On the basis of conflicting evidence about the role of serotonin (5-hydroxytryptamine, 5-HT) in anxiety (Reference Charney, Woods and GoodmanCharney et al, 1987; Reference Deakin and GraeffDeakin & Graeff, 1991), we have suggested that 5-HT reduces unconditioned anxiety but increases conditioned anxiety and that this may relate to its effects in panic and anticipatory/generalised anxiety, respectively (Reference Deakin and GraeffDeakin & Graeff, 1991). Normal volunteer studies have been consistent with this ‘dual’ role for 5-HT in human anxiety (Reference Deakin, Guimaraes, Graeff, Palomo and ArcherDeakin et al, 1994). A previous small study in patients with panic disorder had reported that reducing 5-HT function using acute tryptophan depletion had no effect on resting anxiety (Reference Goddard, Sholomskas and WaltonGoddard et al, 1994). In this study we investigated the effect of tryptophan depletion in a larger group of patients with panic disorder and in controls receiving a panic challenge. Our hypothesis was that tryptophan depletion would decrease resting anxiety while exacerbating anxiety responses to 5% CO2 inhalation.
MATERIALS AND METHODS
Subjects
Twenty patients with DSM—III—R (American Psychiatric Association, 1987) panic disorder, diagnosed by the Structured Clinical Interview for DSM—III—R (SCID; Reference Spitzer, Williams and GibbonSpitzer et al, 1990a ), were recruited by referral or newspaper advertisement (Reference Dratcu and BondDratcu & Bond, 1993). Patients were required to report at least weekly panic attacks, confirmed by diary-keeping, and to be without comorbid psychiatric diagnoses (except secondary major depression) or alcohol/drug misuse within the last two years. Nineteen controls with no history of psychiatric disorder were recruited by advertisement and screened using the non-patient version of the SCID (Reference Spitzer, Williams and GibbonSpitzer et al, 1990b ). Exclusion criteria for all subjects included any significant medical disorder, taking regular medication or other drugs (within two weeks for patients and two months for volunteers) and drinking more than 20 units of alcohol a week. Benzodiazepine use was asked about but not directly screened for. All subjects had normal physical examination, electrocardiogram and biochemistry, including thyroid function. Baseline measures before the first test consisted of the Spielberger Trait Anxiety Inventory (Reference Spielberger, Gorsuch and LusheseSpielberger et al, 1979), the Hamilton Rating Scale for Anxiety (Reference HamiltonHamilton, 1969), the Hamilton Rating Scale for Depression (Reference HamiltonHamilton, 1960) and the Beck Depression Inventory (Reference Beck, Ward and MendelsonBeck et al, 1961). Subject details are given in Table 1. The study was approved by the local Research Ethics Committee and all subjects gave written informed consent.
Table 1 Subject characteristics

| Patients | Controls | |
|---|---|---|
| Number of subjects (Male:Female) | 10:10 | 11:8 |
| Age (years) | 38.4 (9.9)** | 29.1 (8.4) |
| Agoraphobia | 17 | - |
| Comorbid DSM-III-R major depression | 7 | - |
| Family history of panic disorder | 8 | 0 |
| Number of panic attacks per weeks | 2 (1-3.75) | - |
| Spielberger Trait Anxiety Inventory score | 52.0 (13.9)*** | 30.8 (7.8) |
| Hamilton Rating Scale for Anxiety score | 19.1 (7.9)*** | 1.1 (1.6) |
| Hamilton Rating Scale for Depression score (17-item) | 14.3 (7.6)*** | 1.1 (1.6) |
| Beck Depression Inventory score | 21.7 (11.7)*** | 3.2 (3.9) |
Experimental procedures
Subjects were tested on two occasions separated by at least four days; for females this occurred during the first 14 days of the menstrual cycle or in the contraceptive pill-free week. On arrival, a forearm vein was cannulated and kept patent using heparinised saline. After 45 minutes the subjects received either a tryptophan-free 100 g amino acid drink or a control drink containing 2.3 g of tryptophan at 09.00 (time zero). Each test session lasted a further 330 minutes. A 5% CO2 panic challenge (see below) was administered 270 minutes after the amino acid drink on both test days. Psychological ratings and blood samples were taken before the amino acid drink and then hourly until 240 minutes, followed by half-hourly until 330 minutes. The subjects sat in a quiet testing room and were not allowed to sleep, eat or smoke but could drink water and read emotionally neutral material. A meal was provided at the end of the experiment.
