Background
Among patients with major depressive disorder (MDD), guidelines recommend starting antidepressant treatment with a selective serotonin reuptake inhibitor (SSRI), a serotonin norepinephrine reuptake inhibitor (SNRI) or mirtazapine.Reference Bayes and Parker1 Although tricyclic antidepressants (TCA) represent the most efficient group of antidepressants,Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson and Ogawa2, Reference Pereira and Hiroaki-Sato3 safety concerns usually reserve initial treatment with a TCA for patients experiencing severe depression. However, despite state-of-the-art treatment, approximately 50% will not respond sufficiently to their first-line antidepressant.Reference Trivedi, Rush, Wisniewski, Nierenberg, Warden and Ritz4 In order to achieve response, several approaches are relevant. This includes dose increase,Reference Bose, Tsai and Li5 augmentationReference Nierenberg, Fava, Trivedi, Wisniewski, Thase and McGrath6 or switching to another antidepressant,Reference Thase, Rush, Howland, Kornstein, Kocsis and Gelenberg7 with the latter including switching within the same classReference Rush, Trivedi, Wisniewski, Stewart, Nierenberg and Thase8 or to a different class of antidepressants.Reference Thase, Rush, Howland, Kornstein, Kocsis and Gelenberg7
Despite switching between antidepressants representing a frequent and important clinical approach, little research has been performed on this important aspect of clinical decision-making. Recent meta-analyses found that several studies have investigated switching, but only eight randomised trials have compared the effect of switching antidepressant medication versus continuation.Reference Bschor and Baethge9, Reference Bschor, Kern, Henssler and Baethge10 Interestingly, the results showed no difference between switching and continuation.Reference Bschor, Kern, Henssler and Baethge10 Indeed, one randomised trial (n = 189) found that continuation showed significantly better effects compared with switching.Reference Souery, Serretti, Calati, Oswald, Massat and Konstantinidis11 In addition, the recent VAST-D trial, which included 1522 US veteran patients with MDD and non-response to at least one antidepressant course,Reference Mohamed, Johnson, Chen, Hicks, Davis and Yoon12 found that augmentation with aripiprazole showed significant better remission compared with individuals randomised to bupropion switching. However, the response rates were rather modest (22–29%) and the study population consisted mainly of older males (85.2%; mean age 54.4 years).
Nevertheless, if continuation is not possible, several switching approaches may improve treatment effects after non-response or side-effects to SSRIs, for example switching to SNRIsReference Bose, Tsai and Li5, Reference Rush, Trivedi, Wisniewski, Stewart, Nierenberg and Thase8 or vortioxetine.Reference Brignone, Diamand, Painchault and Takyar13 Regarding TCA treatment, it is often assumed that the most efficient antidepressant has been given. Therefore, it may not be beneficial to switch to a first-line antidepressant, for example an SSRI. Nevertheless, one large trial included patients with chronic depression who failed to respond to a 12-week treatment with the SSRI sertraline or the TCA imipramine.Reference Thase, Rush, Howland, Kornstein, Kocsis and Gelenberg7 Switching from sertraline to imipramine (n = 117) or imipramine to sertraline (n = 51) resulted in response among more than 50% of the patients in both groups. Another trial found beneficial effects for switching to the SSRI fluoxetine (n = 142) after non-response to the TCA nortriptyline.Reference Shelton, Williamson, Corya, Sanger, Van Campen and Case14 However, this randomised study found no difference compared with nortriptyline continuation (n = 68).
Hence, more research in this clinically highly relevant area is needed and several switching combinations and specific drugs have not been investigated.Reference Bschor, Kern, Henssler and Baethge10, Reference Brignone, Diamand, Painchault and Takyar13, Reference Connolly and Thase15 Furthermore, many patients experience side-effects, particularly to TCA treatment, necessitating switching to another antidepressant. In addition, it has been suggested that switching after 2 weeks may be beneficial among patients with early non-response.Reference Nakajima, Uchida, Suzuki, Watanabe, Hirano and Yagihashi16 Other findings indicate that continuation and dose increase may lead to better response rates.Reference Bose, Tsai and Li5 The clinically important aspect of switching options after a failed first antidepressant treatment has to be explored in different populations and other SSRI and TCA compounds need to be studied including the effect of the timing of switching. Hence, our aim was to investigate whether switching from the TCA nortriptyline to the SSRI escitalopram or vice versa resulted in improved treatment effects among patients with MDD after a failed first antidepressant treatment.
