14
Method
Participants
The WAFACS evaluated effects of a combination pill of folic acid (2.5 mg/day), vitamin B6 (50 mg/day), and vitamin B12 (1 mg/day) in prevention of major vascular events among women at high CVD risk. The trial began in 1998, when the folic acid and B-vitamin component was added to the Women’s Antioxidant Cardiovascular Study (WACS), then an ongoing 2 × 2 × 2 factorial trial of vitamins C and E and β-carotene. The design of WAFACS reflected biologically plausible synergy between homocysteine-lowering and antioxidant supplements for CVD prevention. Details of the design and the main results from the WAFACS and WACS have been published previously (www.clinicaltrials.gov Identifier NCT00000541). Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13,Reference Bassuk, Albert, Cook, Zaharris, MacFadyen and Danielson15,Reference Cook, Albert, Gaziano, Zaharris, MacFadyen and Danielson16
In the WACS, 8171 female health professionals were randomised between June 1995 and October 1996 to receive vitamin C (500 mg/day), vitamin E (600 IU every other day), and β-carotene (50 mg every other day) v. matching placebos. Eligible women were ≥40 years old, postmenopausal or had no intention of becoming pregnant and had a self-reported history of CVD (myocardial infarction, stroke, coronary revascularisation or angina) or at least three traditional CVD risk factors. In April 1998, 5442 of these women, who were willing and eligible for participation in WAFACS, were randomised to an active B-vitamin/folate pill or a matching placebo. Details of the randomisation scheme are provided elsewhere. Reference Bassuk, Albert, Cook, Zaharris, MacFadyen and Danielson15 Briefly, participants were assigned to active treatment and matching placebo arms using computer-generated random permuted blocks; there were eight participants in each block and 64 strata (i.e. eight 5-year age groups × eight possible prior treatment groups (from the 2 × 2 × 2 factorial WACS groups)); this block randomisation scheme mitigated risk of unbalanced entry into the study arms during recruitment. Approval for the WAFACS and WACS, and for the current analysis, was obtained from the institutional review board of Brigham and Women’s Hospital (Boston, Massachusetts, USA). All participants gave written informed consent. The WAFACS was sponsored by the National Institutes of Health; study pills were provided by BASF Corporation (Mount Olive, New Jersey, USA).
Population for analysis
Participants with a history of depression before WAFACS randomisation (n = 1111) were excluded from this analysis, leaving 4331 women (Fig. 1). History of depression at baseline was determined by: (a) self-report of ever having physician-diagnosed depression; (b) self-reported use of select antidepressants
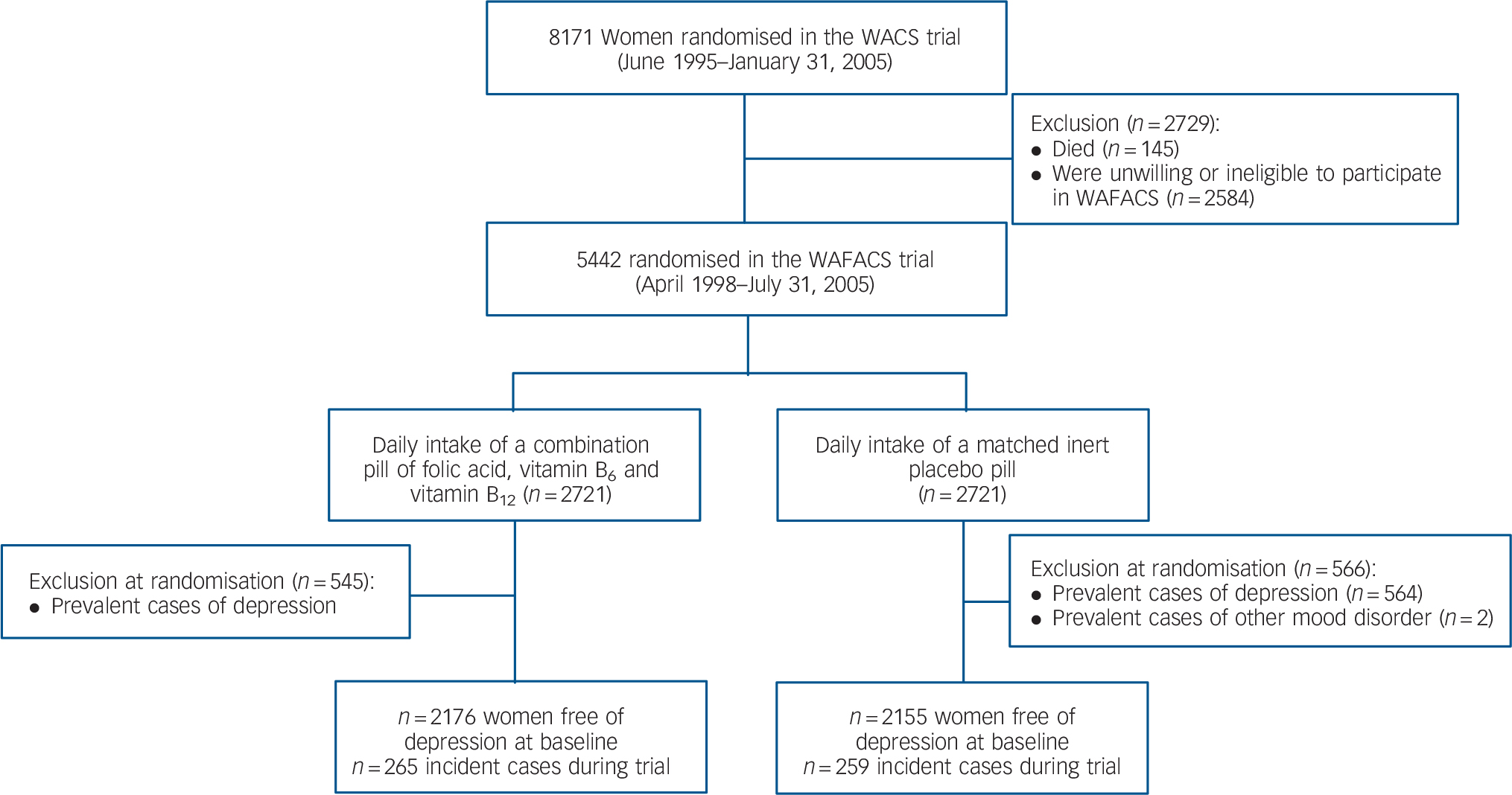
Fig. 1 Flow diagram of participation and incident depression in the folic acid/B6/B12 combination pill and placebo arms of the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS) trial.
(described further below), along with an appropriate (ICD-9-CM) 17 depressive disorder code (i.e. 296.2x, 296.3x, 300.4, 309.0, 309.1, 309.28, 311), as entered by trained data coders. Women who had ICD-9-CM codes for bipolar disorder or non-affective psychosis were also excluded. ICD-9-CM codes could be generated because participants specified physician diagnoses for which they were prescribed medications; participants did so by either writing down the diagnosis or verbally relaying this information to trained research staff over the phone.