Acute tryptophan depletion
Oral administration of a tryptophan-free amino acid load leads to a profound reduction in plasma tryptophan concentration, thus reducing its availability for brain 5-HT synthesis (Reference Reilly, McTavish and YoungReilly et al, 1997). In humans there is preliminary evidence for reduced 5-HT synthesis (Reference Nishizawa, Benkelfat and YoungNishizawa et al, 1997) and reduced cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid (Reference Carpenter, Anderson and PeltonCarpenter et al, 1998) after tryptophan depletion. Following a standard protocol (Reference Young, Smith and PihlYoung et al, 1985; Reference Benkelfat, Ellenbogen and DeanBenkelfat et al, 1994), subjects ate a low-protein diet (20 g) for 24 hours, fasted overnight and received one of two amino acid drinks, double-blind, in a balanced order in the morning. The drinks differed only in whether they lacked or contained 2.3 g of tryptophan (tryptophan-free drink and control drink, respectively). The other amino acids were: L-alanine, 5.5 g; L-arginine, 4.9 g; L-cysteine, 2.7 g; glycine, 3.2 g; L-histidine, 3.2 g; L-isoleucine, 8 g; L-leucine, 13.5 g; L-lysine monohydrochloride, 11 g; L-methionine, 3 g; L-phenylalanine, 5.7 g; L-proline, 12.2 g; L-serine, 6.9 g; L-threonine, 6.9 g; L-tyrosine, 6.9 g; and L-valine, 8.9 g. Methionine, cysteine and arginine were encapsulated because of their unpleasant taste. Females received 80% of the above amounts because of their lower body weight. The powdered amino acids were mixed with 150 ml of water, 100 ml of chocolate syrup and two desert spoons of sugar immediately before administration. Subjects drank the mixture quickly through drinking straws and then chewed sugar-free gum to minimise the unpleasant taste.
Panic challenge with 5% CO2
The CO2 challenge is a widely used panic challenge, with evidence for increased sensitivity to its anxiogenic action in patients with panic disorder (Reference Sanderson and WetzlerSanderson & Wetzler, 1990) and those at genetic risk for panic disorder (Reference Perna, Cocchi and BertaniPerna et al, 1995). This may reflect derangement of a brainstem suffocation-alarm system (Reference KleinKlein, 1993). Using the method of Roth et al (Reference Roth, Margraf and Ehlers1992), compressed air (21% oxygen and 79% nitrogen from the British Oxygen Company) was administered for 10 minutes through a positive pressure mask before switching to continuous 5% CO2 (5% CO2, 21% oxygen and 74% nitrogen; British Oxygen Company) for 20 minutes. Single breaths of 5% CO2 were given at two and seven minutes to maximise blindness. The gas cylinders were in an adjacent room and connected to the subject through a Y-valve and CO2-impervious tubing, with a reservoir bag connected to the tubing just before the mask. End-tidal CO2 pressure was monitored. To minimise psychological effects on panic rates (Reference Sanderson, Rapee and BarlowSanderson et al, 1989), the experimental conditions were standardised by familiarising the subjects with the procedure on the first visit, providing oral and written information on the effects of breathing 5% CO2 on both test days and ensuring that the experimenter was visible during the challenge. A prior decision was made to exclude subjects from analysis of the effects of panic challenge if they panicked before CO2 was administered.
Psychological measurements
Rating scales consisted of the Visual Analogue Scales (VAS: sad, anxious, panicky, lightheaded, happy, drowsy, nauseated, irritable) on a 100 mm line (0 mm=not at all; 100 mm-extremely), the Spielberger State Anxiety Inventory (STAI—S; Reference Spielberger, Gorsuch and LusheseSpielberger et al, 1979) and Profile of Mood States (POMS; Reference McNair, Lorr and DroppelmanMcNair et al, 1971), given in that order. Before and after the 5% CO2 challenge, subjects completed a modified version of the Acute Panic Inventory (API; Reference Dillon, Gorman and LiebowitzDillon et al, 1987), consisting of 24 items formed from the 13 DSM—III—R panic symptoms and apprehension rated on a five-point severity scale (0=not present; 4=extremely severe). After CO2 challenge, subjects were asked to rate the peak severity that occurred during the challenge and also the similarity to their usual panic attacks. Because of the difficulties in assessing laboratory panic attacks (Reference Sanderson and WetzlerSanderson & Wetzler, 1990), we applied two definitions of panic attack. First, the patients' own report of a panic attack rated at least ‘quite similar’ to their usual attacks. Second, an increase over baseline of four DSM—III—R panic symptoms rated at least moderately severe on the API, together with an increased Anxiety VAS or Panic VAS of 15 mm or greater.
Biochemical measurements
Blood samples were taken into lithium heparin tubes and centrifuged within an hour for 10 minutes at 2400 rpm and 4°C. The separated plasma was frozen and stored at —20°C before analysis. Plasma was assayed for total and free tryptophan (at 0, 240 and 300 minutes) and for cortisol (at 270, 300 and 330 minutes). Plasma tryptophan concentration was measured by a semi-automated high-performance liquid chromatography with fluorescence end-point detection. Intra- and interassay coefficients of variation were 8% and 13%, respectively, and the limit of detection was 1.3 pg/ml. Cortisol was analysed by standard radioimmunoassay. Intra- and inter-assay coefficients of variation were 4.3% and 5.6%, and the limit of detection was 0.1 μg/100 ml.