Method
Study design and participants
The GENome-Based Therapeutic Drugs for Depression (GENDEP) study is a 12-week partly randomised multicentre clinical trial (trial registration: EudraCT No.2004-001723-38 (http://eudract.emea.europa.eu) and ISRCTN No.03693000 (http://www.controlled-trials.com)) comparing treatment with escitalopram with that of nortriptyline (a detailed flow chart is available in Uher et al Reference Uher, Farmer, Maier, Rietschel, Hauser and Marusic17). A total of 811 adults diagnosed with MDD of at least moderate severity established in the Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview,Reference Wing, Sartorius and Üstun18 including people both with first-time depression and those with recurrent depression who previously may have received treatment, were recruited in nine European countries. Exclusion criteria were a personal or family history (first-degree relative) of bipolar disorder or schizophrenia, a personal history of hypomanic or manic episodes or mood incongruent psychotic symptoms, active substance dependence, primary organic disease, previous non-response, side-effects or contraindications to both study medications and ethnicity other than White, the latter being because of the genetic part of GENDEP.
We assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human participants/patients were approved by ethics boards in all participating centres. Written informed consent was obtained from all participants. Verbal consent was witnessed and formally recorded.
Interventions
Participants without contraindications for escitalopram or nortriptyline were randomly allocated to receive one of the two antidepressants using a random number generator, stratified by centre and performed independently of the assessing clinician: 233 were randomised to escitalopram and 235 to nortriptyline. Patients with a history of non-response, side-effects or contraindications for one of the drugs were allocated non-randomly to the other antidepressant: 225 to escitalopram and 118 to nortriptyline.
Escitalopram was initiated at 10 mg daily and increased to a target dose of 15 mg daily within the first 2 weeks and could be further increased to 20 mg daily (and up to 30 mg in individuals where there was clinical agreement that a higher dose was needed). Nortriptyline was initiated at 50 mg daily and titrated to a target dose of 100 mg daily within the first 2 weeks and could be further increased to 150 mg daily (and up to 200 mg individuals where there was clinical agreement that a higher dose was needed). Other psychotropic medications were not allowed with the exception of occasional use of hypnotics. Adherence was monitored weekly by self-reported pill count, and plasma levels of antidepressants were measured at week 8. Overall, individuals treated with escitalopram or nortriptyline improved to a similar degree regarding depressive symptoms.Reference Uher, Maier, Hauser, Marusic, Schmael and Mors19 If a patient experienced poor tolerance or no response was observed during the first 12 weeks on the initially assigned drug, patients were offered to switch to the other drug. This decision was based on a clinical evaluation considering both therapeutic and adverse effects, as assessed with the antidepressant side-effect checklist (ASEC),Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen20 and no cut-off criteria for switching were defined. In total, 72 patients switched from nortriptyline to escitalopram and 36 switched from escitalopram to nortriptyline and were followed for up to 26 weeks after switching. These 108 patients represent the study population for the present study.
Measures
Depression severity was measured at inclusion and weekly throughout the first 12 weeks, both during the first antidepressant trial and after switching, with a final visit 26 weeks after switching. Three established scales were applied: the clinician-rated 10-item Montgomery–Åsberg Depression Rating Scale (MADRS)Reference Montgomery and sberg21 and the 17-item Hamilton Rating Scale for Depression (HDRS),Reference Hamilton22 and the self-report 21-item Beck Depression Inventory (BDI).Reference Beck, Ward, Mendelson, Mock and Erbaugh23 We included response, defined as a reduction ≥50% on the MADRS or HDRS and remission, defined as MADRS ≤11 or HDRS ≤7, respectively.
The rating scales were administered by trained psychologists and psychiatrists who achieved high interrater reliability on recorded interviews that did not differ between centres (Cronbach's α ≥0.9 for MADRS and BDI and Cronbach's α≥0.8 for HDRS).Reference Uher, Farmer, Maier, Rietschel, Hauser and Marusic17 In a previous GENDEP studyReference Uher, Maier, Hauser, Marusic, Schmael and Mors19 we found that depressive symptoms could be described in more detail by three symptom dimensions derived by categorical item factor analyses: observed mood, cognitive symptoms and neurovegetative symptoms. The observed mood dimension comprises clinician-rated items assessing core mood symptoms, anxiety and activity. The cognitive symptom dimension includes items assessing pessimism, guilt, suicidality and the majority of self-reported items from the BDI. The neurovegetative symptom dimension comprises insomnia, poor appetite, weight loss and decreased libido.
