Electroconvulsive therapy (ECT) is one of the most effective treatments for depression but the mechanisms underlying its therapeutic effects are unknown. Brain serotonin2 (5-hydroxytryptamine2; 5-HT2) receptors were considered potential targets; however, pre-clinical studies showed that electroconvulsive shock up-regulates brain 5-HT2 receptors in rodents; which is in contrast to the effects of various antidepressant medications such as tricyclics, monoamine oxidase inhibitors, atypical antidepressants such as mianserin, and selective serotonin reuptake inhibitors (SSRIs), all of which cause down-regulation of brain 5-HT2 receptors. Reference Kellar, Cascio, Butler and Kurtzke1–Reference Blackshear and Sandersbush4 Some argue that it is this unique ability of ECT to up-regulate brain 5-HT2 receptors that accounts for its superior efficacy, whereas others suggest that brain 5-HT2 receptors are irrelevant to therapeutic effects of antidepressant treatments given the fact that the two most effective treatments for depression induce changes in these receptors in opposite directions. The most definitive way to resolve this controversy is to assess the effects of these treatments on brain 5-HT2 receptors in vivo in humans, more specifically, in individuals with depression. Accordingly, taking advantage of the ability of positron emission tomography (PET) to visualise brain 5-HT2 receptors in humans, three recent studies, each using an antidepressant from a different class, reported that treatment of participants with depression with a selective noradrenaline reuptake inhibitor desipramine, Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5 an SSRI paroxetine, Reference Meyer, Kapur, Eisfeld, Brown, Houle and DaSilva6 or an SSRI plus 5-HT2 antagonist nefazodone, Reference Mischoulon, Dougherty, Bottonari, Gresham, Sonawalla and Fischman7 down-regulated brain 5-HT2 receptors. The missing piece of the puzzle is the effects of ECT on brain 5-HT2 receptors in individuals with depression. Therefore, in order to address this controversy, we used PET and [18F]setoperone to assess the effects of a course of ECT on brain 5-HT2 receptors in individuals with depression who were refractory to treatment with antidepressant medications. We chose [18F]setoperone as a ligand for quantifying brain 5-HT2 receptors because of its selectivity and sensitivity for 5-HT2 receptor binding in cerebral cortex Reference Meyer8 in vivo and for its higher (>2.5) total to non-specific binding ratio. Reference Blin, Sette, Fiorelli, Bletry, Elghozi and Crouzel9,Reference Crouzel, Guillaume, Barre, Lemaire and Pike10
Method
Participants
All participants were assessed by a structured clinical interview for DSM–IV diagnosis (SCID). Reference First, Spitzer, Gibbon and Williams11 The DSM–IV 12 diagnosis of major depressive disorder was arrived at by the consensus of a research team based on information from an unstructured clinical interview as well as SCID. Individuals who met criteria for a major depressive disorder, refractory to previous antidepressant therapy and referred to a tertiary care mood disorders centre for ECT treatment were approached for participation in the study. The study was approved by the Clinical Research Ethics Board of the University of British Columbia. All participants provided written informed consent prior to completing any study procedures. Participants had no other Axis I diagnoses, substance or alcohol misuse within the past 6 months. All study participants had adequate trials with at least two antidepressant medications from two different classes and most of them had trials with several antidepressants and augmenting agents for the current episode. They were physically healthy and had a psychotropic medication washout period of at least 1 week prior to the baseline PET scan. The duration of washout period was determined for each individual based on the half-life of psychotropic medication they had been taking and the minimum washout period required was equivalent to at least five half-lives of the psychotropic medication. Individuals, however, were allowed to receive benzodiazepines for night sedation. Each participant had a magnetic resonance imaging (MRI) scan to exclude cerebral pathology and for co-registration of PET images. The severity of depressive symptoms in individuals before and after treatment with ECT was quantified using Hamilton Rating Scale for Depression (HRSD). Reference Hamilton13,Reference Williams, Link, Rosenthal and Terman14
PET scans
Each participant had two [18F]setoperone PET scans, one before the first ECT and the second within 1 week of the last ECT treatment. The [18F]setoperone was synthesised and the PET scans were performed as previously described. Reference Yatham, Liddle, Shiah, Scarrow, Lam and Adam15 The participants were positioned in the scanner to obtain most of the cerebral cortex (frontal, temporal, parietal) apart from the upper regions near the vertex and some cerebellum. After participants had a transmission scan for 10 min to correct PET images for attenuation, they were injected with 4 to 7 mCi of [18F]setoperone intravenously and following this, 15 frame emission scans were performed over 110 min as previously described. Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5 Radioactivity in the brain was measured with a PET camera ECAT/953B (Siemens, Knoxville, Tennessee, USA) that collects 31 axial slices. The spatial resolution of PET images is about 9 × 9 × 6 mm3.