Analysis
Two time periods were analysed using SPSS for Windows Release 6 (SPSS Inc., Chicago, IL): the pre-challenge period (0-270 minutes), reflected a resting period; and the CO2 challenge period (270-330 minutes). The principal analysis for continuous data was by repeated measured analysis of variance (ANOVA) using the Huynh—Feldt correction with a between-subjects factor (group) and two within-subject factors of occasion (tryptophan-free or control drink) and time. Controls and patients were analysed separately by ANOVA following significant interactions by group or by occasion. Post hoc t-tests were used to aid interpretation of data. Categorical data were analysed using McNemar's test (Reference Armitage and BerryArmitage & Berry, 1987) and correlations using Spearman's correlation coefficient. Data are presented as mean (s.d.) values.
RESULTS
The control and tryptophan-free drinks were indistinguishable by the subjects and experimenter (H.E.J.M.) and generally well tolerated, although drowsiness, nausea and abdominal fullness did occur up to 120 minutes. No order effects were detected in the results.
Tryptophan measures
There were no significant differences in baseline measures (Table 2). Significant occasion × time interactions for total tryptophan (F(2,74)=174.23, P<0.001) and free tryptophan (F(2,74)=4.123, P<0.001) occurred, with plasma tryptophan decreasing on the tryptophan depletion occasion (total tryptophan: patients, 83% and volunteers, 85%; free tryptophan: patients, 62% and volunteers, 74%) and increasing on the control occasion (total tryptophan: patients, 283% and volunteers, 256%; free tryptophan: patients, 164% and volunteers, 185%).
Table 2 Effect of control and tryptophan-free drinks on total and free plasma tryptophan

| Control drink | Tryptophan-free drink | |||||
|---|---|---|---|---|---|---|
| 0 | 240 min | 300 min | 0 | 240 min | 300 min | |
| Total tryptophan | ||||||
| Controls | 9.8 (1.6) | 25.0 (7.6)*** | 21.7 (7.8)*** | 10.5 (2.5) | 2.0 (1.2)*** | 1.6 (1.2)*** |
| Patients | 11.3 (3.4) | 31.8 (14.2)*** | 24.4 (11.6)*** | 12.4 (4.2) | 2.5 (1.8)*** | 2.2 (1.5)*** |
| Free tryptophan | ||||||
| Controls | 0.74 (0.45) | 1.37 (0.66)** | 1.34 (0.57)*** | 0.74 (0.65) | 0.22 (0.36)*** | 0.19 (0.21)*** |
| Patients | 0.77 (0.46) | 1.26 (0.86)** | 1.22 (0.70)*** | 0.69 (0.44) | 0.34 (0.4)** | 0.26 (0.18)*** |
Pre-challenge period (time 0-270 minutes)
Caseline behavioural ratings
Patients had significantly higher anxiety and depression ratings than controls (group effect on ANOVA P<0.01 for all measures; Fig. 1).
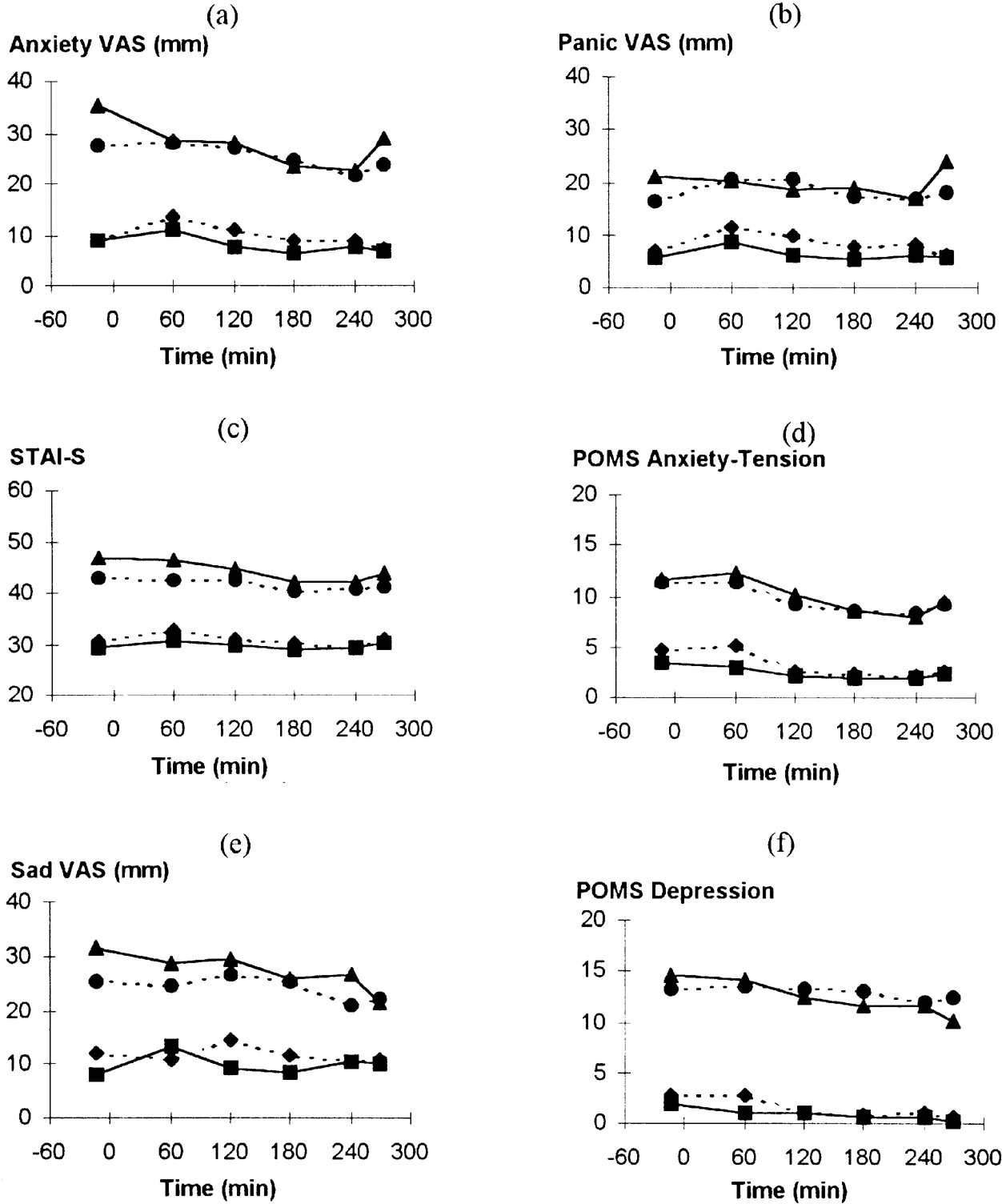