Statistical analysis
To investigate treatment outcome after switching, we performed mixed-effects linear regression models with full information maximum likelihood estimation and report β-coefficients including 95% CIs. These models allow inclusion of all relevant covariates across repeated measurements and efficiently handle missing data.Reference Lane24 MADRS was the primary outcome measure as in previous GENDEP studies.Reference Uher, Farmer, Maier, Rietschel, Hauser and Marusic17, Reference Uher, Maier, Hauser, Marusic, Schmael and Mors19 Regarding missing data, we found no differences between the switching groups regarding early drop-out before week 12 after switching (n = 40 dropped out before week 12, P = 0.481 for group difference). Furthermore, we had 26 observations with missing single visits on the MADRS and there was a significant difference showing more missing values among those who switched to nortriptyline (n = 16) compared with those who switched to escitalopram (n = 10) (P = 0.007).
First, we performed analyses among all 108 individuals to explore the overall effect of switching. Second, we investigated switchers to escitalopram and switchers to nortriptyline separately on the overall treatment effect and on the treatment effect at every visit during the study period. Third, we performed analyses using the HDRS and the BDI as the dependent variables including analyses on the symptom dimension scores (i.e. the observed mood, cognitive and neurovegetative symptom dimensions). Finally, we explored the effect of switching on response and remission. For this, we performed t-tests among all 108 individuals and logistic regression analyses among the 68 individuals who completed 12 weeks of follow-up after switching.
We performed all the above-mentioned analyses in an unadjusted model and in a model adjusting for age, gender, severity of depression at switching and centre. For comparison of demographic and baseline clinical characteristics we performed t-tests and report means and standard deviations (s.d.). We used Stata version 14 for all analyses.
Sensitivity analyses
First, we restricted the analyses to individuals with at least 6 weeks of follow-up after switching to the second antidepressant (n = 84). Second, to minimise selection bias, we performed all analyses among individuals who were randomised to treatment (i.e. excluding those 22 patients who had not been randomised). Third, we performed all analyses among individuals with non-response (n = 94) to the first medication. This analysis included those individuals who experienced non-response (n = 34) and those who experienced both non-response and side-effects (n = 60). Fourth, to explore the timing of switching, we investigated whether a specific week of switching was associated with a better response. Fifth, since the two switching groups differed regarding age at onset, we performed analyses where we additionally adjusted for age at onset. Sixth, we performed analyses among those individuals who switched because of side-effects only and among those who switched because of non-response only.
Results
Participant characteristics
Characteristics of participants at study entry can be found in Table 1. A total of 72 patients switched from nortriptyline to escitalopram after a mean of 6.2 weeks and 36 from escitalopram to nortriptyline after a mean of 7.0 weeks (range 1–12 weeks for both groups; no difference in switching week, P = 0.197) (Table 2). The reasons for switching were side-effects (n = 12), non-response (n = 34) or both (n = 60), whereas for two individuals there was no information regarding reason for switching (Table 2).
Table 1 Characteristics of the study population at study entry (i.e. at the initiation of the first antidepressant trial)
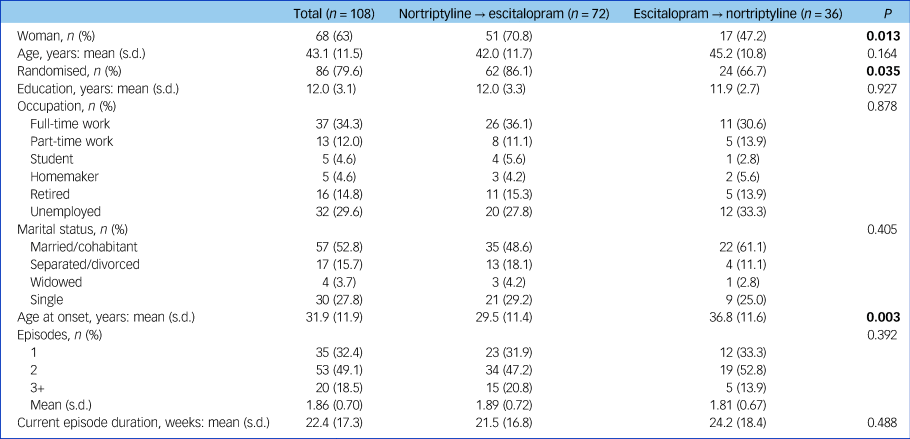
Results in bold are significant.