The above exclusion process provided reassurance for assembling a baseline sample that was at risk for true incident depression. Regarding the requirement that antidepressant use be accompanied by a relevant ICD-9-CM code, this procedure was driven by clear evidence in our data of varying susceptibility to misclassification by class/type of antidepressant. For example, among all participants reporting use of selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs, excluding low-dose (5 mg daily) selegiline), tri- or tetracyclics widely known for mood indications (for example nortriptyline, desipramine, imipramine, maprotiline) or newer/atypical antidepressants (for example bupropion, nefazodone), over 70% had a depression code and nearly 80% had a mood, anxiety or other mental health-related code. By contrast, only ∼25% of participants reporting use of certain tricyclic antidepressants (for example doxepin, amitriptyline) or trazodone had any mental health-related code – depression or otherwise; these women were frequently prescribed these medication for sleep or pain conditions. Thus, our data suggested that antidepressant use alone could not function as a reliable proxy of depression and likely reflected the shift away from tricyclic antidepressants in favour of newer agents during the post-1990s period of the WAFACS trial. Reference Pirraglia, Stafford and Singer18
WAFACS parent trial follow-up procedures
Participants were followed-up annually via mailed questionnaires to update information on occurrence of major illnesses or adverse events, numerous health and lifestyle factors and study adherence. Importantly, all participants had been administered at baseline a semiquantitative food-frequency questionnaire, previously developed and validated in highly comparable samples of health professionals, Reference Willett, Sampson, Stampfer, Rosner, Bain and Witschi19,Reference Feskanich, Rimm, Giovannucci, Colditz, Stampfer and Litin20 to assess nutrient intakes; folate intake was calculated as total intake of folate from diet, including supplements and post-mandatory-fortification folate. Follow-up continued until the WAFACS’ planned end on 31 July 2005, for a total duration of 7.3 years; average adherence (i.e. taking at least two-thirds of study pills as assigned) was 83%, with no differences between active v. placebo groups. Morbidity and mortality information was complete for ≥98% of person-years of follow-up. Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13
Among a subset of participants, WAFACS measured plasma levels of relevant biomarkers to determine the influence of the active agent on nutrient levels, as well as to examine possible influences of background folate fortification among those receiving placebo (mandatory folate fortification of the US food supply commenced in 1998). Reference Jacques, Selhub, Bostom, Wilson, Rosenberg and Collaboration21 Over 70% of women in the WAFACS provided a blood sample prior to the initiation of fortification. Among those adherent with study medications, 300 (150 in the active agent group and 150 in the placebo group) were randomly selected to provide blood samples at the end of randomised treatment. As detailed previously, Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 baseline median plasma folate and homocysteine levels were similar between active and placebo groups. At the end of follow-up, folate levels increased significantly in both groups, but the relative increase was greater in the active treatment group. Despite significant increases in post-fortification folate levels, there was no reduction in homocysteine levels when comparing values at the beginning v. the end of the trial among the placebo group. By contrast, a significant decrease in plasma homocysteine levels was observed in the active treatment group.