Fig. 1 Effect of tryptophan depletion on psychological ratings during the resting phase: (▴) patients with panic disorder on tryptophan depletion occasion; (•) patients with panic disorder on control occasion; (▪) normal volunteers on tryptophan depletion occasion; (♦) normal volunteers on control occasion. (a) Anxiety VAS, time: F(5,185)=4.27; P=0.003. (b) Panic VAS, time: F(5,185)=1.29; P=0.28. (c) STAI—S, time: F(5,185)=5.73; P<0.001. (d) POMS Anxiety-Tension, time: F(5,185)=8.70; P<0.001. (f) POMS Depression, time: F(1,37)=4.60, P=0.007.
Effect of tryptophan depletion on behavioural ratings
Anxiety scores, apart from Panic VAS, fell during the pre-challenge period (Figs 1a-d). There were no significant interactions, indicating no effect of tryptophan depletion and no difference between patients and controls. Anxiety increased between 240 and 270 minutes (Fig. 1), suggesting acute anticipatory anxiety before the CO2 challenge. Analysis of variance of these two time points showed increases in STAI—S (time: F(1,37)=5.43; P=0.025) and POMS Anxiety-Tension (time: F(1,37)=4.00; P=0.053). Patients, but not controls, had increased Anxiety VAS ratings (group × time: F(1,37)=5.89; P=0.02) with a similar but non-significant pattern with Panic VAS (Fig. 1). For Panic VAS alone there was a greater increase on the tryptophan depletion occasion compared with the control occasion (occasion × time: F(1,37)=4.37; P=0.043).
Depression-related ratings fell during this period but this was only significant for POMS Depression. There were no significant interactions between group, time or occasion. No significant effect of tryptophan depletion was found in separate analyses of patients with panic disorder according to the presence (n=7) or absence (n=13) of current major depression or a past history of major depression in the absence of current depression (n=8) (results not shown).
Other behavioural ratings generally showed a maximal effect at 60 minutes due to the drink, returning towards baseline by the end of the resting period. There were significant effects of time for Nausea VAS, Lightheaded VAS, Drowsiness VAS and POMS Vigour (all P<0.001) but not POMS Fatigue or POMS Confusion. There were no significant differences over time between patients and volunteers or occasions.
Carbon dioxide challenge period (270-330 minutes)
One patient discontinued the gas inhalation before receiving CO2 (control occasion) and one panicked at the time of putting on the mask (tryptophan depletion occasion), leading to exclusion. One control subject was excluded from analysis of API scores only because of a very high pre-inhalation API score inconsistent with all other anxiety ratings.
Cortisol measures
Plasma samples from four controls were missing at 330 minutes and results were therefore available from 32 subjects. There were no significant effects of CO2 inhalation and no difference between patients and controls or occasion. Patients who had a panic attack (by either definition) did not differ from those not panicking (results not shown).