Table 2 Clinical characteristics of individuals who switched from nortriptyline to escitalopram (n = 72) or escitalopram to nortriptyline (n = 36) after non-response or side-effects to the first medication
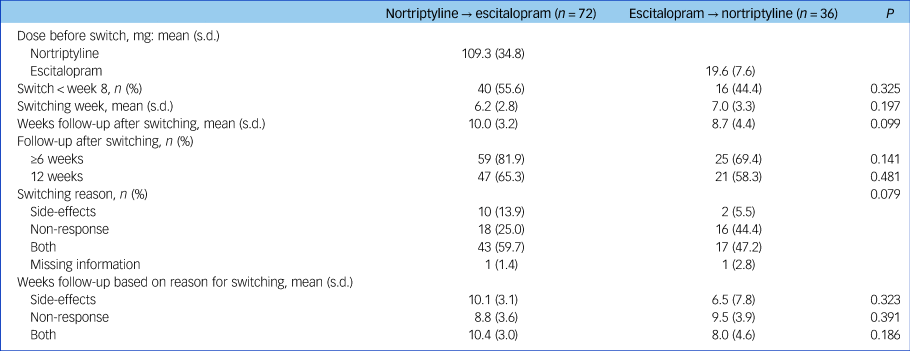
Commonly reported adverse reactions to escitalopram included nausea and vomiting (15%) and sexual dysfunction (30%). Common adverse effects of nortriptyline included dry mouth (80%), orthostatic dizziness (32%), drowsiness (27%) and constipation (24%). Individuals who switched from nortriptyline to escitalopram were more often women (71% v. 47%, P = 0.013), had a younger age at onset of depressive symptoms (29.5 v. 36.8 years, P = 0.003) and were more often randomised to treatment (86.1% v. 66.7%, P = 0.035) (Table 1).
The mean MADRS score at switching was 21.5 (s.d. = 8.3) for those switching from nortriptyline to escitalopram and 24.2 (s.d. = 7.7) for those switching from escitalopram to nortriptyline (Table 3). We found no significant differences in MADRS, HDRS, BDI, observed mood or cognitive symptoms at the time of switching, but those who switched from escitalopram to nortriptyline had higher neurovegetative symptoms (0.6 v. 0.1; P = 0.0009) (Table 3).
Table 3 Treatment effects among individuals switching from nortriptyline to escitalopram (n = 72) or escitalopram to nortriptyline (n = 36) after non-response or side-effects to the first medicationa
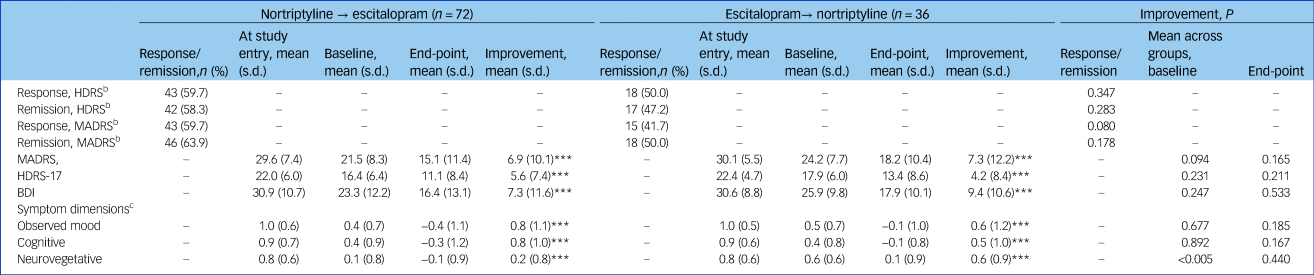
HDRS, Hamilton Rating Scale for Depression, 17-item; MADRS, Montgomery–Åsberg Depression Rating Scale; BDI, Beck Depression Inventory.
a. Study entry is before start of the first drug; baseline is after treatment with the first drug and before switch to the second drug.
b. Response: MADRS or HDRS reduced ≥50%; remission: MADRS ≤11, HDRS ≤7.
c. The score for the symptom dimensions was based on the item-response theory approach including the score of the several items from the three applied rating scales, i.e. the MADRS, HDRS and the BDI.
*** P<0.0001.
Treatment effect after switching
Of the 108 individuals, a total of 84 (77.8%), 81 (75.0%) and 68 (63.0%) completed 6, 8 and 12 weeks of follow-up after switching medication, respectively. In addition, 40 (37.0%) individuals completed the additional follow-up visit 26 weeks after switching. The mean follow-up after switching was 9.5 weeks (s.d. = 3.7). We found no difference regarding length of follow-up after switching or reason for switching between the two switching groups (Table 2). Similar proportions of individuals who switched because of non-response, side-effects or both completed 6 weeks (P = 0.458) or 12 weeks (P = 0.559) of the second course of treatment, respectively.
