Electroconvulsive therapy (ECT) is highly effective in the treatment of severe depression (UK ECT Review Group, 2003). During the past decade, transcranial magnetic stimulation has emerged as a new anti-depressant treatment (e.g. Reference Berman, Narasímhan and SanacoraBerman et al, 2000). Some randomised trials suggest that repetitive transcranial magnetic stimulation (rTMS) might be as effective as ECT in the treatment of non-psychotic depression (Grunhaus et al, Reference Grunhaus, Dannon and Schreiber2000, Reference Grunhaus, Schreiber and Dolberg2003; Reference Janicak, Dowd and MartisJanicak et al, 2002). However, recent reviews and meta-analyses show temperate enthusiasm for transcranial magnetic stimulation as an alternative to ECT (e.g. Reference Martin, Barbanoj and SchlaepferMartin et al, 2003; Reference Schlaepfer, Kosel and NemeroffSchlaepfer et al, 2003) and emphasise the need for further studies.
In weighing the benefits and risks of different treatment methods, cognitive side-effects are an important issue. Electroconvulsive therapy has been shown to induce anterograde amnesia, retrograde amnesia and subjective memory complaints (e.g. Reference Squire and SlaterSquire & Slater, 1983; Reference Lisanby, Maddox and PrudicLisanby et al, 2000). Although such deficits tend to cease within weeks to months, a review of patients’ perspectives on ECT (Reference Rose, Wykes and LeeseRose et al, 2003) indicates that persistent memory impairment following this therapy may be more frequent than is evident from testing with standard neuropsychological batteries. In contrast, rTMS seems not to have any substantial cognitive side-effects (e.g. Reference Triggs, McCoy and GreerTriggs et al, 1999). However, a comprehensive comparison of cognitive side-effects is lacking so far.
METHOD
Participants
Thirty patients referred to the Psychiatric University Hospital of Bonn with treatment-resistant, non-psychotic major depressive disorder participated in the study. All patients met the following inclusion criteria:
-
(a) a DSM–IV diagnosis of major depressive disorder (American Psychiatric Association, 1994), as assessed by an experienced clinical psychiatrist;
-
(b) no additional Axis I diagnosis;
-
(c) unsuccessful treatment response to at least two different types of antidepressants, each given in a sufficient dosage range for at least 4 weeks;
-
(d) age over 18 years;
-
(e) no previous treatment with ECT or rTMS.
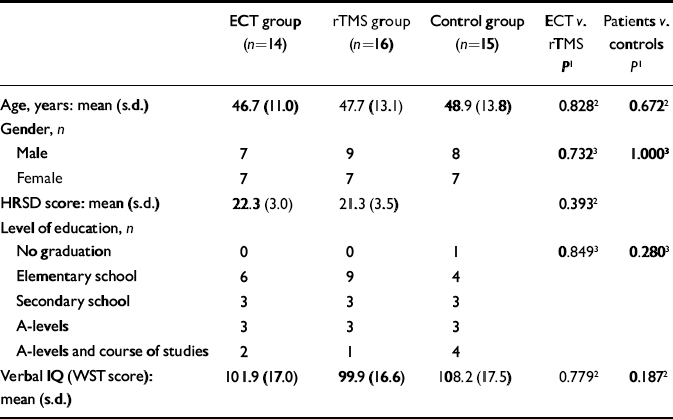
The patient sample consisted of 16 men and 14 women; the average age was 47 years (range 25–69) (Table 1).
Table 1 Demographic and clinical variables
| ECT group (n=14) | rTMS group (n=16) | Control group (n=15) | ECT v. rTMS p 1 | Patients v. controls p 1 | |
|---|---|---|---|---|---|
| Age, years: mean (s.d.) | 46.7 (11.0) | 47.7 (13.1) | 48.9 (13.8) | 0.8282 | 0.6722 |
| Gender, n | |||||
| Male | 7 | 9 | 8 | 0.7323 | 1.0003 |
| Female | 7 | 7 | 7 | ||
| HRSD score: mean (s.d.) | 22.3 (3.0) | 21.3 (3.5) | 0.3932 | ||
| Level of education, n | |||||
| No graduation | 0 | 0 | 1 | 0.8493 | 0.2803 |
| Elementary school | 6 | 9 | 4 | ||
| Secondary school | 3 | 3 | 3 | ||
| A-levels | 3 | 3 | 3 | ||
| A-levels and course of studies | 2 | 1 | 4 | ||
| Verbal IQ (WST score): mean (s.d.) | 101.9 (17.0) | 99.9 (16.6) | 108.2 (17.5) | 0.7792 | 0.1872 |
All patients were referred for ECT or rTMS at the University Hospital of Bonn after prior treatments with antidepressant drugs had failed. The department offers both of these therapies, and is one of only a few specialised treatment centres in Germany to do so. All participants approached our clinic with the specific wish–or a referring doctor's recommendation – to receive either rTMS or ECT, and received the treatment of their choice if no clinical exclusion criterion was present. The study consisted of consecutively admitted cases within a specified period, going into either the rTMS or the ECT group with comparable likelihood. Fourteen patients were treated with ECT and 16 received rTMS. The two groups did not show significant differences on any of the variables measured; most importantly, they were comparable with regard to age, gender, level of depression, level of education and verbal IQ (Table 1). Furthermore, the cognitive status levels of the two groups at baseline were almost identical, allowing us to study the changes induced by ECT or rTMS. All participants participants gave informed consent to repeated neurocognitive assessment.