Psychological measures
As noted for other anxiety ratings (see above), patients had significantly higher API scores at challenge baseline (270 minutes) than controls (see Fig. 3; group: F(1,34)=18.62; P<0.001). Carbon dioxide challenge caused increases in all ratings of anxiety (Fig. 2). Significant group × time interactions occurred for all anxiety ratings due to patients' ratings increasing more than controls (Fig. 2). Panic VAS showed a significant group × occasion × time interaction (F(2,70)=5.69; P=0.005) due to a greater increase following tryptophan depletion in patients but not controls. When controls and patients were considered separately, the anxiogenic effect of CO2 was small in controls and only Anxiety VAS increased significantly (F(2,36)=4.36; P=0.024); indeed, STAI—S decreased throughout the period (F=(2,36)9.27; P=0.001). Tryptophan depletion had no effect on anxiety ratings in controls. In contrast, patients showed consistent increases in anxiety ratings following CO2 (Fig. 2), with greater responses seen on the tryptophan depletion occasion for Panic VAS (F(2,34)=6.14; P=0.005) and a trend for Anxiety VAS (F(2,34)=3.07; P=0.060). Increases in POMS Anxiety-Tension and STAI—S were non-significantly greater on the depletion occasion.
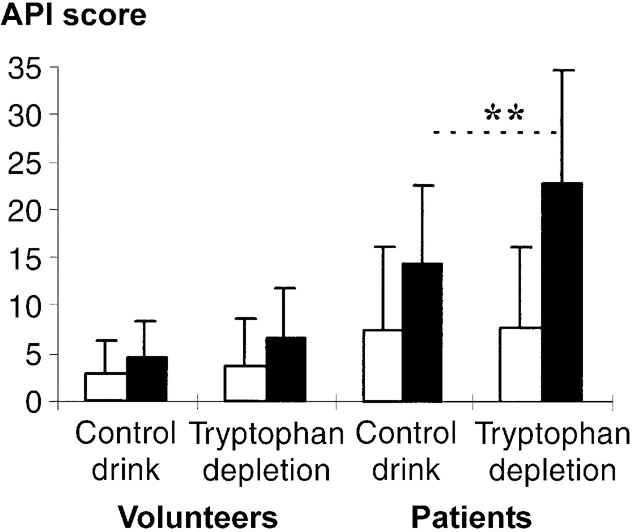
Fig. 3 Effect of tryptophan depletion on Acute Panic Inventory (API) scores before and after 5% CO2 challenge: (□) pre-CO2 challenge; (▪) post-CO2 challenge. Values are means with standard deviations; ** P<0.001 for occasion × time interaction for patients with panic disorder.
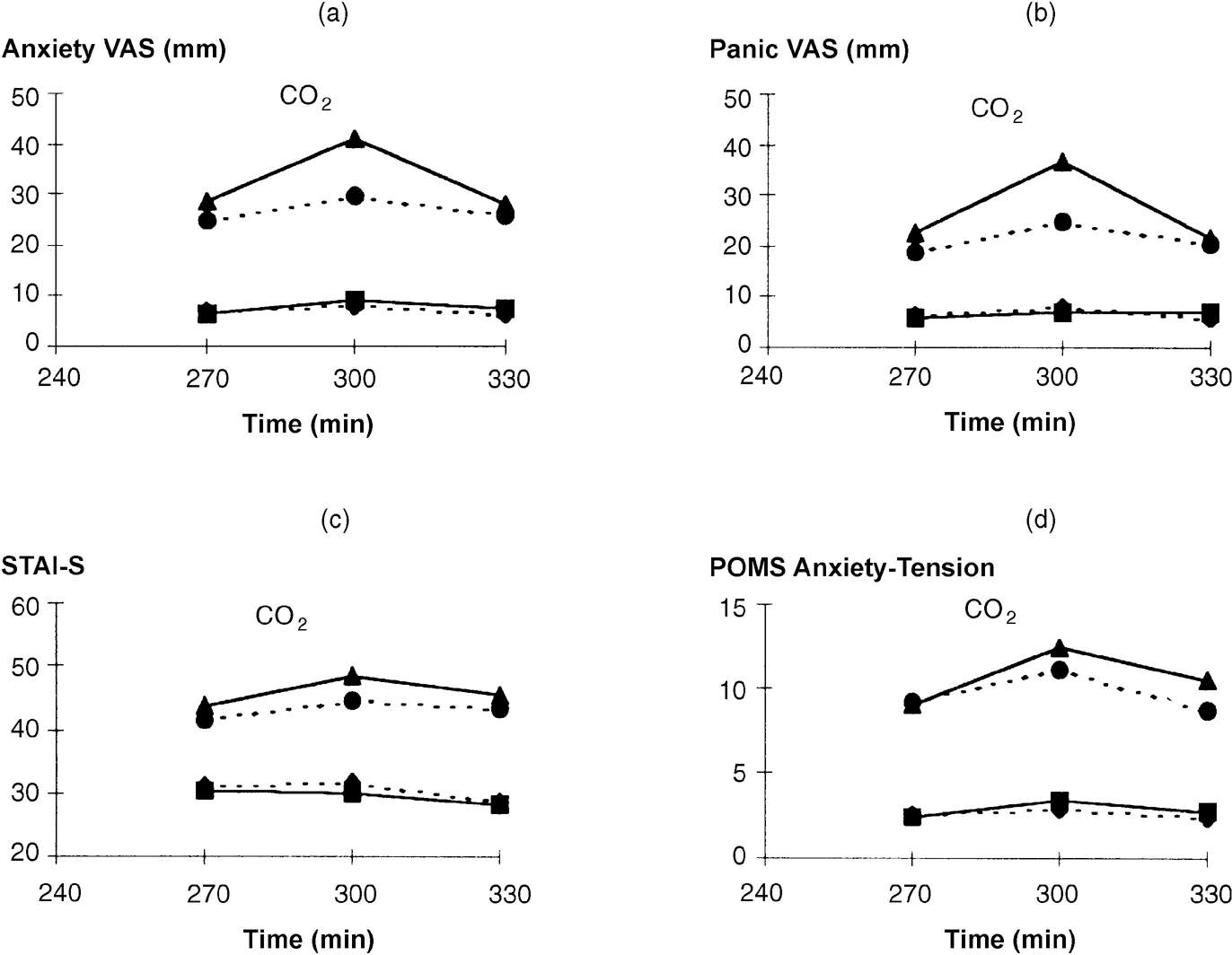
Fig. 2 Effect of tryptophan depletion on psychological ratings during 5% CO2 challenge. For meaning of symbols, see legend to Fig. 1. (a) Anxiety VAS, time: F(1,35)=16.87, P<0.001; group time: F(2,70)=4.35, P=0.017. (b) Panic VAS, time: F(1,35)=11.78, P<0.001; group × time: F(2,70)=7.93, P=0.001. (c) STAI—S, time: F(1,35)=28.89, P<0.001; group × time; F(2,70)=10.09, P<0.001. (d) POMS Anxiety-Tension, time: F(1,35)=25.12, P<0.001; group × time: F(2,70)=5.23, P=0.008.
Carbon dioxide inhalation increased API scores particularly in patients and on the tryptophan depletion occasion (group × occasion × time: F(1,34)=7.90; P=0.008; Fig. 3). In controls analysed separately there was a modest CO2-induced increase in API scores (time: F (1,17)=6.54; P=0.021) but no effect of tryptophan depletion (occasion × time: F (1,17)=0.46; P=0.508). In patients there was a robust effect of CO2 inhalation (time: F(1,17)=46.08; P<0.001) and a marked effect of tryptophan depletion (occasion × time: F(1,34)=23.06; P<0.001).