No significant difference was found at the end point between the two switching groups on the three rating scales or the three symptom dimensions (Table 3). The development of symptom scores on the MADRS, HDRS, BDI and the three symptom dimensions before and after switching is illustrated in Fig. 1. Overall, switching resulted in a significant decrease on the MADRS among all 108 patients as indicated by an adjusted β of −0.35 (95% CI −0.41 to −0.28, P<0.001). Both groups improved similarly on the MADRS (escitalopram to nortriptyline: n = 36, β = −0.38, 95% CI −0.51 to −0.25, P<0.001; nortriptyline to escitalopram: n = 72, β = −0.34, 95% CI −0.41 to −0.26, P<0.001). We found significant improvements on the HDRS and BDI for both switching groups (Table 3 and Fig. 1, all P<0.001). Furthermore, both switching groups significantly improved on the three symptom dimensions, i.e. on observed mood, cognitive and neurovegetative symptoms (Fig. 2, all P<0.001). All analyses were adjusted for age, gender, severity of depression at switching, and centre.
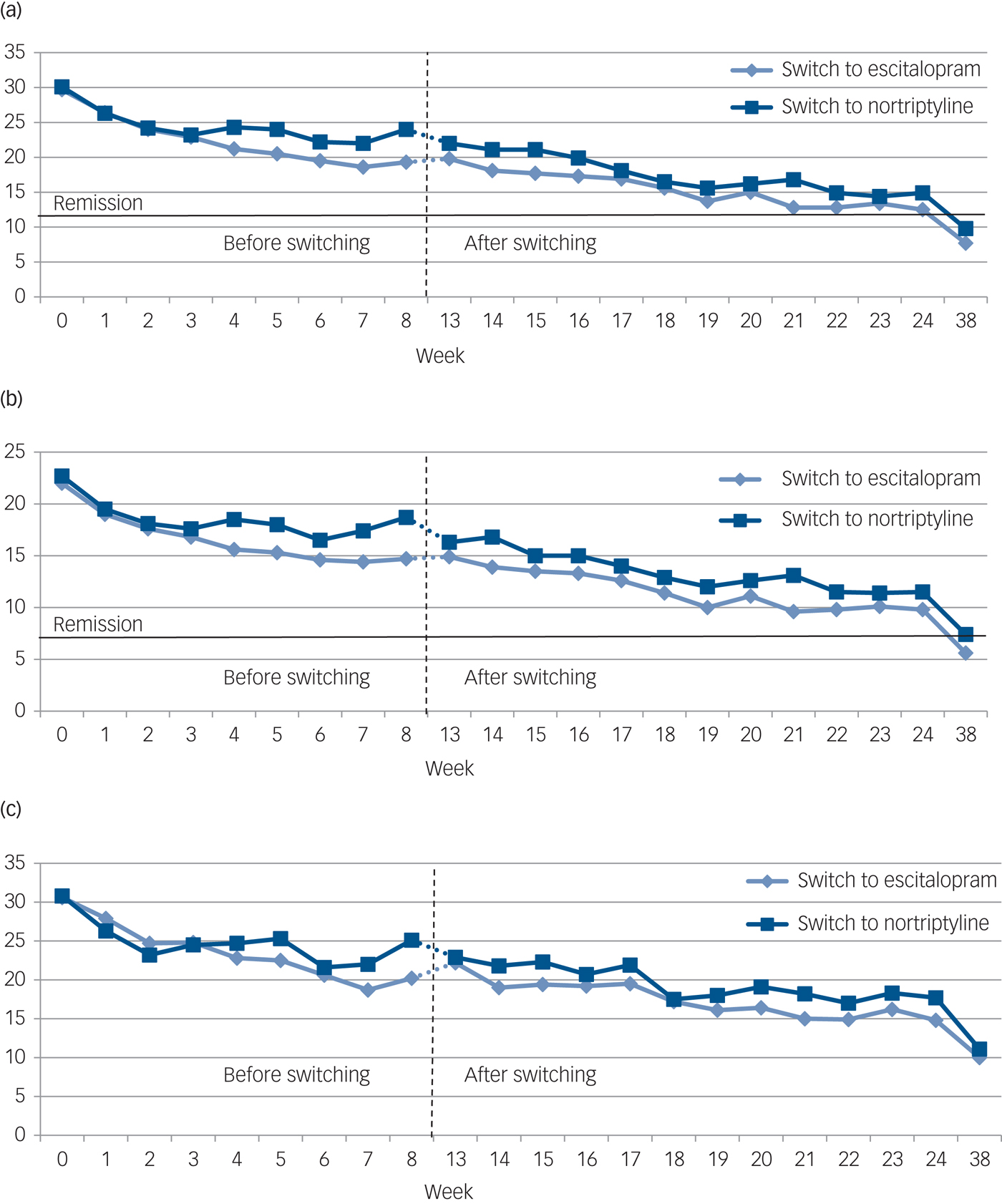
Fig. 1 The development of mean scores on three standard rating scales (a–c) among patients with major depressive disorder (MDD) before (week 0–8) and after (week 13–38) switching from nortriptyline to escitalopram (n = 72) or from escitalopram to nortriptyline (n = 36).