Ascertainment of incident depression
Incident depression was defined as physician/clinician-diagnosed depression or presence of clinically significant depressive symptoms. Information used to determine incident depression was obtained on the WACS 24-, 48- 72-, 84-, 96- and 120-month study questionnaires as either self-reported physician/clinician-diagnosed depression or self-reported depressive symptoms, based on the Mental Health Index (MHI). Reference Cuijpers, Smits, Donker, ten Have and de Graaf22 Among participants in our sample for this depression substudy, the WACS 24-month questionnaire was the baseline/pre-randomisation survey for the B-vitamin/folate factorial arm. Thus, during follow-up, incident depression was classified as the first occurrence of self-reported: (a) physician/clinician-diagnosed depression (asked at the 72-, 84-, 96- and 120-month questionnaires) or (b) clinically significant depressive symptoms (asked at the 48- and 96-months questionnaires using the MHI).
Regarding the capture of physician/clinician-diagnosed depression, participants also had the ability to use write-in space to indicate a diagnosis, along with month/year of diagnosis, on any of the annual questionnaires during the entire follow-up period; however, physician/clinician depression diagnosis was only explicitly ascertained on the questionnaire years specified above.
Regarding clinically significant depressive symptoms, these were operationalised as follows: features of depression, by mood quality, duration and level of dysfunction, that are indicative of – at minimum – depressive disorder NOS (not otherwise specified) or minor depression. 23 Specifically, in order to be classified as having clinically significant depressive symptoms, participants had to report feeling downhearted or blue most or all the time, for the preceding continuous 4 weeks, and endorse difficulty with work and/or social activities because of their emotional symptoms.
Finally, available data in study-event files (i.e. from phone or letter contacts with participants) were used to supplement the above end-points; these files included ICD-9-CM depressive disorder diagnoses entered by trained coders, who used participants’ self-reported physician diagnosis descriptions to perform coding. Depression event dates were calculated as the month/year for physician/clinician diagnosis and as the questionnaire return date for events determined on the basis of clinically significant depressive symptoms; if participants could be classified as depressed by more than one method, the earliest date was used.
Validity of the depression measures
Symptom data on our questionnaires came from a three-item version of the MHI, which has been validated elsewhere. Reference Cuijpers, Smits, Donker, ten Have and de Graaf22 However, we applied a case definition even more rigorous than one based solely on cut-points, as we required both that participants endorsed the core feature of depressed quality of mood most or all of the time and that the emotional symptoms during that same 4-week time frame interfered with social and/or occupational activities. Our approach in using self-reported clinical data or symptoms to classify depression is consistent with published work Reference Pan, Okereke, Sun, Logroscino, Manson and Willett24,Reference Li, Mirzaei, O'Reilly, Winkelman, Malhotra and Okereke25 and has yielded depression prevalence and incidence rates among community-dwelling older women in our prior studies Reference Li, Mirzaei, O'Reilly, Winkelman, Malhotra and Okereke25,Reference Pan, Sun, Czernichow, Kivimaki, Okereke and Lucas26 that are similar to those that have been determined using structured, live interview methods. Reference Steffens, Skoog, Norton, Hart, Tschanz and Plassman27,Reference Luijendijk, van, Berg, Dekker, van Tuijl, Otte and Smit28
Statistical analysis
Primary analyses focused on depression incidence by randomised group. After exclusion of n = 1111 at baseline because of history of depression, data from all remaining 4331 randomised participants were analysed on an intention-to-treat basis. Participants were followed until the occurrence of the depression end-point, death or the end of the trial, whichever came first.
Baseline characteristics were compared by randomised groups using two-sample t- or Wilcoxon tests for continuous variables and χ2 and Fisher exact statistics for categorical variables. Kaplan-Meier curves were used to estimate cumulative incidence for the active treatment and placebo groups; the log-rank test was used to compare the curves. The primary analysis used Cox proportional hazards models to estimate relative risks (RRs) and 95% confidence intervals for active treatment v. placebo, adjusting for the design variables of age and the other randomised agents (vitamin E, vitamin C and β-carotene). In a multivariable model, we further adjusted for baseline covariates with specific relevance: dietary intakes of folate, B6 and B12; alcohol intake (which affects folate absorption); medical comorbidity, summarised by the Charlson (Deyo) index. Reference Deyo, Cherkin and Ciol29 However, of note, we did not observe significant evidence of imbalance in the distributions of these key factors by randomised treatment group among these 4331 women eligible for incident depression. In extended models, we included additional demographic, lifestyle and health factors (education, smoking, physical activity and menopausal status and hormone therapy); however, results were identical for the models above and are not detailed here. Finally, because of our specific scientific interest regarding influences of B-vitamin/folate supplementation on depression in late-life, we repeated the primary analysis (as well as secondary analyses described below) with further stratification by age at 65 years.