In order to disentangle treatment-related changes in performance from test repetition effects, a control group of 15 healthy volunteers, matched by age, gender, level of education and verbal IQ to the patient groups (see Table 1), was also assessed twice, with the same interval between testings as the patients.
Clinical ratings and neuropsychological tasks
Patients and controls were assessed with an extensive test battery before and about a week after completion of the treatment. Pre-treatment testing took place 1–3 days before the first treatment session, and the post-treatment testing was done 8.8 days on average after the last ECT or rTMS session with equal intervals between the last treatment and post-treatment testing for both groups (P=0.68, NS). The clinical effects of ECT and rTMS were assessed using the 17-item Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1967; German version by the Collegium Internationale Psychiatriae Scalarum, 1978) and the Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBeck et al, 1961; German version by Reference Hautzinger, Bailer and WorallHautzinger et al, 1994). The ratings were made by the treating clinical psychiatrist. Cognitive effects of ECT and rTMS were assessed with a comprehensive neuro-psychological battery (see Appendix) with special emphasis on memory functions. Cognitive testing was done by a psychologist masked to the treatment assignments.
Treatments
Electroconvulsive therapy
Electroconvulsive therapy was given in accordance with current clinical guidelines (American Psychiatric Association, 1990; Reference FolkertsFolkerts, 1997), using a Thymatron III DG stimulator (Somatics Inc., Illinois, USA), which delivers a brief-pulse bi-directional current. All treatments were given under anaesthesia with propofol (2 mg/kg), muscle relaxation with suxamethonium (1 mg/kg) and 100% oxygenation. Medication was not changed during treatment. Antidepressants, low-potency neuroleptics and non-benzodiazepine hypnotics were allowed. Stimulation was always unilateral on the right hemisphere (all patients were reported to be right-handed for several manual activities). Seizure threshold was determined by a titration method and was age-based. Stimulation intensity was 2–2.5 times the seizure threshold. Therapy was given twice-weekly with a minimum interval of 48 h between treatments. Decisions concerning the number of treatments were made by the psychiatrist in attendance; participants received a mean of 9.9 (s.d.=2.7) treatments.
Transcranial magnetic stimulation
Transcranial magnetic stimulation was given with a Magstim Rapid machine (Magstim Co. Ltd, Whitland, UK). To make the frequencies of ECT and rTMS comparable, patients were treated two or three times per week; they received a mean of 10.8 (s.d.=1.4) treatments. Medication was kept constant as well. Stimulation was applied over the left dorsolateral prefrontal cortex with an intensity of 100% and a frequency of 10 Hz (20–30 trains of 2 s duration per treatment session, 5 s intertrain interval). Stimulation frequency was identical to that in previous studies comparing rTMS and ECT (Grunhaus et al, Reference Grunhaus, Dannon and Schreiber2000, Reference Grunhaus, Schreiber and Dolberg2003; Reference Janicak, Dowd and MartisJanicak et al, 2002).
Data analysis
Data analysis was done using the Statistical Package for the Social Sciences, for Windows version 10.0. To compare the two treatment methods, analyses of variance (ANOVAs) with repeated-measures, between-group t-tests (Welch-corrected for unequal variances) and within-group t-tests were performed. In addition, the numbers of responders in both groups were compared using a chi-squared test. Patients were considered to be responders to treatment if their HRSD scores had decreased by at least 50% from baseline levels. One person in the ECT group withdrew from the study because of severe orientation and memory problems after two ECT treatments; these data were not included in the analysis.