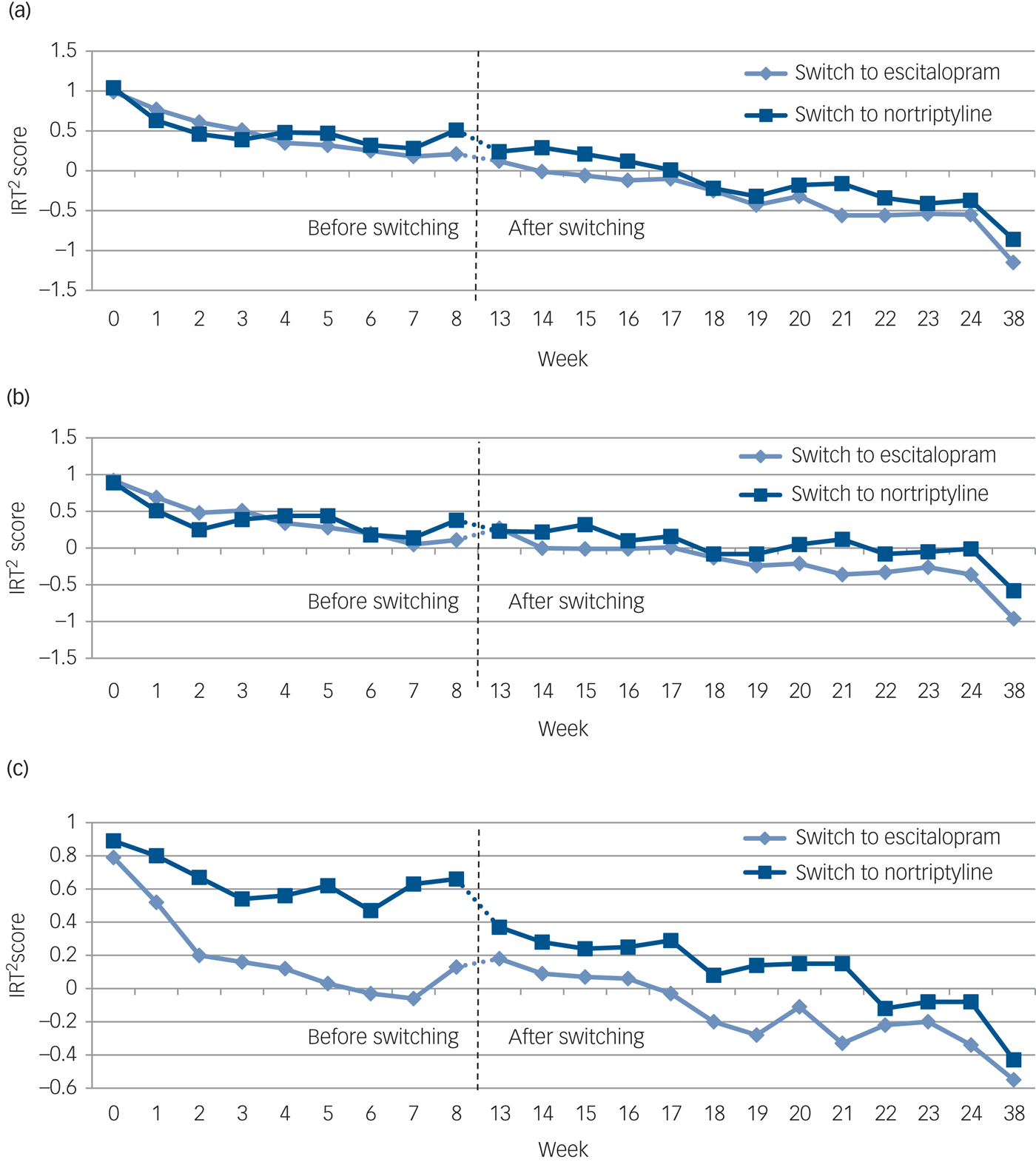
Fig. 2 The development of mean scores on three symptom dimension scores (a–c) among patients with major depressive disorder (MDD) before (week 0–8) and after (week 13–38) switching from nortriptyline to escitalopram (n = 72) or from escitalopram to nortriptyline (n = 36).