ECT procedure
The procedure for ECT administration was similar to that described previously. Reference Shiah, Yatham, Srisurapanont, Lam, Tam and Zis16 A Thymatron ECT apparatus was used to deliver electrical stimulus to individuals who had general anaesthesia induced with sodium thiopental and muscle relaxation with succinylcholine. All of them were given right unilateral ECT. Seizure threshold was estimated during the first treatment. Subsequent treatments were given at three times the seizure threshold, and further adjustments in stimulus intensity (an increase of 10–20%) were made for each ECT treatment to elicit a seizure of at least 20 s duration (measured with single strip electroencephalogram recording) and good amplitude with each treatment. Participants received ECT treatments three times a week and the number of ECT treatments for each individual was determined by the treating clinician based on clinical judgement of treatment response or non-response. The standard practice at the UBC Hospital is to give at least two more ECT treatments after remission of depression, but in those that had not shown any improvement, ECT is typically discontinued after eight ECT treatments. Each participant had a second PET scan between 3 and 7 days after the last ECT treatment.
Image processing and data analysis
The PET images were processed as previously described using Statistical Parametric Mapping software 2 (SPM2; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, www.fil.ion.ucl.ac.uk/spm/software/spm2) run on Matlab 6 (Mathworks Inc., Natick, Massachusetts, USA). Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5 Briefly, the PET scan frames 14 and 15 corresponding to the last 40 min of emission data were realigned to each other to create a mean image for baseline scan and a mean image for post-ECT scan. These two mean images were realigned to create a composite image that was then co-registered with the individuals' MRI. The PET and MRIs were then transformed into the standard coordinate frame used for templates in SPM2 using the spatial normalisation method specified in SPM2.
The SPM2 was used to determine the significance of the change in regional [18F]setoperone binding with ECT. Because non-specific binding for setoperone in cerebellum differs from that in cortex, Reference Petit-Taboue, Landeau, Barre, Onfroy, Noel and Baron17 we used a reference region comprising all cortical grey matter voxels, excluding voxels in which there was a change (either decrease or increase) in binding significant at P<0.1 Reference Desjardins, Kiehl and Liddle18 and the rationale for this is as follows: the ratio of concentration of ligand in a local region to that in a reference region is given by
where BP denotes binding potential, and k 6 and k 6 are the rate constants for attachment to and dissociation from non-specific binding sites. If the local and reference regions are in cerebral cortex, it is reasonable to assume that (k 5/k 6) is similar in both regions. If the reference region is defined as that cortical region in which there is no change in binding between pre- and post-treatment scans and furthermore, if it is assumed that (k 5/k 6) is not affected by treatment, the change in
where ΔBP local is the change in local binding potential and
Therefore, a change in (C local /C reference), while systematically underestimating ΔBP local, remains proportional to it. The reference region in which there was no change was identified by iterative exclusion of all voxels in which there was trend towards either an increase or decrease in setoperone binding during treatment, at level P<0.1 as stated earlier. Reference Desjardins, Kiehl and Liddle18
For inclusion of voxels in the SPM analysis, grey matter threshold was set at 120% of the mean image intensity as this threshold eliminated most white matter voxels without excluding any grey matter voxels. The theory of Gaussian fields as implemented in SPM2 was applied to assess the statistical significance of cluster of contiguous voxels in which change during treatment exceeded a threshold of P<0.025. This threshold was chosen because we predicted a widespread reduction in brain 5-HT2 receptors with ECT. The Worsley's method in SPM takes into account extent within the cluster and applies correction for multiple comparisons in calculating cluster significance. The corrected cluster significance was set at P<0.01. We also assessed the significance of change in [18F]setoperone for each voxel using the Worsley's method based on the theory of Gaussian fields Reference Worsley19 as implemented in SPM. Reference Friston, Frith, Liddle and Frackowiak20 The SPM analysis provides corrected P-values based on family-wise error control (FWE-corr) as well as false-discovery rate. The former controls chances of any false positives in the brain, whereas the latter controls for the expected proportion of false positives among suprathreshold voxels. The false-discovery rate is more sensitive and is adaptive to the amount of signal observed in the data. Reference Genovese, Lazar and Nichols21
Computation of binding potential using Logan graphical method
Since the ratio method is susceptible to changes in ligand delivery/blood flow, we also computed the pre- and post-treatment values of the 5-HT2 binding potential (BP ND) for the regions identified as yielding a significant difference in the ratio values, using the Logan graphical method. Reference Logan, Fowler, Volkow, Wang, Ding and Alexoff22 The BP ND refers to the ratio at equilibrium of specifically bound radioligand to that of non-displaceable radioliogand in tissue and represents the ratio between the maximum free receptor density (B max) and the dissociation constant (K d). Since studies have shown Reference Klimek, Zak-Knapik and Mackowiak23 no change in K d with treatment, it can be assumed that a change in BP ND is directly related to a change in receptor density.