RESULTS
Clinical effectiveness
Both treatment methods resulted in a marked reduction of depression, as assessed by HRSD score, BDI score and response rates. In the ECT group the mean HRSD scores decreased by 35% from 22.4 (s.d.=3.1) to 14.5 (s.d.=5.7), P<0.001. In the rTMS group the mean HRSD score decreased by 39%, from 21.3 (s.d.=3.5) to 13.0 (s.d.=4.9), P<0.001 (Fig. 1). The ANOVA showed a significant time effect (F (1,27)=65.25, P<0.001), but no group effect and no interaction. Similar results were found for self-ratings of depression on the BDI. In the ECT group the mean BDI scores decreased by 7.6 points (24%) and in the rTMS group the decrease was 6.4 points (27%). The ANOVA revealed a significant time effect (F (1,24)=8.40, P<0.008), but no significant group effect and no interaction.
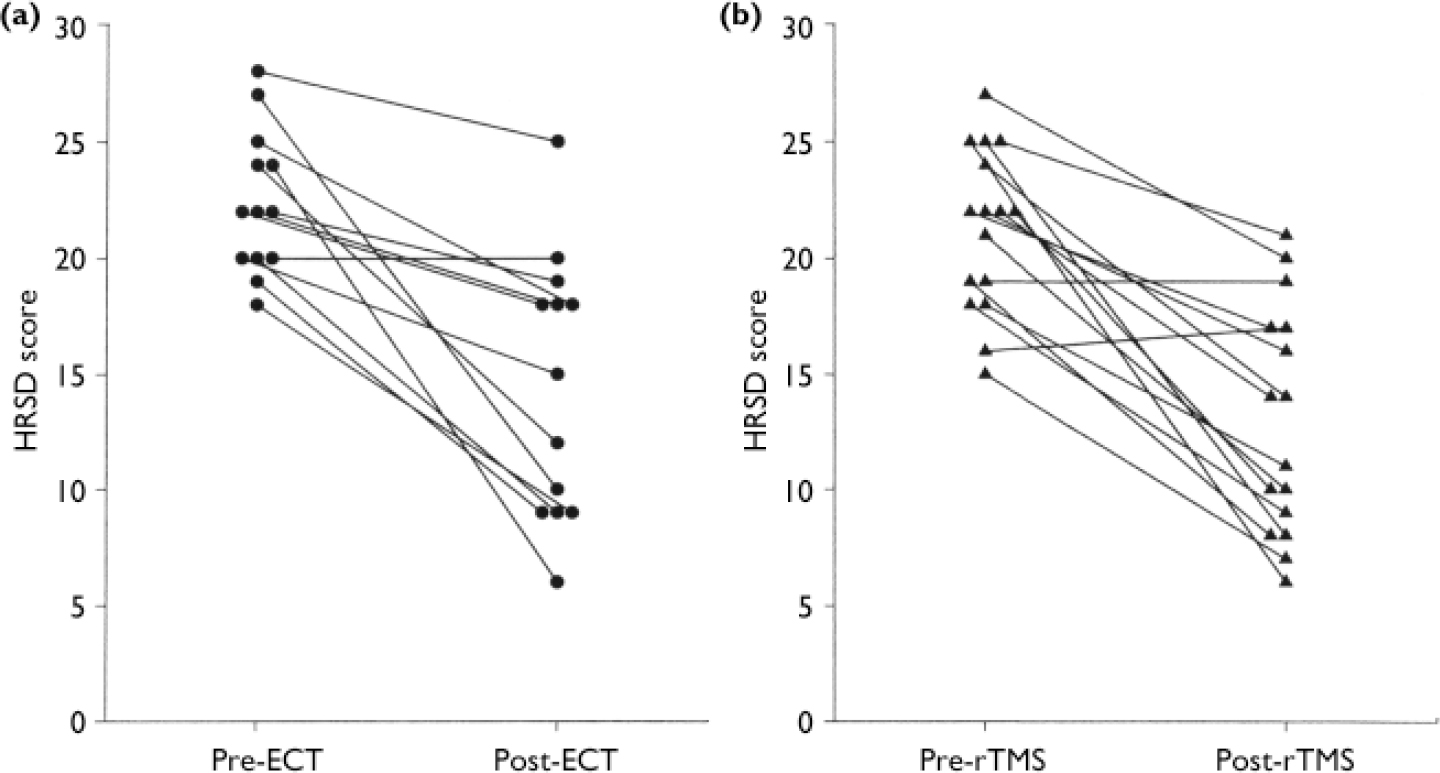
Fig. 1 Clinical improvement shown by reductions in Hamilton Rating Scale for Depression (HRSD) scores following (a) electroconvulsive therapy (ECT) and (b) transcranial magnetic stimulation (rTMS).
Patients were considered to be responders to treatment if their final HRSD score had decreased by 50% or more from baseline. According to this criterion, 46% of the ECT group and 44% of the rTMS group were treatment responders (χ2 (1)=0.02, P=0.90, NS).