Response and remission
During the entire follow-up period after switching, a total of 58 (53.7%) individuals achieved responses on the MADRS and 61 (56.5%) on the HDRS, respectively, whereas 64 (59.3%) achieved remission on the MADRS and 59 (54.6%) on the HDRS, respectively. We found no significant differences between the two switching groups regarding response and remission based on t-tests (Table 3).
When analysing the 68 individuals who completed 12 weeks of follow-up after switching (47 switched to escitalopram and 21 switched to nortriptyline), a total of 28 (59.6%) switchers to escitalopram experienced response on the MADRS and 22 (46.8%) experienced remission. Among individuals who switched to nortriptyline, 7 (33.3%) experienced response and 8 (38.1%) remission. Fully adjusted logistic regression showed no difference between the two switching groups regarding response (P = 0.125) or remission (P = 0.440) after 12 weeks, respectively.
Sensitivity analyses
When only analysing individuals with at least 6 weeks of follow-up after switching, we found significant treatment effects on the MADRS scale among switchers from nortriptyline to escitalopram (n = 59, β = −0.34, 95% CI −0.41 to −0.26, P<0.001) and switchers from escitalopram to nortriptyline (n = 25, β = −0.35, 95%CI −0.49 to −0.22, P<0.001).
Second, when restricting to individuals who were randomised to treatment, we found significant treatment effects on the MADRS among switchers from nortriptyline to escitalopram (n = 62, β = −0.34, 95% CI −0.42 to −0.26, P<0.001) and switchers from escitalopram to nortriptyline (n = 24, β = −0.41, 95% CI −0.58 to −0.24, P<0.001).
Third, we found effects for switching among those 94 individuals who switched due to non-response, which was present among individuals who switched from nortriptyline to escitalopram (n = 61, β = −0.39, 95% CI −0.47 to −0.31, P<0.001) and individuals who switched from escitalopram to nortriptyline (n = 33, β = −0.37, 95% CI −0.49 to −0.25, P<0.001).
Fourth, we performed analyses based on the week of switching (Supplementary Table 1 available at https://doi.org/10.1192/bjp.2018.302). We found no indication that a specific period, for example early or late switching, showed a better response pattern. Switching in all weeks was associated with better response except for the five individuals who switched in week 4.
Fifth, analyses additionally adjusting for age at onset supported the primary results. Switchers to escitalopram improved by β = −0.38 (95% CI −0.51 to −0.25, P<0.001), whereas switchers to nortriptyline improved by β = −0.34 (95% CI −0.41 to −0.26, P<0.001).
Sixth, analyses among those individuals who switched because of side-effects only (n= 12) showed improved treatment effects in both groups (nortriptyline to escitalopram, n = 2, β = −1.03, 95% CI −1.87 to −0.19, P = 0.016; escitalopram to nortriptyline, n = 10, β = −0.34, 95% CI −0.50 to −0.17, P<0.001), and 9 (75%) out of the 12 achieved remission. The 34 individuals who switched because of non-response only showed improved treatment effects in both groups (nortriptyline to escitalopram, n = 18, β = −25, 95% CI −0.35 to −0.15, P<0.001; escitalopram to nortriptyline, n = 16, β = −0.38, 95% CI −0.47 to −0.29, P<0.001) and 17 (50%) out of the 34 achieved remission.
Discussion
Among 108 individuals with MDD who experienced non-response or side-effects to a first treatment course with escitalopram or nortriptyline, switching to the other drug resulted in significant improvement in depression scores and more than 50% of patients achieving response or remission. We found significant improved treatment effects on several rating scales and different symptom dimensions. Particularly, our finding that switching from a TCA to an SSRI improved treatment outcomes is contra intuitive to clinical consensus but in line with two previous trials.Reference Thase, Rush, Howland, Kornstein, Kocsis and Gelenberg7, Reference Shelton, Williamson, Corya, Sanger, Van Campen and Case14 However, we had no control group, for example individuals randomised to continuation treatment. Nevertheless, our results suggest that switching from nortriptyline to escitalopram or vice versa may be a viable approach to improve treatment outcomes after non-response or side-effects to a first antidepressant treatment course. Our finding that individuals who switched to escitalopram were younger may be explained by the fact that the first treatment assignment was based on a clinical evaluation, and the rate of contraindications for TCAs increases with age.