Results
A total of 16 participants completed both baseline and post-ECT scans. Of these, the PET scan image for one person was corrupted and could not be processed for analysis. So, the data for the remaining 15 people (6 male and 9 female) was analysed.
Participants had a mean age of 44.26 (s.d. = 10.4) years. The duration of current depressive episodes ranged from 12 weeks to 13 years. Only two participants had no previous depressive episodes, whereas the others had episodes ranging from two to more than ten episodes. Two participants had unremitting depressive symptoms for 10–13 years. The mean baseline HRSD score was 33.6 (s.d. = 7.71) and depressive symptoms improved significantly with a course of ECT treatment (post-ECT mean HRSD score 11.26 (s.d. = 10.2, t = 7.57, d.f. = 14, P<0.0001). The duration of drug-free period prior to baseline PET scan ranged from 7 to 84 days (mean 18.5, s.d. = 18.7) and the number of ECTs participants received ranged from 5 to 13 (mean 8.6, s.d. = 2.6). Ten out of 15 participants met criteria for response (≥50% reduction in symptoms) and nine individuals met criteria for remission (≤12) on HRSD. There were no differences in the number of ECTs received or number and duration of previous depressive episodes between responders and non-responders. Further, there was no correlation between the number of ECTs and changes in brain 5-HT2 receptors.
Electroconvulsive therapy significantly reduced the [18F]setoperone binding in various cortical regions. The reduction in [18F]setoperone binding was evident in an extensive cluster of voxels (P = 1.3 × 10–9, after correction for multiple comparisons in the entire brain volume), which embraced bilateral occipital cortex, medial parietal cortex (peak in the lingual gyrus), limbic cortex (with peak in the right parahippocampal gyrus) as well as bilateral prefrontal cortex (with a peak in the right inferomedial prefrontal cortex). This cluster included 31 908 voxels (Fig. 1).

Fig. 1 Maximum intensity projection of the significant cluster in which setoperone binding decreased between pre- and post-treatment scans.
Cluster inclusion threshold P<0.025 uncorrected; the cluster of 31 908 voxels was significant after correction for multiple comparisons; P = 8 × 10 –11.
The mean change in [18F]setoperone binding in the cluster was 3.8%. The largest percentage change in signal was observed in right parahippocampal gyrus (6.7%) but because of lesser variance in the effect between participants, the most significant change observed was in left lateral occipital gyrus (5.4%). The reduction in right medial frontal gyrus was 5%.
There were many voxels in this cluster that had shown a significant reduction in [18F]setoperone binding (P<0.025, false-discovery rate criterion) regardless of their membership in the cluster. The location and t-values for the voxels at local maxima that satisfied the false-discovery rate criterion are provided in Table 1 (see also Fig. 2).