Subgroup analyses
We addressed whether certain subgroups of women markedly varied in depression risk with B-vitamin/folate treatment. Subgroups were based on baseline status of select factors of interest: i.e. age (<65 or ≥65 years), the other randomised treatments (yes/no), low intakes of B vitamins (yes/no), daily alcohol use (yes/no) and high medical comorbidity (Charlson <2 or ≥2 points). Regarding low nutrient intakes, we applied cut-offs as described in an earlier study: Reference Kang, Cook, Manson, Buring, Albert and Grodstein30 low intake was defined as <1.9 mg/d for vitamin B6 and as <279 μg/d for folate, using cut-offs based on intakes that were found to be significantly associated with elevated homocysteine among older adults; low intake was defined as <2.4 μg/d for vitamin B12 by using the reference dietary intake for older persons, as we lacked similar information for B12. We created an indicator for women with either a low intake of any one of the three B vitamins (n = 1287) v. adequate intakes of all three. In addition, we performed formal tests of effect modification using multiplicative interaction terms between the subgroup indicators and randomised assignment.
Sensitivity analyses
Sensitivity analyses were undertaken to address the robustness of findings. First, we utilised an alternative definition of depression that included as cases only participants with physician/clinician-diagnosed depression, but not those defined by depressive symptoms alone. Second, we conducted an adherence analysis in which women were censored on the date closest to when they stopped taking at least two-thirds of study pills, started to use outside (non-study) B-vitamin/folate supplements on ≥4 days per month, or were missing study pill adherence information. Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 Third, we used alternative definitions for folate intake (i.e. from food only, with fortification; from food only, without fortification; from food and supplements, without fortification) and for comorbidity (i.e. in the multivariable models, medical comorbidity burden was defined continuously as the number of points on the Charlson index, as opposed to being defined dichotomously as having a Charlson score of <2 or ≥2 points). This allowed us to assess whether results were sensitive to alternative definitions of these variables.
All statistical analyses were performed with SAS version 9.2 for Unix. Two-sided tests, with a significance level of α = 0.05 (P<0.05), were used. For Cox models, the proportional hazards assumption was confirmed analytically.
Results
Baseline characteristics
During ∼7 years of follow-up (average person-time 6.6 years), 524 women developed depression. Average total follow-up by death-censored person-time was identical in this sample (7.0 years) to that in the full WAFACS (7.0 years). As with the main WAFACS, Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 there were no significant differences in characteristics between the active treatment and placebo groups in this sample (see online Table DS1). Regarding adherence, 84.1% achieved
Table 1 Relative risks and 95% confidence intervals of depression, by randomised groupFootnote a
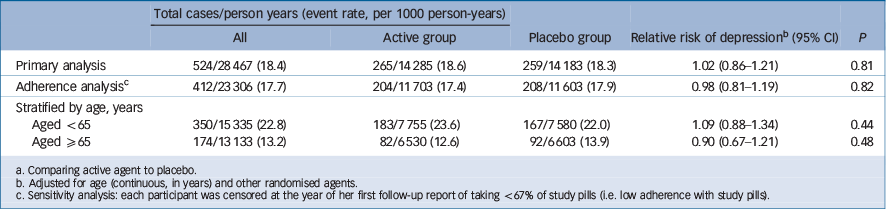
| Total cases/person years (event rate, per 1000 person-years) | |||||
| All | Active group | Placebo group | Relative risk of depressionFootnote b (95% CI) | P | |
| Primary analysis | 524/28 467 (18.4) | 265/14 285 (18.6) | 259/14 183 (18.3) | 1.02 (0.86–1.21) | 0.81 |
| Adherence analysisFootnote c | 412/23 306 (17.7) | 204/11 703 (17.4) | 208/11 603 (17.9) | 0.98 (0.81–1.19) | 0.82 |
| Stratified by age, years | |||||
| Aged <65 | 350/15 335 (22.8) | 183/7 755 (23.6) | 167/7 580 (22.0) | 1.09 (0.88–1.34) | 0.44 |
| Aged ≥65 | 174/13 133 (13.2) | 82/6 530 (12.6) | 92/6 603 (13.9) | 0.90 (0.67–1.21) | 0.48 |
a. Comparing active agent to placebo.
b. Adjusted for age (continuous, in years) and other randomised agents.
c. Sensitivity analysis: each participant was censored at the year of her first follow-up report of taking <67% of study pills (i.e. low adherence with study pills).
good or better adherence during follow-up. There were no differences in adherence by assignment to active agent (84.1%) v. placebo (84.2%). Adherence was similar within the 524 incident cases (82.8%).
Main effects of folic acid and B vitamins on incident late-life depression
Overall, there was no significant effect of the folic acid/B6/B12 combination on risk of depression, compared with the placebo group (Table 1 and Fig. 2). There were 265 participants with depression in the active treatment group (18.6/1000 person-years) and 259 in the placebo group (18.3/1000 person-years). The relative risk (RR) was 1.02 (95% CI 0.86–1.21, P = 0.81) after adjusting for design variables. Multivariable-adjusted results were the same: RR = 1.01 (95% CI 0.85–1.21, P = 0.90) (data not shown). Although there were significant differences in depression incidence by age – rates were 22.8/1000 person-years among those aged <65 years (n = 2338) and 13.2/1000 person-years among those aged 65+ years (n = 1993) – there were no differences in the effect of B vitamins/folate on depression risk according to age (Table 1).