Neuropsychological effects
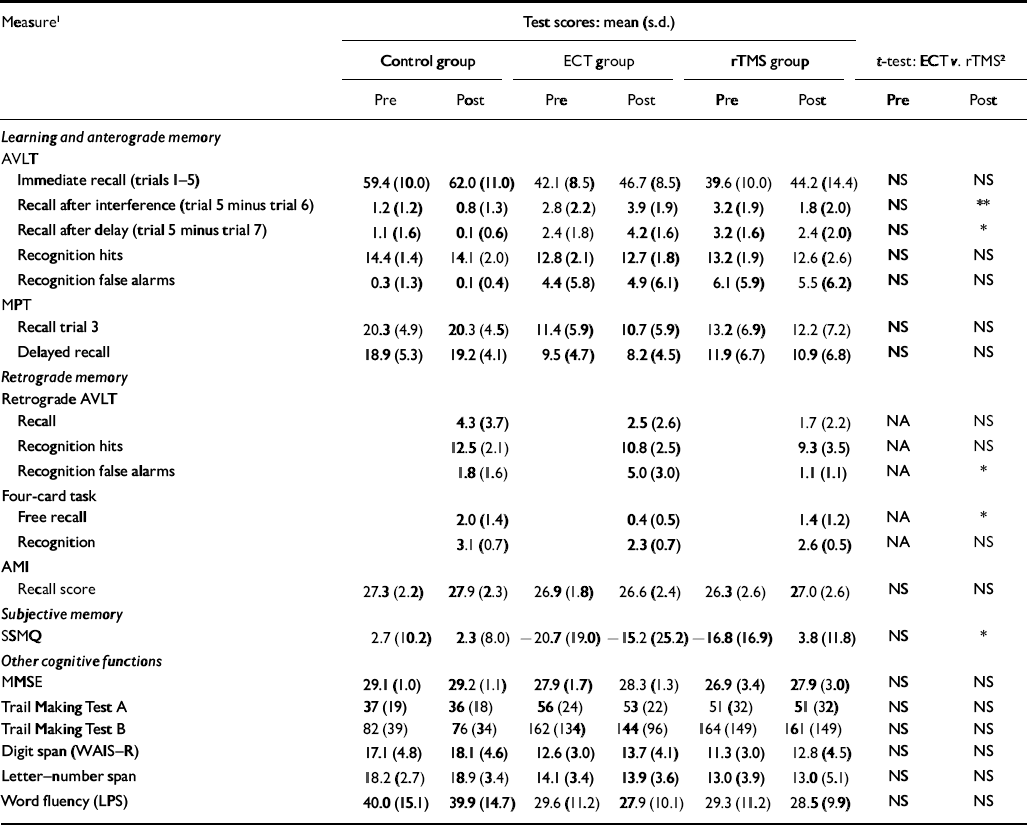
Before treatment, the two groups with depressive disorder did not differ from each other on any of the neuropsychological measures (Table 2). After treatment, significant differences between the ECT and rTMS treatment groups emerged for specific memory functions; these differences were consistently in favour of rTMS. Significant differences between patient groups after treatment were found in five measures of long-term memory recall or recognition, but not for any non-memory measure (Table 2).
Table 2 Cognitive measures in the three study groups
| Measure1 | Test scores: mean (s.d.) | |||||||
|---|---|---|---|---|---|---|---|---|
| Control group | ECT group | rTMS group | t-test: ECTv. rTMS2 | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Learning and anterograde memory | ||||||||
| AVLT | ||||||||
| Immediate recall (trials 1-5) | 59.4 (10.0) | 62.0 (11.0) | 42.1 (8.5) | 46.7 (8.5) | 39.6 (10.0) | 44.2 (14.4) | NS | NS |
| Recall after interference (trial 5 minus trial 6) | 1.2 (1.2) | 0.8 (1.3) | 2.8 (2.2) | 3.9 (1.9) | 3.2 (1.9) | 1.8 (2.0) | NS | ** |
| Recall after delay (trial 5 minus trial 7) | 1.1 (1.6) | 0.1 (0.6) | 2.4 (1.8) | 4.2 (1.6) | 3.2 (1.6) | 2.4 (2.0) | NS | * |
| Recognition hits | 14.4 (1.4) | 14.1 (2.0) | 12.8 (2.1) | 12.7 (1.8) | 13.2 (1.9) | 12.6 (2.6) | NS | NS |
| Recognition false alarms | 0.3 (1.3) | 0.1 (0.4) | 4.4 (5.8) | 4.9 (6.1) | 6.1 (5.9) | 5.5 (6.2) | NS | NS |
| MPT | ||||||||
| Recall trial 3 | 20.3 (4.9) | 20.3 (4.5) | 11.4 (5.9) | 10.7 (5.9) | 13.2 (6.9) | 12.2 (7.2) | NS | NS |
| Delayed recall | 18.9 (5.3) | 19.2 (4.1) | 9.5 (4.7) | 8.2 (4.5) | 11.9 (6.7) | 10.9 (6.8) | NS | NS |
| Retrograde memory | ||||||||
| Retrograde AVLT | ||||||||
| Recall | 4.3 (3.7) | 2.5 (2.6) | 1.7 (2.2) | NA | NS | |||
| Recognition hits | 12.5 (2.1) | 10.8 (2.5) | 9.3 (3.5) | NA | NS | |||
| Recognition false alarms | 1.8 (1.6) | 5.0 (3.0) | 1.1 (1.1) | NA | * | |||
| Four-card task | ||||||||
| Free recall | 2.0 (1.4) | 0.4 (0.5) | 1.4 (1.2) | NA | * | |||
| Recognition | 3.1 (0.7) | 2.3 (0.7) | 2.6 (0.5) | NA | NS | |||
| AMI | ||||||||
| Recall score | 27.3 (2.2) | 27.9 (2.3) | 26.9 (1.8) | 26.6 (2.4) | 26.3 (2.6) | 27.0 (2.6) | NS | NS |
| Subjective memory | ||||||||