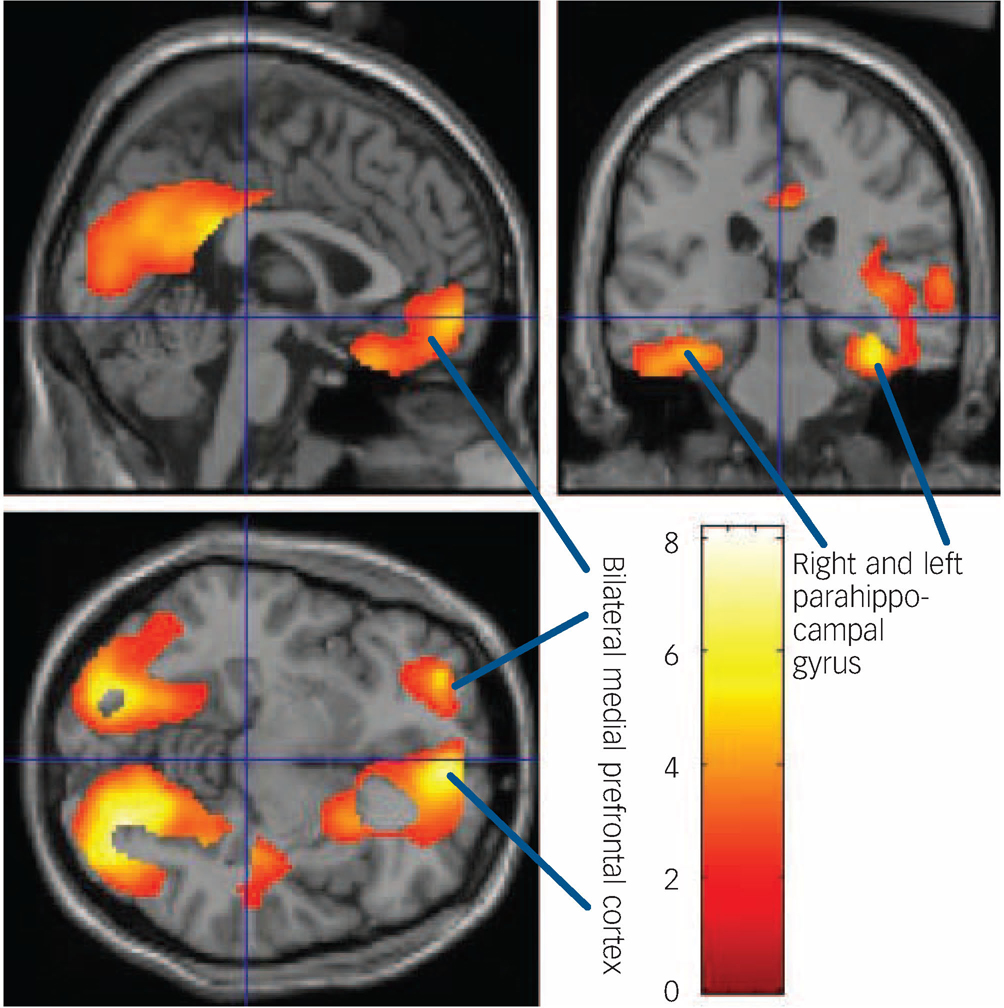
Fig. 2 Areas of significant decreases in [18F]setoperone binding on the sagittal, coronal and transverse renderings of the brain.
Arrows indicate reductions in binding in parahippocampal gyrus and bilateral medial prefrontal cortex.
Table 1 Brain regions that showed significant decreases in [18F]setoperone binding

| Voxels | ||||||
|---|---|---|---|---|---|---|
| Family-wise error, corrected P | False-discovery rate, corrected P | Coordinatesa | ||||
| Brain regions | t | x | y | z | ||
| Left lateral occipital gyrus | 8.17 | 0.011 | 0.002 | -18 | -94 | 18 |
| Right lingual gyrus | 8.07 | 0.011 | 0.002 | 30 | -76 | -16 |
| Left cuneus | 7.44 | 0.022 | 0.002 | -12 | -76 | -22 |
| Right medial frontal gyrus | 7.07 | 0.033 | 0.002 | 6 | 52 | -10 |
| Right parahippocampal gyrus | 5.79 | 0.155 | 0.002 | 38 | -26 | -20 |
To ascertain if changes in regional [18F]setoperone binding were related to improvement in clinical symptoms, we tested for regression coefficients within 15 mm radius spherical regions centred on the five local maxima shown in Table 1. Regression coefficients significant at the level P = 0.001 (uncorrected) were observed in three (right parahippocampal gyrus, right medial prefrontal cortex and right lingual gyrus) of these five regions of interest, as shown in Table 2 and Figs 3(a)–(c). However, after correction for multiple comparisons within the volume of interest (using the small volume correction method of Worsely et al) Reference Worsley19 these regression coefficients only achieved a trend level of significance (P≤0.1). The results with the Logan method Reference Logan, Fowler, Volkow, Wang, Ding and Alexoff22 confirmed that the setoperone BP ND was significantly lower (P = 0.009) following ECT in the same regions as the ratio method and that the reduction in BP ND was 8.1%.