Results from subgroup and sensitivity analyses
There were no significant differences in depression risk according to B-vitamin/folate randomisation across subgroups; all interaction tests were statistically non-significant (Table 2). Regarding low nutrient intakes of the agents, we specifically addressed whether the effect of B-vitamin/folate randomised treatment would vary according to low intake on any of the three v. adequate intake of all three; again, relative risks did not vary by these groups. When further subsetting into the approximate halves of participants below v. at-or-above 65 years, results were unchanged – with the exception of vitamin C: among women aged ≥65 years, there was a significant interaction between vitamins B and C randomised treatments, with a 30% lower relative risk of depression among active B-vitamin/folate recipients taking active vitamin C v. vitamin C placebo: age- and design variable-adjusted RR = 0.67 (95% CI 0.45–1.00, P = 0.047, P-interaction = 0.03); multivariable-adjusted RR = 0.68 (95% CI 0.45–1.02, P = 0.061, P-interaction = 0.03) (data not shown). However, there was no other evidence of varying effects of B vitamins on depression risk by age.
In sensitivity models that used the stricter definition of depression (n = 470 cases) in order to minimise potential bias as a result of inadvertent inclusion of misclassified cases, B-vitamin/folate treatment was not significantly related to depression risk (multivariable-adjusted RR = 0.94, 95% CI 0.78–1.13); results by age-65 dichotomisation were also the same. Results from the adherence sensitivity analysis similarly showed no effects of randomised treatment on depression risk (n = 412
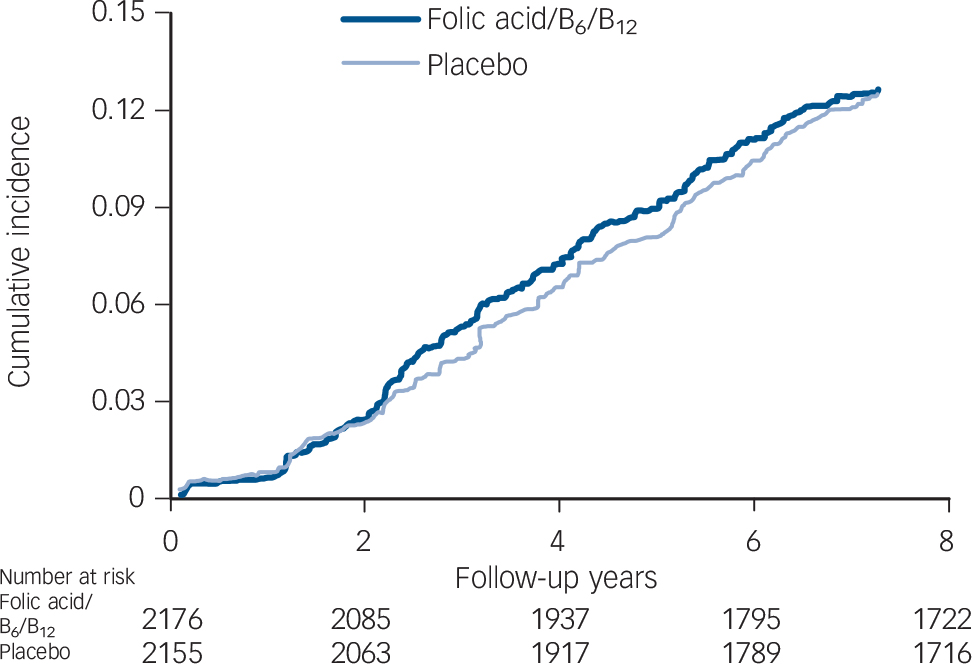
Fig. 2 Cumulative incidence of depression by randomised treatment assignment (active agent v. placebo).
cases): multivariable-adjusted RR = 0.98 (95% CI 0.80–1.19, P = 0.84). Finally, in the separate analyses using alternative definitions of folate intake and medical comorbidity, results for interactions of randomised treatment with low nutrient intakes and with elevated comorbidity were unchanged (data not shown).