| SSMQ | 2.7 (10.2) | 2.3 (8.0) | -20.7 (19.0) | -15.2 (25.2) | -16.8 (16.9) | 3.8 (11.8) | NS | * |
| Other cognitive functions | ||||||||
| MMSE | 29.1 (1.0) | 29.2 (1.1) | 27.9 (1.7) | 28.3 (1.3) | 26.9 (3.4) | 27.9 (3.0) | NS | NS |
| Trail Making Test A | 37 (19) | 36 (18) | 56 (24) | 53 (22) | 51 (32) | 51 (32) | NS | NS |
| Trail Making Test B | 82 (39) | 76 (34) | 162 (134) | 144 (96) | 164 (149) | 161 (149) | NS | NS |
| Digit span (WAIS—R) | 17.1 (4.8) | 18.1 (4.6) | 12.6 (3.0) | 13.7 (4.1) | 11.3 (3.0) | 12.8 (4.5) | NS | NS |
| Letter—number span | 18.2 (2.7) | 18.9 (3.4) | 14.1 (3.4) | 13.9 (3.6) | 13.0 (3.9) | 13.0 (5.1) | NS | NS |
| Word fluency (LPS) | 40.0 (15.1) | 39.9 (14.7) | 29.6 (11.2) | 27.9 (10.1) | 29.3 (11.2) | 28.5 (9.9) | NS | NS |
Anterograde memory function
Significant differences to the disadvantage of ECT were found in two variables of the Auditory Verbal Learning Test (AVLT; Reference ReyRey, 1964): loss after interference (trial 5 minus trial 6) and loss after delay (trial 5 minus trial 7). In the ANOVA, the group × time interaction was significant for both measures (F (1,23)=7.81, P=0.010 and F (1,23)=15.56, P=0.001), whereas the effects of time and group were not significant (Fig. 2(a)).
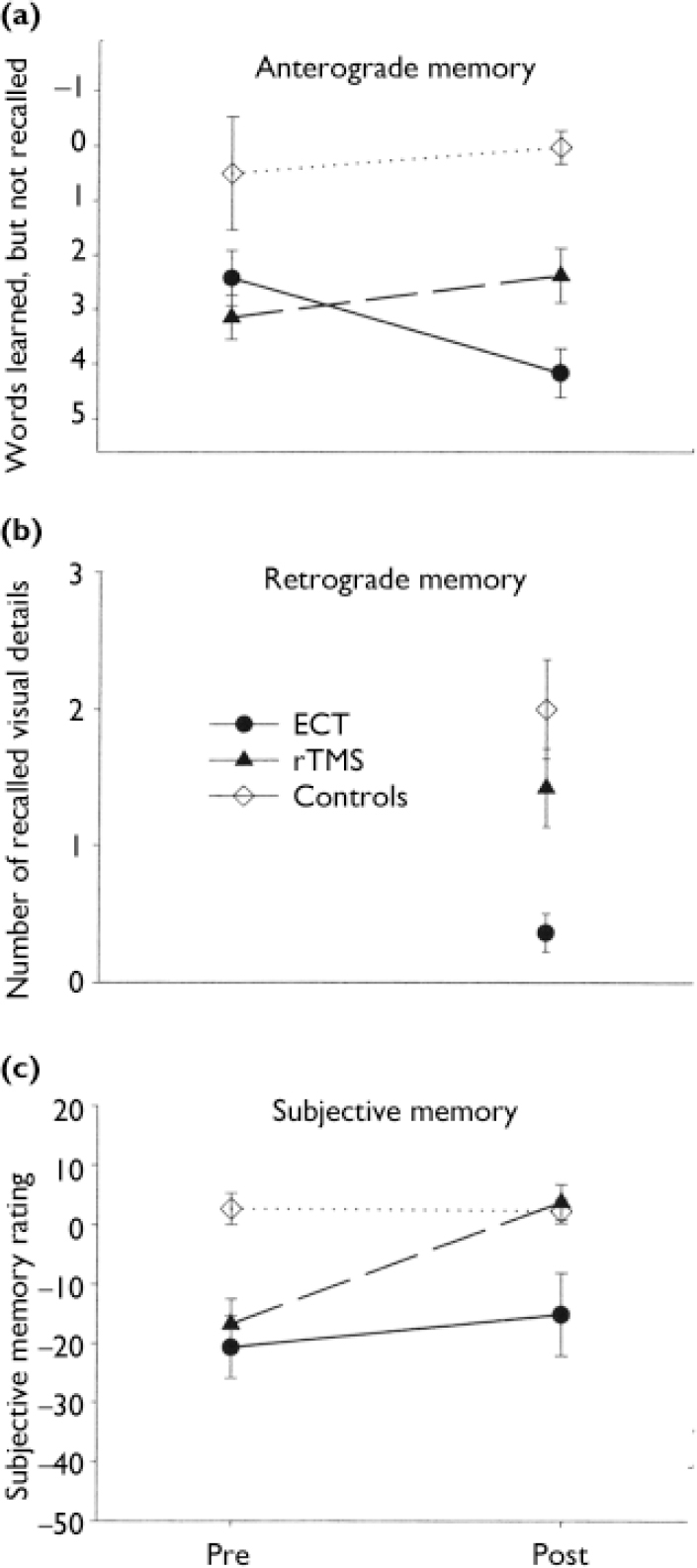
Fig. 2 Memory functions and memory complaints before (Pre) and about 1 week after (Post) treatment with electroconvulsive therapy (ECT) or repetitive transcranial magnetic stimulation (rTMS). Control group participants were tested twice at comparable intervals. (a) Anterograde memory: Auditory Verbal LearningTest, delayed recall, trial 5 minus trial 7. (b) Retrograde memory (four-card task): recall of visual material seen before treatment. (c) Subjective memory, relative to premorbid state (patients) or relative to 1 year earlier (controls): Squire Subjective Memory Questionnaire score (0, normal memory). Means and standard errors are shown.
Retrograde memory function
After treatment, participants in the ECT group made significantly more errors than those in the rTMS group in recognising words learned before treatment (P=0.025). After treatment, they also recalled significantly fewer items from the visual card task administered before treatment, compared with the rTMS group (P=0.012) (Fig. 2(b)).
Subjective memory complaints