Fig. 3 Correlations between reduction in [18F]setoperone binding in brain regions and improvement in depressive symptoms as measured by the difference in Hamilton Rating Scale for Depression (HRSD) scores between baseline and post-electroconvulsive therapy treatment. (a) Right medial prefrontal cortex, (b) right lingual gyrus, (c) right parahippocampal gyrus.
Table 2 Correlations between change in Hamilton Rating Scale for Depression score and change in [18F]Setoperone binding in 15 mm radius spherical regions centred on local maxima of the change in [18F]setoperone binding during treatment (reported in Table 1)

| Voxels | |||||||
|---|---|---|---|---|---|---|---|
| Pearson correlation coefficients | Small volume correction for multiple comparisons, P | Uncorrected P | Coordinates | ||||
| Anatomical location | t | x | y | z | |||
| Right lingual gyrus | 0.71 | 3.65 | 0.10 | 0.001 | 22 | -64 | -12 |
| Right medial prefrontal cortex | 0.73 | 3.84 | 0.08 | 0.001 | 6 | 60 | 2 |
| Right parahippocampal gyrus | 0.73 | 3.82 | 0.08 | 0.001 | 36 | -38 | -28 |
Discussion
To our knowledge, this is the first study to examine the effects of ECT on brain 5-HT2 receptors. The results showed that, in contrast to the effects of electroconvulsive shock on brain 5-HT2 receptors in rodents that show up-regulation, Reference Kellar, Cascio, Butler and Kurtzke1,Reference Kellar and Stockmeier24,Reference Vetulani, Lebrecht and Pilc25 our findings indicate that ECT in individuals with depression, like antidepressant medications, Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5–Reference Mischoulon, Dougherty, Bottonari, Gresham, Sonawalla and Fischman7 down-regulates 5-HT2 receptors in several cortical regions. Species differences in 5-HT2 receptor regulation might be one explanation for discrepancy in findings between rodent studies and the present study in humans. Indeed, a study in monkeys showed that electroconvulsive shock down-regulates brain 5-HT2 receptors in all cortical regions, Reference Strome, Clark, Zis and Doudet26 which supports the possibility that 5-HT2 receptor regulation is different in rodents and primates.
Is 5-HT2 receptor down-regulation intrinsic to mechanism of action of antidepressant treatments?
The finding that ECT and three different antidepressants with differing modes of action (desipramine – a noradrenaline reuptake inhibitor; Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5 paroxetine – a serotonin reuptake inhibitor (SSRI); Reference Meyer, Kapur, Eisfeld, Brown, Houle and DaSilva6 and nefazodone – an SSRI and a 5-HT2 receptor antagonist) Reference Mischoulon, Dougherty, Bottonari, Gresham, Sonawalla and Fischman7 all down-regulate brain 5-HT2 receptors, suggests that down-regulation of 5-HT2 receptors is either an intrinsic feature of the mechanism of antidepressant action or is a direct consequence of antidepressant action. Furthermore, this study of ECT and all three previous studies with antidepressants Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5–Reference Mischoulon, Dougherty, Bottonari, Gresham, Sonawalla and Fischman7 have reported down-regulation of 5-HT2 receptors in limbic and prefrontal areas, although with paroxetine this was seen mainly in younger participants. Reference Meyer, Kapur, Eisfeld, Brown, Houle and DaSilva6 Limbic and frontal areas are implicated in the neurobiology of depression. Reference Mayberg, Goldapple and MacIntosh27,Reference Mayberg28 The possibility that down-regulation of 5-HT2 receptors in limbic and frontal areas is intrinsic to the mechanism of antidepressant action is further supported by the finding of a trend towards correlation between ECT-related changes in [18F]setoperone binding in medial prefrontal cortex as well as right parahippocampal gyrus and clinical improvement in depression as measured by changes in HRSD scores.