Discussion
Main findings
In this large RCT among 4331 women with either prior history of CVD or multiple risk factors, we found no significant effect of
Table 2 Relative risks and 95% confidence intervals of clinical depression, according to stratified subgroupsFootnote a
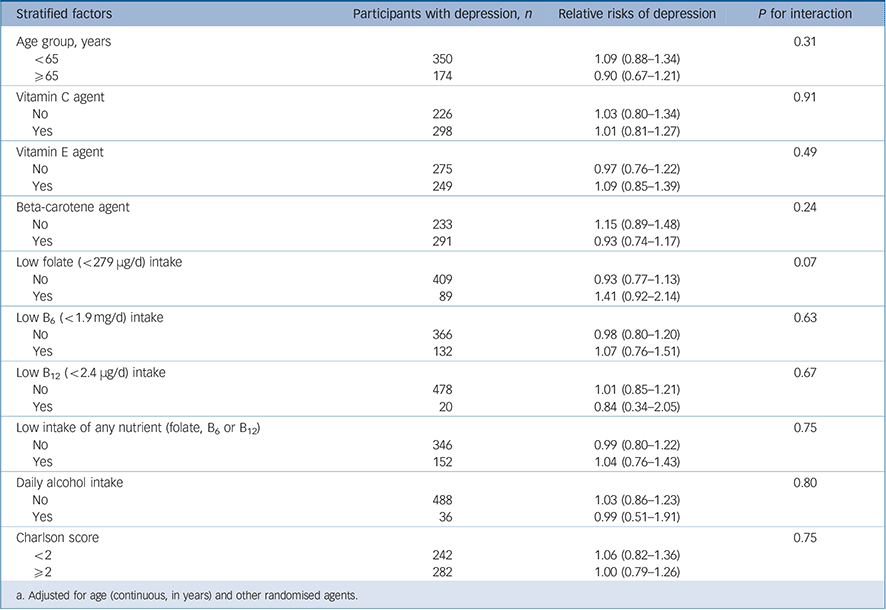
| Stratified factors | Participants with depression, n | Relative risks of depression | P for interaction |
|---|---|---|---|
| Age group, years | 0.31 | ||
| <65 | 350 | 1.09 (0.88–1.34) | |
| ≥65 | 174 | 0.90 (0.67–1.21) | |
| Vitamin C agent | 0.91 | ||
| No | 226 | 1.03 (0.80–1.34) | |
| Yes | 298 | 1.01 (0.81–1.27) | |
| Vitamin E agent | 0.49 | ||
| No | 275 | 0.97 (0.76–1.22) | |
| Yes | 249 | 1.09 (0.85–1.39) | |
| Beta-carotene agent | 0.24 | ||
| No | 233 | 1.15 (0.89–1.48) | |
| Yes | 291 | 0.93 (0.74–1.17) | |
| Low folate (<279 μg/d) intake | 0.07 | ||
| No | 409 | 0.93 (0.77–1.13) | |
| Yes | 89 | 1.41 (0.92–2.14) | |
| Low B6 (<1.9 mg/d) intake | 0.63 | ||
| No | 366 | 0.98 (0.80–1.20) | |
| Yes | 132 | 1.07 (0.76–1.51) | |
| Low B12 (<2.4 μg/d) intake | 0.67 | ||
| No | 478 | 1.01 (0.85–1.21) | |
| Yes | 20 | 0.84 (0.34–2.05) | |
| Low intake of any nutrient (folate, B6 or B12) | 0.75 | ||
| No | 346 | 0.99 (0.80–1.22) | |
| Yes | 152 | 1.04 (0.76–1.43) | |
| Daily alcohol intake | 0.80 | ||
| No | 488 | 1.03 (0.86–1.23) | |
| Yes | 36 | 0.99 (0.51–1.91) | |
| Charlson score | 0.75 | ||
| <2 | 242 | 1.06 (0.82–1.36) | |
| ≥2 | 282 | 1.00 (0.79–1.26) | |
a. Adjusted for age (continuous, in years) and other randomised agents.
combined folic acid/vitamin B6/vitamin B12 supplementation on risk of depression over an average of 7 years of treatment. Subgroup analyses addressing high-risk groups – for example, those with low dietary intakes of B vitamins or high medical comorbidity – and numerous sensitivity analyses, including use of alternative outcome classification and adherence analysis, also did not reveal significant differences between active agent and placebo. Also, B-vitamin/folate treatment did not appear to reduce risk specifically of late-life depression – i.e. among participants aged ≥65 years.
Comparison with findings from other studies
These null results are observed in the context of strong biological plausibility for a role of folate and other B vitamins in mood and brain health. Reference Fava and Mischoulon31 These nutrients are critical for maintaining supply of methyl donor groups and for formation of neurotransmitters. Reference Farah32 Furthermore, epidemiological data support mood benefits of homocysteine-lowering nutrients; low biochemical levels and/or intake of B vitamins as well as high levels of homocysteine have been associated with depressed mood in older adults. Reference Beydoun, Shroff, Beydoun and Zonderman2,Reference Kim, Stewart, Kim, Yang, Shin and Yoon4,Reference Ng, Feng, Niti, Kua and Yap7,Reference Almeida, McCaul, Hankey, Norman and Jamrozik33,Reference Folstein, Liu, Peter, Buell, Arsenault and Scott34 However, limitations of observational studies – for example, cross-sectional design, potential residual confounding (such as by physical health status) or reverse causation bias (i.e. people with depression may have poorer nutrition) – highlighted the importance of experimental approaches. Thus, there has been a recent growth in RCTs addressing the impact of folate and B vitamins on depression risk in humans.