After treatment, participants given ECT reported no change in memory problems compared with before treatment (P=0.38, NS), whereas the rTMS patients judged their own memory much more positively after treatment (P=0.002). The ANOVA of the subjective memory measure showed a significant time effect (F (1,23)=11.04, P=0.003) and a group×time interaction approaching significance (F (1,23)=3.68, P=0.067) in the absence of any group effect (Fig. 2(c)).
In summary, significant between-group differences were found in anterograde verbal memory, in two retrograde memory parameters and in participants’ subjective estimation of their own memory abilities. In contrast to memory, other cognitive functions measured remained constant in both treatment groups; we found no significant group, time or interaction effect in these variables (Table 2).
Association of memory and depression
Weiner et al (Reference Weiner, Rogers and Davidson1986) found no relationship between subjective and objective memory measures in patients given ECT, and concluded that self-rated memory changes may be more a function of clinical symptoms than of objectively demonstrable changes in memory function. We explored this issue, correlating the subjective memory complaints, rated with the Squire Subjective Memory Questionnaire (Reference Squire, Wetzel and SlaterSquire et al, 1979), with the level of depression (BDI and HRSD scores) and with the assessed neuropsychological variables. The patients’ memory complaints correlated with depression as well as with several cognitive measures. The patients’ self-ratings of memory functions correlated significantly with their self-ratings of depression (BDI: r=-0.67, P<0.01), but less so with the clinician ratings of depression (HRSD: r=-0.31, P=0.13). Self-rating of memory also correlated with the ability to learn and recall new verbal and visual material (AVLT sum of trials 1–5: r=0.42, P<0.05; AVLT trial 7 after delay: r=0.46, P<0.05; Memory for Persons Test, delayed recall, r=0.48, P<0.05), and with the ability to correctly recognise words learned before treatment (retrograde AVLT, false alarms: r=–0.61, P<0.05), but not with their autobiographical memory (r=0.07, P=0.75, NS). Importantly, the correlations between subjective and objective memory did not change markedly when controlling for self-rated (BDI) depression by partial correlations.