5-HT2 receptors and right hemisphere
Although reduction in brain 5-HT2 receptors was observed in both right and left cortical regions, changes were slightly prominent on the right side. Since all participants in this study received right unilateral ECT, it raises the possibility that slightly more prominent reduction in 5-HT2 receptors in right cortical regions might be as a result of the fact that the electrical stimulus was delivered to the right brain. However, previous studies that assessed the effects of antidepressants on brain 5-HT2 receptors have also observed such asymmetry with changes slightly more prominent on the right side. Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5,Reference Mischoulon, Dougherty, Bottonari, Gresham, Sonawalla and Fischman7 Furthermore, some studies have also reported changes in brain 5-HT2 receptors only on the right side in untreated individuals with depression. Reference D'haenen, Bossuyt, Mertens, Bossuyt-Piron, Gijsemans and Kaufman29,Reference Biver, Wikler, Lotstra, Damhaut, Goldman and Mendlewicz30 Given that the density of 5-HT2 receptors in human brain appears to be similar in both hemispheres, Reference Schotte, Maloteaux and Laduron31 these findings suggest that changes in brain 5-HT2 receptors in the right brain might be more relevant to neurobiology of depression and therapeutic effects of treatments.
Brain 5-HT2 receptors and efficacy of ECT in refractory depression
Interestingly, the percentage reduction in brain 5-HT2 receptors was only 3.8% for the entire cluster, 5% in medial frontal gyrus and 6.7% in right parahippocampal gyrus. These percentage reductions in 5-HT2 receptor binding with ECT are somewhat smaller than those reported with desipramine (8–15.7%) Reference Yatham, Liddle, Dennie, Shiah, Adam and Lane5 and paroxetine (10%). Reference Meyer, Kapur, Eisfeld, Brown, Houle and DaSilva6 However, it should be noted that many of the participants in this study had already received extensive treatment with antidepressants before ECT, so it is possible that the reductions related to ECT were superimposed on prior reduction associated with antidepressant medication. Furthermore, some Reference Yatham, Liddle, Shiah, Scarrow, Lam and Adam15,Reference Mintun, Sheline, Moerlein, Vlassenko, Huang and Snyder32 but not all Reference Meyer, McMain, Kennedy, Korman, Brown and DaSilva33,Reference Bhagwagar, Hinz, Taylor, Fancy, Cowen and Grasby34 studies suggest that untreated individuals with major depression have up to 29% reduction in brain 5-HT2 receptors compared with age- and gender-matched healthy individuals and we have previously hypothesised that such down-regulation might be a compensatory homeostatic response of the brain to the state of depression. Reference Yatham, Liddle, Shiah, Scarrow, Lam and Adam15 Thus, it is plausible that, in some individuals, natural homeostatic processes might produce sufficient reduction in brain 5-HT2 receptors to alleviate depression; in others, antidepressant medication is required, whereas in treatment-refractory cases, ECT is required to produce additional reduction in 5-HT2 receptor density. This hypothesis is supported by the findings of this study that showed that the antidepressant-refractory participants had further reduction in 5-HT2 receptors and that there was a trend for a correlation between reduction in [18F]setoperone binding in medial prefrontal cortex as well as right parahippocampal gyrus and clinical improvement in depression with ECT. The reductions in 5-HT2 receptors observed in this study in participants who were refractory to antidepressant treatment may explain not only its superior efficacy to antidepressants but also its benefit in those non-responsive to pharmacotherapy.