Panic attacks in patients were more frequent on the tryptophan depletion occasion for both subjective panic attacks (eight v. two, P<0.05) and DSM—III—R panic attacks (nine v. two, P<0.05). Patients commented that the security of the experimental situation had reduced cognitive symptoms and severity compared with their usual panic attack. This is reflected in relative low mean panic attack similarity ratings, which were higher on the tryptophan depletion occasion (1.39 (s.d.=0.85) v. 0.78 (s.d.=0.65), P=0.012).
On the tryptophan depletion occasion, patients with a DSM—III—R panic attack had higher mean anxiety scores immediately before CO2 inhalation (270 minutes) than non-panickers; this was statistically significant for API scores (11.9 (s.d.=10.9) v. 3.8 (s.d.=2), P=0.049), STAI—S (49.2 (s.d.=7.9) v. 38.4 (s.d.=9.2), P=0.017) and POMS Anxiety-Tension (12.1 (s.d.=5.0) v. 6.0 (s.d.=4.1), P=0.012). However, there were no significant differences at 240 minutes on any anxiety measures, suggesting that those who experienced greater acute anticipatory anxiety were more likely to panic when challenged by 5% CO2.
Analysis of the two respiratory items on the API separately showed no significant effect of tryptophan depletion (increases on control v. tryptophan depletion occasion: 2.0 (s.d.=1.7) v. 2.6 (s.d.=2.0), P=0.165 in patients and 0.8 (s.d.=1.5) v. 1.4 (s.d.=1.2), P=0.127 in volunteers).
Correlations
In patients, anxiety responses to 5% CO2 (the difference score between 270 and 300 minutes) correlated with a number of values at baseline (time zero) and 270 minutes on the tryptophan depletion occasion. Anxiety VAS responses to 5% CO2 tended to correlate with: Anxiety VAS values at time zero (rho=0.43, P=0.078) and 270 minutes (rho=0.44, P=0.065); Panic VAS values at time zero (rho=0.57, P=0.050) and 270 minutes (rho=0.51, P=0.031); and STAI—S at time zero (rho=0.56, P=0.016). Panic VAS responses also correlated with STAI—S at time zero (rho=0.49, P=0.039). No significant correlations between baseline ratings and anxiety responses to 5% CO2 challenge were seen on the control occasion or in volunteers.
There were no consistent patterns of association between bichemical measures and anxiety responses to 5% CO2.
DISCUSSION
Our main finding was that acute tryptophan depletion increased anxiety responses induced by inhalation of 5% CO2 in drug-free patients with panic disorder. In contrast, resting levels of anxiety and other mood measures were not affected by tryptophan depletion. We saw no effects in normal volunteers apart from a mildy anxiogenic effect of 5% CO2 inhalation.