Comparison with healthy controls
The group of healthy controls was included to control for test repetition effects and to see whether (and which) impaired cognitive functions would return to normal levels after treatment in both groups. A comparison of the cognitive functions of the participants with depression and the healthy controls at baseline showed highly significant deficits on the part of the patients in almost all measures. Repeated-measures ANOVAs comparing each treatment group separately with the healthy control group revealed that the performance gap between the ECT and control groups with regard to anterograde memory increased (group time interaction, P<0.05 for recall after interference and P<0.001 for recall after delay). In contrast, the difference between the rTMS and control groups remained unchanged (the group×time interactions were not significant).
After treatment, the ECT and control groups differed considerably in their ability to remember words or cards from pre-treatment testing (retrograde AVLT: P=0.049; four-card task: P=0.001), whereas the rTMS group showed retrograde memory functions identical to those of the control group (retrograde AVLT: P=0.33, NS; four-card task: P=0.26, NS). Similarly, the self-rating of memory functions by the rTMS group differed significantly from that in the control group only before treatment (P=0.001); after treatment the rTMS group rated their memory functions to be as good as those of the healthy group (P=0.69, NS). In contrast, the ECT group rated their memory abilities more negatively than the control group both before (P=0.001) and after treatment (P=0.039). The group×time interaction in the ANOVA comparing control and rTMS groups was significant (P<0.001), but this interaction was not significant in the ANOVA comparing the control and ECT groups (P=0.30, NS).
DISCUSSION
The major conclusion to be drawn from this study pertains to the cognitive effects of unilateral ECT and left prefrontal rTMS in patients with severe depression, since this study was designed to assess these effects with sensitive neuropsychological measures. Because the antidepressant effect of both treatments was identical, and the groups did not differ prior to treatment, the cognitive changes over time and the post-treatment group differences can be attributed to the different treatments. In the ECT group, not a single cognitive variable improved after treatment, and the recall of newly learned material even became worse. In the rTMS group, some objective memory measures and the subjective memory rating improved in parallel with the improvement in mood, and reached normal performance levels.
Clinical effectiveness of ECTand rTMS
The two treatments appeared to be clinically equivalent in this group of patients with treatment-resistant, non-psychotic depression. Although the study was not randomised, its finding of comparable anti-depressant efficacy of ECT and rTMS is in line with the results of all three randomised comparison studies published so far (Grunhaus et al, Reference Grunhaus, Dannon and Schreiber2000, Reference Grunhaus, Schreiber and Dolberg2003; Reference Janicak, Dowd and MartisJanicak et al, 2002). The rates of those responding to unilateral ECT are in the expected range for medication-resistant non-psychotic depression (Reference McCallMcCall, 2001), but might have been higher if a higher ECT dosage had been used (Reference Sackeim, Prudic and DevanandSackeim et al, 2000). However, a higher dosage would probably increase the risk of cognitive adverse effects. A definitive answer to the question of clinical equipotency of ECT and rTMS will have to await further studies.
Anterograde memory
Patients treated with ECT showed more anterograde memory problems at the post-treatment assessment than did either patients treated with rTMS or healthy controls. In particular, they remembered fewer words only after learning the interference word list of the Auditory Verbal Learning Test (between Trials 5 and 6 of the AVLT), indicating a recall deficit rather than a working memory or learning deficit. This extends findings by Hasse-Sander et al (Reference Hasse-Sander, Müller and Schurig1998), who reported impaired verbal delayed recall 1–2 days after unilateral ECT (no longer follow-up was made), and of Cronholm & Ottosson (Reference Cronholm and Ottosson1961), who described specific deficits in the delayed recall of newly learned words, figures and persons 1 week after bilateral ECT. For the rTMS group, the lack of anterograde memory effects is in line with previous studies (e.g. Reference Triggs, McCoy and GreerTriggs et al, 1999).
Retrograde memory
The ECT patients, in contrast to the patients treated with rTMS or the control group, also showed retrograde memory problems after treatment. They remembered fewer of the pictures and made more errors in recalling words learned before treatment. No difference emerged for auto-biographical memory, which is in line with previous studies demonstrating deficits in recall of past events only after bilateral ECT, not after unilateral therapy (Reference Squire, Slater and MillerSquire et al, 1981; Reference Weiner, Rogers and DavidsonWeiner et al, 1986; Reference Lisanby, Maddox and PrudicLisanby et al, 2000). The verbal and visual retrograde memory tasks used here might be more sensitive to ECT-induced impairments than the Autobiographical Memory Interview, possibly because recent memory traces formed during the days before treatment are more vulnerable to ECT effects than are more remote memories (Reference Squire, Slater and MillerSquire et al, 1981; Reference Lisanby, Maddox and PrudicLisanby et al, 2000).