Brain 5-HT2 receptors and improvement in depression
If ECT and antidepressant medications all down-regulate brain 5-HT2 receptors, and if such down-regulation mediates antidepressant effects, other treatments that down-regulate 5-HT2 receptors should also work as antidepressants. Indeed, atypical antipsychotics, which block and down-regulate brain 5-HT2 receptors, Reference Tarazi, Zhang and Baldessarini35 have recently been shown to have significant antidepressant properties in clinical trials of people with bipolar depression. Reference Tohen, Vieta, Calabrese, Ketter, Sachs and Bowden36,Reference Calabrese, Keck, Macfadden, Minkwitz, Ketter and Weisler37 In contrast, conventional antipsychotics such as haloperidol do not alter brain 5-HT2 receptors Reference Burnet, Chen, McGowan, Franklin and Harrison38 and have no significant antidepressant properties or, indeed, can induce depressive symptoms. Reference Tohen, Goldberg, Arrillaga, Azorin, Vieta and Hardy-Bayle39 Furthermore, in another PET study, we have shown that, in healthy volunteers, 5-HT2 receptor down-regulation is associated with lack of depressive symptoms induced by tryptophan depletion. Reference Yatham, Liddle, Shiah, Lam, Adam and Zis40
Limitations
Some limitations of our study warrant comment. First, although setoperone binds with higher affinity to 5-HT2 receptors (K i = 0.37 nmol/l), it has some affinity to α1 adrenergic as well as dopamine D2 receptors. Reference Blin, Sette, Fiorelli, Bletry, Elghozi and Crouzel9,Reference Crouzel, Guillaume, Barre, Lemaire and Pike10 However, pre-treatment with α1 antagonist prazosin or D2 antagonist sulpiride did not change specific/non-specific binding ratios in humans, indicating that setoperone signal in humans is reflective of 5-HT2 binding. Reference Blin, Sette, Fiorelli, Bletry, Elghozi and Crouzel9 Setoperone also has a good specific to non-specific binding ratio in cortex and hence is considered a suitable ligand for assessing brain 5-HT2 receptors in humans. Reference Blin, Sette, Fiorelli, Bletry, Elghozi and Crouzel9,Reference Crouzel, Guillaume, Barre, Lemaire and Pike10 It is likely that setoperone binding provides an estimate of brain 5-HT2A receptors as it was fully displaced by EMD281014, a more selective 5-HT2A antagonist. Reference Mamo, Sedman, Tillner, Sellers, Romach and Kapur41 Second, since each ECT treatment was given under general anaesthesia, one could argue that the anaesthetic agent contributed to reduction in brain 5-HT2 receptors observed in this study. As no study to date examined the effects of barbiturates anaesthesia on brain 5-HT2 receptors, we cannot exclude this possibility. However, a previous study that examined the effects of other anaesthetic agents such as ketamine, zoletile mixture, isoflurane or halothane reported that none had any effect on either 5-HT2 receptors or cerebellar non-specific binding. Reference Elfving, Bjornholm and Knudsen42 Third, we cannot exclude the effects of the muscle relaxant succinylcholine on brain 5-HT2 receptors as no previous study assessed its effects. Fourth, the method used to assess changes in 5-HT2 receptors in this study does not provide an absolute quantification of 5-HT2 receptor binding. In fact, the changes in 5-HT2 receptor binding reported here are relative changes between pre- and post-treatment scans in total bound concentration of ligand. Provided that non-specific binding did not change during treatment (an assumption also required if we had used a cerebellar reference region), these changes reflect a change in specific binding. Further, these reported changes are relative to total pre-treatment binding at the reported locations. Assuming the changes in non-specific binding are negligible, our method, although providing an estimate of change in specific binding that is proportional to the change in 5-HT2 binding potential, systematically underestimates the true change in 5-HT2 receptors. This may have been one of the reasons for a smaller observed effect of ECT on 5-HT2 receptors in this study. Fifth, in the absence of a control group, we cannot exclude the possibility of a systematic change in 5-HT2 binding over time that is unrelated to treatment. However, the observation by Chow et al Reference Chow, Mamo, Uchida, Graff-Guerrero, Houle and Smith43 of test–retest variability of 1.9% in frontal lobes and 3.3% in temporal lobes without evidence of significant systematic change makes it unlikely that the change we observed (3.8% averaged over the entire cluster, with a 6.7% peak reduction) was as a result of systematic change unrelated to treatment.
In conclusion, this study provides evidence that, in contrast to the effects of electroconvulsive shock on 5-HT2 receptors in rodents, ECT in individuals with depression down-regulates brain 5-HT2 receptors in the limbic and prefrontal cortical brain areas that have previously been implicated in the neurobiology of depression. The findings of this study taken in conjunction with the findings of previous studies of the effects of antidepressants on brain 5-HT2 receptors, consolidates the role of brain 5-HT2 receptors in mediating the antidepressant effects and may put to rest the controversy over the last four decades.
Funding
This study was funded by National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award as well as a Canadian Institutes of Health Research (CIHR) operating grant to L.N.Y.








eLetters
No eLetters have been published for this article.