In a depression substudy within the VITATOPS RCT, Almeida et al Reference Almeida, Marsh, Alfonso, Flicker and Davis10 found a ∼50% reduction in relative risk of major depression among 273 patients (mean age 63 years) with recent stroke or transient ischemic attack who received a daily folic acid (2 mg)/vitamin B6 (25 mg)/vitamin B12 (0.5 mg) combination over a 7.1-year average follow-up period. However, despite such exciting findings, the null results in WAFACS are more consistent with the majority of larger RCTs, which have identified no effects of B vitamins on depression risk. For example, Ford et al Reference Ford, Flicker, Thomas, Norman, Jamrozik and Almeida8 found no significant differences, comparing combined folic acid (2 mg/d), B6 (25 mg/d) and B12 (400 μg/d) to placebo, in depressive symptoms or incidence of clinically significant depression (on the Beck Depression Inventory) over 2 years among 299 men, aged 75+ years, with history of hypertension. Later, in a larger trial involving 909 community-based older adults (aged 60–74 years) Walker et al Reference Walker, Mackinnon, Batterham, Jorm, Hickie and McCarthy9 reported no differences, comparing combined folic acid (400 μg/d) and B12 (100 μg/d) to placebo, in depressive symptoms (measured using the Patient Health Questionnaire (PHQ)-9) over a 2-year study period; however, the authors Reference Walker, Mackinnon, Batterham, Jorm, Hickie and McCarthy9 cautioned that under-recruitment and reduced statistical power were possible limitations and that the supplement doses were insufficient to lower participants’ homocysteine levels relative to baseline. Similarly, Andreeva et al Reference Andreeva, Galan, Torrès, Julia, Hercberg and Kesse-Guyot11 found no differences in depressive symptoms on the Geriatric Depression Scale by allocation to B vitamins (0.56 mg/d 5-methyl-tetrahydrofolate and vitamins B6 (3 mg/d) and B12 (0.02 mg/d)) v. placebo among 2000 CVD survivors (aged 45–80 years) in an ancillary study of the SU.FOL.OM3 trial of secondary CVD prevention, Reference Andreeva, Galan, Torrès, Julia, Hercberg and Kesse-Guyot11 after a median treatment duration of 4.7 years.
When considering the results from these RCTs, key differences in study populations could explain the apparently conflicting findings and also suggest that folic acid/B-vitamin supplementation may be more important in select groups. For example, the VITATOPS-depression sample included only people meeting strict criteria for recent stroke or transient ischemic attack and, thus, was enriched for those at particularly high risk for depression; indeed, Robinson et al Reference Robinson, Jorge, Moser, Acion, Solodkin and Small35 similarly demonstrated a significant impact of escitalopram and problem-solving therapy in reducing depression incidence in this very high-risk population. Thus, the VITATOPS-depression sample may not be directly comparable with the more mixed groups of community-dwelling people in WAFACS and other larger-scale trials.
Possible explanations for our findings
Alternative explanations for our findings must also be considered. First, it is possible that this study – despite the large sample, high dosing and long treatment duration – failed to identify a true difference in depression risk by folic acid and B-vitamin supplementation. Indeed, prior trials were able to report on outcomes of depressive symptoms – as opposed to only the binary outcome of incident depression in WAFACS; use of continuous outcomes provides for higher statistical power. Thus, it is not known whether depressive symptom trajectories might have differed significantly by treatment status in WAFACS. Second, intervention effects could have been muted in the context of folate fortification; however, there are important reasons why this is unlikely. Changes in homocysteine levels in response to folate fortification were explicitly addressed in WAFACS: Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 although significant elevation in plasma folate was detected – as expected – post-mandatory fortification, plasma homocysteine levels themselves changed little in the placebo group; by contrast, homocysteine levels were lowered by ∼18.5% (2.27 μmol/l) in the active treatment group. Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 Thus, inadequate homocysteine reduction does not appear to be an issue for our study. Nevertheless, we cannot exclude the possibility that more substantial homocysteine reductions are required to observe mood benefits, and this issue may be of particular relevance among those with biochemical nutrient deficiency. Third, efficiency of the supplement’s effects on the brain may vary by the form of folate used: Reference Fava and Mischoulon31 both WAFACS and an earlier larger-scale high-dose trial Reference Ford, Flicker, Thomas, Norman, Jamrozik and Almeida8 utilised folic acid; however, a recent RCT Reference Papakostas, Shelton, Zajecka, Etemad, Rickels and Clain36 of l-methylfolate (5-methyl-tetrahydrofolate) 15 mg/d, used as adjunctive therapy with SSRIs, yielded reduced depression severity among patients with major depression. Fourth, it is possible that genetic variations in enzymes in homocysteine metabolism (for example, MTHFR (methylenetetrahydrofolate reductase) 677C→T polymorphism), not measured in the current study, may modify effects of B-vitamin/folate treatment on depression risk. Reference Lewis, Lawlor, Davey Smith, Araya, Timpson and Day6 Although prevalence of gene variants will be balanced in the active treatment v. placebo groups by randomisation, there may be subgroups with specific variants that could benefit from these agents; future trials would need to be explicitly designed to test this hypothesis.