Tryptophan depletion and resting anxiety and depression
We only measured resting anxiety up to 270 minutes, which could be too short to detect an effect because maximum plasma tryptophan reduction does not occur until about 300 minutes (Reference Young, Smith and PihlYoung et al, 1985; Reference Delgado, Charney and PriceDelgado et al, 1990; see Table 2). However, our results agree with those of Goddard et al (Reference Goddard, Sholomskas and Walton1994) who also found little effect of tryptophan depletion in a small study with eight drug-free patients with panic disorder assessed up to 420 minutes. They are also consistent with findings in other psychiatric conditions where tryptophan depletion does not alter anxiety (e.g. Reference Delgado, Price and MillerDelgado et al, 1994; Reference Aronson, Black and McDougleAronson et al, 1995; Reference Benkelfat, Seletti and PalmourBenkelfat et al, 1995), unless there is exacerbation of the primary disorder (e.g. Reference Delgado, Charney and PriceDelgado et al, 1990; Reference Menkes, Coates and FawcettMenkes et al, 1994; Reference Weltzin, Fernstrom and McConahaWeltzin et al, 1994). The lack of effect of tryptophan depletion on anxiety in normal volunteers in our study is also consistent with most other studies (e.g. Reference Young, Smith and PihlYoung et al, 1985; Smith et al, 1987; Reference Weltzin, Fernstrom and McConahaWeltzin et al, 1994; Reference Goddard, Charney and GermineGoddard et al, 1995; Reference Koszycki, Zacharko and Le MelledoKoszycki et al, 1996).
Our findings appear to conflict with evidence suggesting that tryptophan depletion should be anxiolytic. Reducing 5-HT function is anxiolytic in a number of paradigms in animals (Reference Coplan, Gorman and KleinCoplan et al, 1992) and ritanserin, a 5-HT2 antagonist, is anxiolytic in a human model of generalised anxiety and in patients with mixed anxiety and depression (Reference Deakin, Graeff, Guimaraes, Marsden and HealDeakin et al, 1992). Conversely, increasing 5-HT functioning using m-chlorophenylpiperazine (Reference Charney, Woods and GoodmanCharney et al, 1987) and fenfluramine (Reference Targum and MarshallTargum & Marshall, 1989) challenge causes anxiety in patients with panic disorder, as can initial treatment with clomipramine and selective serotonin reuptake inhibitors (e.g. Reference Ramos, Gentil and GorensteinRamos et al, 1993). These data are difficult to reconcile but the present study suggests that the increased levels of anxiety generally seen in patients with panic disorder are not simply due to tonically increased 5-HT function.
Our lack of effect of tryptophan depletion on depressive symptoms is consistent with the previous study in patients with panic disorder (Reference Goddard, Sholomskas and WaltonGoddard et al, 1994), whereas in other patient groups there have been variable findings with regard to induction of depression (Delgado et al, Reference Delgado, Charney and Price1990, Reference Delgado, Price and Miller1994; Reference Barr, Goodman and McDougleBarr et al, 1994; Reference Smith, Fairburn and CowenSmith et al, 1997), possibly related to diagnosis and drug status. Similarly variable results in lowering mood have been seen in normal volunteers studies (e.g. Reference Young, Smith and PihlYoung et al, 1985; Reference Oldman, Walsh and SalkovskisOldman et al, 1994; Reference Weltzin, Fernstrom and McConahaWeltzin et al, 1994; Reference Goddard, Charney and GermineGoddard et al, 1995; Reference Koszycki, Zacharko and Le MelledoKoszycki et al, 1996).
Tryptophan depletion and provoked anxiety
We found 5% CO2 inhalation to be panicogenic in patients but only mildly anxiogenic in controls. Like Roth et al (Reference Roth, Margraf and Ehlers1992), we found that patients who panicked had higher anxiety levels immediately before challenge than non-panickers. They argued that this could be explained by anticipatory anxiety increasing above a threshold of tolerance (i.e. situational panic). In contrast, other studies have found no relationship between baseline anxiety and subsequent panic attacks (e.g. Reference Gorman, Fyer and GoetzGorman et al, 1988; Reference Sanderson and WetzlerSanderson & Wetzler, 1990), suggesting a model of spontaneous panic attacks. To what extent prior anxiety may predict panic to CO2 challenge therefore remains unresolved, but our results suggest that it may act as a factor.