Evidence for switching between antidepressants
Clinicians frequently encounter the situation where continuation with an antidepressant is not possible because of non-response or side-effects. It is important to choose an evidence-based switching strategy with the potential of improving treatment effects. Switching to an antidepressant targeting the same receptors, for example from one SSRI to another SSRI, may seem less likely to result in fewer side-effects and better treatment response. The GENDEP study was designed to investigate two drugs with different mechanisms of action (i.e. escitalopram targeting mainly serotonergic pathways versus nortriptyline mainly targeting noradrenergic pathways). Hence, the important finding of the present study is that switching to an antidepressant with a different receptor profile can improve the antidepressant treatment effects. This is supported by a meta-analysis of clinical trials.Reference Papakostas, Fava and Thase25 Although only including four trials, the results indicated that after SSRI non-response, switching to a different class of antidepressants showed better response rates compared with switching to a different SSRI. In addition, two prior trials found beneficial effects after switching between a TCA and an SSRI.Reference Thase, Rush, Howland, Kornstein, Kocsis and Gelenberg7, Reference Shelton, Williamson, Corya, Sanger, Van Campen and Case14 However, the only randomised trial found no differences between switching to the SSRI fluoxetine (n = 142) and nortriptyline continuation (n = 68).Reference Shelton, Williamson, Corya, Sanger, Van Campen and Case14
Finally, the timing of switching represents an important clinical aspect. A small study found that among early non-responders to sertraline (≤20% reduction in MADRS within the first 2 weeks), randomisation to paroxetine switching (n = 20) after 2 weeks resulted in better response and remission compared with sertraline continuation (n = 21).Reference Nakajima, Uchida, Suzuki, Watanabe, Hirano and Yagihashi16 On the other hand, a larger randomised trial found that after non-response to 2 weeks on escitalopram, continuation and up-titration (n = 229) resulted in better response compared with duloxetine switching (n = 245).Reference Bose, Tsai and Li5 In the present study, we could not identify any timing of switching that indicated a better response; however, we were not able to investigate specific hypotheses as the before mentioned studies. Hence, present evidence supports following guidelines before considering switching. Guidelines recommend antidepressant continuation for at least 4–6 weeks to be able to evaluate clinical response.Reference Bayes and Parker1 Indeed, continuation and up-titration can lead to response as late as after 8 weeks.Reference Uher, Mors, Rietschel, Rajewska-Rager, Petrovic and Zobel26
Future trials may investigate mechanisms potentially explaining better response after switching to specific antidepressant drugs and whether an earlier time of switching may result in better treatment response. Particularly the finding that switching from a TCA to an SSRI improved treatment outcome was unexpected and may be explained by many factors, for example hepatic metabolism or effects on the immune system.
Strengths and limitations
GENDEP is a large, real-world partly randomised trial, representing patients seen in everyday practice, that is, with comorbid disorders, prior (failed) antidepressant treatment attempts and suicidal ideation. The close monitoring with weekly visits during 12 weeks of treatment and the large study population (only slightly smaller compared with previous trials that investigated switching between TCAs and SSRIsReference Thase, Rush, Howland, Kornstein, Kocsis and Gelenberg7, Reference Shelton, Williamson, Corya, Sanger, Van Campen and Case14) support our findings. Furthermore, the investigated drugs, escitalopram and nortriptyline, target different receptors, which is an important clinical aspect to consider when switching medication.
Regarding limitations, we did not compare the effect of switching with patients randomised to continuation treatment or other approaches such as augmentation. Hence, we are not able to distinguish specific drug response from spontaneous remission and we cannot evaluate whether other treatment approaches, such as augmentation, may have resulted in better treatment response. Furthermore, patients switched at very different time points based on clinical evaluation. This limited the possibility to investigate whether switching at specific time points may result in better treatment effects. In addition, adherence was high (98.4%) but primarily measured via self-reported pill counts.
Implications
In the case of a patient with side-effects or non-response despite sufficient dose and treatment duration, switching to an antidepressant with a different receptor profile may be one strategy to improve antidepressant treatment response. Our findings indicate that even switching from a TCA to an SSRI may improve the antidepressant treatment effects. Future large trials need to include patients with MDD and non-response after antidepressant treatment of sufficient length and dose and compare: (a) different randomised intervention groups (for example continuation/up-titration, augmentation or switching), (b) include several antidepressant drugs with different receptor profiles, and (c) explore the importance of specific timing of the new intervention (for example early versus late switching).
Funding
The Genome-Based Therapeutic Drugs for Depression (GENDEP) study was funded by a European Commission Framework six grant (EC Contract Ref LSHB-CT-2003-503428). Lundbeck provided both nortriptyline and escitalopram free of charge for the GENDEP study. The funders had no role in the design and conduct of the study, in data collection, analysis, or interpretation or in writing the report.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.302.








eLetters
No eLetters have been published for this article.