Finally, the statistically significant interaction between randomised B and C vitamins among older participants was intriguing and may suggest particular importance of B vitamins under conditions of high oxidative stress, which has relevance in the ageing process. Reference Harman37 Decreasing oxidative stress is a potential mechanism for mood benefits of homocysteine reduction; Reference Leonard and Maes38,Reference Papatheodorou and Weiss39 yet, high-doses of vitamin C have been linked to paradoxical pro-oxidant effects. Reference Podmore, Grifiths, Herbert, Mistry and Mistry40 However, the fact that this was an interaction among the older half of the sample – and the multivariable-adjusted RR estimate within this subgroup was itself of marginal statistical significance (P = 0.061) – warrants considerable caution, as the finding could be as a result of chance.
Strengths and limitations
This study had the advantages of a large sample size, lengthy duration and a complement of B vitamins at doses with significant homocysteine-lowering effects, as well as superior cohort follow-up and adherence rates. Limitations also warrant consideration.
First, reliance on self-reported data may have led to outcome misclassification. However, to the extent that this occurred, the proportions of misclassified cases would have been similar in the active treatment and placebo groups because participants were randomised and group assignment was double-masked; such misclassification would have been non-differential, but could have resulted in attenuated estimates. Furthermore, the fact that this B-vitamin/folate combination has previously been shown not to have an impact on incident heart disease, Reference Albert, Cook, Gaziano, Zaharris, MacFadyen and Danielson13 cancer Reference Zhang, Cook, Albert, Gaziano, Buring and Manson41 or cognitive decline Reference Kang, Cook, Manson, Buring, Albert and Grodstein30 in this cohort is highly advantageous – as influences on interim development of these outcomes would be the most likely sources of the remote possibility of differential misclassification of depression.
A second issue was that, even with a relatively large sample, statistical power in this universal prevention paradigm remained limited. Post hoc power analysis shows that with a sample size of 4300, the observed incidence rate of 18–19 cases per 1000 person-years (comparable to community-based, gender-specific, late-life incident depression rates found elsewhere Reference Luijendijk, van, Berg, Dekker, van Tuijl, Otte and Smit28 ), a follow-up period of 7 years and the observed 84% pill adherence rate, the minimum relative risk reduction detectable at ≥80% power was approximately 25% (RR = 0.75); thus, power for much subtler effects, on the order of a 10 or 15% relative risk reduction, was lower.
Indeed, a third limitation is that the study was not designed to address whether these agents would be of benefit among those with baseline biochemical nutrient deficiency or very high homocysteine levels. Thus, our findings speak to the impact of these agents on depression risk in the setting of adequate nutrient levels among a majority of participants. However, in this respect, our study is similar to other recent larger RCTs Reference Ford, Flicker, Thomas, Norman, Jamrozik and Almeida8,Reference Walker, Mackinnon, Batterham, Jorm, Hickie and McCarthy9,Reference Andreeva, Galan, Torrès, Julia, Hercberg and Kesse-Guyot11 that involved measures of blood nutrient and homocysteine levels but were not designed to test the impact on mood of B vitamins among biochemically nutrient-deficient populations.
Fourth, the use of the combination pill did not permit investigation of individual components, or interactions among them, with respect to depression risk. Fifth, we lacked biomarkers in most participants to assess effect modification by baseline plasma nutrient and homocysteine levels; similarly, we could not address interactions with biomarkers of inflammation or oxidative stress – two key biological links to both the effects of homocysteine reduction Reference Papatheodorou and Weiss39 and the aetiology of depression. Reference Leonard and Maes38 Sixth, confounding by unidentified factors cannot be fully excluded; however, this is doubtful, given the observed balance in the treatment groups, evidencing effective randomisation within this subset of the WAFACS. Finally, we cannot assume generalisability of results among these mid-life and older women to men or to the broader general population.
Implications
In this randomised trial among over 4300 women with CVD or multiple CVD risk factors, we found no evidence of benefit or harm of combined folic acid, vitamin B6 and vitamin B12 supplementation on the risk of depression in mid- or late-life, over a 7-year treatment period. When considering the current results in the context of existing RCT evidence, it does not appear that long-term daily supplementation with folic acid and B vitamins yields substantial risk reductions in depression among late mid-life and older people, at least in the setting of a simple universal prevention framework. Clarifying the role of these nutrients in depression prevention will require further efforts. For example, future RCTs may focus on specific formulations of the relevant nutrients (for example, l-methylfolate, which can cross the blood–brain barrier Reference Farah32 ) or employ certain design aspects, such as careful consideration of key groups for selective prevention – as individuals with relevant genetic, environmental or biomarker variation.
Acknowledgements
We acknowledge the invaluable contributions of the very dedicated 8171 WACS participants and 5442 WAFACS participants.







eLetters
No eLetters have been published for this article.