Tryptophan depletion increased acute anticipatory and 5% CO2-induced anxiety in patients and it is interesting to relate this to the effect of treatment with 5-HT-enhancing antidepressants, which have been shown to reduce panic patients' hypersensitivity to CO2 challenge (Reference Bertani, Perna and ArancioBertani et al, 1997; Reference Gorman, Browne and PappGorman et al, 1997). Our result supports increased 5-HT neurotransmission playing an important part in the anti-panic effect of these drugs. The lack of effect that we found in volunteers appears to contrast with a recent report of increased anxiety ratings and some panic symptoms following 35% CO2 inhalation after tryptophan depletion (Reference Klaassen, Klumperbeek and DeutzKlaassen et al, 1998). Although methodological differences, including the weak anxiogenic response in our study, may explain this, there have been conflicting results in normal volunteers using other anxiety challenges that do not simply appear related to the severity of the anxiogenic challenge. Tryptophan depletion has been reported to enhance anxiety after yohimbine administration (Reference Goddard, Charney and GermineGoddard et al, 1995) but not after cholecystokinin challenge (Reference Koszycki, Zacharko and Le MelledoKoszycki et al, 1996) or in response to simulated public speaking (Reference Mortimore, Connell and VanMortimore et al, 1997). It therefore remains unclear whether 5-HT modulates stimulated anxiety in normal subjects.
It is of interest that the measures specifically relating to panic (Panic VAS and API) appeared to be more robustly affected than general anxiety ratings in patients. We cannot, however, distinguish between situational panic related to anticipation of a proximal threat (imminent or actual CO2 challenge) and panic produced by a biological effect of CO2. Although the results are generally consistent with the hypothesis that 5-HT acts to restrain panic, a distinction between its role in spontaneous panic compared to acute anticipatory anxiety is difficult to sustain and it may be that the important distinction is between other aspects of anxiety, such as proximal versus distal threat or conditioned versus unconditioned anxiety.
The lack of effect of 5% CO2 challenge on plasma cortisol is generally consistent with the literature (Reference Carr, Sheehan and SurmanCarr et al, 1986; Reference KleinKlein, 1993). Why patients with panic disorder lack a stress hormone response when panicking is unknown, but it could relate to an adaptive change in the hypothalamus-pituitary axis related to chronic anxiety or stress (Reference GrayGray, 1987), or simply a low threshold for reporting panic attacks. In addition, the anxiety responses to 5% CO2 were relatively mild and may have been insufficient to stimulate a stress response.
Methodological considerations
We found a lower rate of panic following 5% CO2 inhalation on the control occasion than that suggested by the literature (about 10% v. 50%; Reference Sanderson and WetzlerSanderson & Wetzler, 1990), possibly because the setting of the experiment and careful explanation minimised the panic rate through cognitive factors (Reference ClarkClark, 1986). We think it unlikely that the control drink suppressed the panic rate, because although it increased both total and free plasma tryptophan, the plasma tryptophan to large neutral amino acid ratio and hence tryptophan entry into the brain would still be expected to be reduced, although much less than following the tryptophan-free drink (Reference Weltzin, Fernstrom and McConahaWeltzin et al, 1994).
The successful blinding of the experimental occasions argues against cognitive factors directly affecting the result. However, there is a theoretical possibility that tryptophan depletion stimulated respiratory function and hence, indirectly, the panic rate through the occurrence of somatic symptoms. In rats, 5-HT depletion stimulates respiration (Reference Olson, Dempsey and McCrimmonOlson et al, 1979) and a similar tendency was seen with tryptophan depletion in humans (Reference Kent, Coplan and MartinezKent et al, 1996). However, arguing for a direct effect on anxiety, tryptophan depletion enhanced acute anticipatory anxiety and there was no significant effect on the respiratory item scores of the API. Unfortunately we did not have direct measures of respiratory effort or tidal volume for a direct answer to the question.
Implications
Our study suggests that 5-HT directly inhibits panic anxiety and that this may help to explain the anti-panic effects of antidepressants such as selective serotonin reuptake inhibitors. The lack of effect of tryptophan depletion on resting anxiety is consistent with 5-HT playing a different role in different types of anxiety, although the results did not suggest a direct role in the maintenance of high resting levels of anxiety in our patients with panic disorder. This implies that the anxiolytic effect of antidepressants in generalised anxiety and non-panic anxiety associated with depression may involve a different mechanism to the anti-panic effect. Studies using different manipulations of 5-HT function and alternative anxiety challenges in patients with anxiety disorders and healthy volunteers are needed in order to shed further light on the role of 5-HT in human anxiety.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Acute reduction in brain 5-HT function appears to enhance anxiety provoked by 5% CO2 inhalation in patients with panic disorder.
-
▪ Direct enhancement of 5-HT neurotransmission may explain the efficacy of some antidepressants in treating panic disorder.
-
▪ The lack of effect of acute tryptophan depletion on 5% CO2-induced anxiety in normal volunteers may indicate different mechanisms operating in pathological and normal anxiety.
LIMITATIONS
-
▪ Reduction in brain 5-HT function as a consequence of acute tryptophan depletion could only be inferred.
-
▪ Anxiety induced under laboratory conditions as a consequence of 5% CO2 inhalation may not be a valid analogue of naturally occurring panic attacks.
-
▪ The lack of effect of acute tryptophan depletion in normal volunteers may reflect their weak anxiogenic response to 5% CO2 inhalation in our study.








eLetters
No eLetters have been published for this article.