Subjective memory
After treatment, the ECT patients complained more about memory problems than the rTMS patients and the controls. Squire et al (Reference Squire, Wetzel and Slater1979), Freeman et al (Reference Freeman, Weeks and Kendell1980) and Squire & Slater (Reference Squire and Slater1983) also found subjective cognitive side-effects after ECT. After rTMS the patients’ subjective memory ratings equalled those of the healthy controls, whereas after ECT the patients’ ratings were very negative. This group difference cannot be explained by different clinical effects of the two treatment methods. At least in part, this group difference in subjective memory appears to be related to objectively measurable memory impairments after ECT, because the subjective memory functions not only correlated with the level of depression, which is a common finding (e.g. Reference Weiner, Rogers and DavidsonWeiner et al, 1986), but also with several objective memory measures (even when statistically controlling for the level of depression). Associations between subjective and objective memory measures after ECT have often found to be lacking (e.g. Reference Prudic, Peyser and SackeimPrudic et al 2000), but some studies suggest that such a relationship may exist, at least for certain forms of memory and for specific periods after ECT (e.g. Reference Freeman, Weeks and KendellFreeman et al, 1980). Thus, complaints of patients about memory deficits (metamemory) may partly result from the experience of objective memory failures after ECT, and should not be dismissed as being simply a sign of depressive complaints.
Clinical implications
With regard to objective and subjective memory function, patients with severe depression appear to be cognitively better off 1 week after a course of rTMS than 1 week after a course of unilateral ECT, despite (in this study) an indistinguishable antidepressive effect of the two treatments. Adverse memory effects after ECT may fully resolve after a longer interval, usually after several months (Reference Weeks, Freeman and KendellWeeks et al, 1980); nevertheless, if rTMS evolves into an alternative treatment for some forms of medication-resistant depression, clinicians and patients should be aware of its reduced risk for adverse memory effects, compared with unilateral ECT. Future comparison studies of ECT, rTMS and magnetic seizure therapy (Reference Kosel, Frick and LisanbyKosel et al, 2003) should include sensitive memory assessments and longer follow-up intervals to evaluate fully the ratio of benefits and risks of these treatment methods.
Limitations
Because our study lacked a sham-treated patient control group and patients were not randomly assigned to treatments, no conclusion should be drawn regarding the absolute or relative antidepressant effectiveness of rTMS or ECT. Although the pattern of cognitive findings, in line with previous work, suggests that unilateral ECT, in contrast to rTMS, specifically impairs several aspects of memory for at least a week after a treatment series, the small number of participants in our study renders this a preliminary finding requiring confirmation in other samples.
APPENDIX
Neurocognitive test battery
Learning and anterograde memory function
-
(a) Auditory Verbal Learning Test (AVLT; Reference ReyRey, 1964); German version by Helmstaedter et al (Reference Helmstaedter, Lendt and Lux2001).
-
(b) Memory for Persons Test (Reference Bulla-Hellwig and SpanhoferBulla-Hellwig & Spanhofer, 1996); a German visual memory test in which each of 12 different faces has to be associated with a name and an occupation.
Retrograde memory function
-
(a) Autobiographical Memory Interview (Reference Kopelman, Wilson and BaddeleyKopelman et al, 1990); shortened German version.
-
(b) Retrograde AVLT: at the post-treatment assessment participants were asked to recall the 15 AVLT words they had learned before treatment. The task involved a free recall and a recognition task. This measure was introduced after the study began, therefore only data from 13 patients (6 receiving electroconvulsive therapy, 7 repetitive transcranial magnetic stimulation) and 15 controls were available.
-
(c) Four-card task: before treatment, participants were asked to reproduce a demonstrated arrangementof four picture cards from the River-mead Behavioural Memory Test (Reference Wilson, Cockburn and BaddeleyWilson et al, 1991); after treatment they were asked details about the ‘test with cards’ (number of cards, kind of task, recall and recognition of depicted objects), and the number of correct details was scored.
Subjective memory function
Squire Subjective Memory Questionnaire (Reference Squire, Wetzel and SlaterSquire et al, 1979): this 18-item self-rated scale of memory functions comprises items such as ‘My ability to hold in my memory things that I have learned is...’. Respondents were asked to compare their present memory with their memory before they became ill (patients) or with their memory 1 year ago (controls), on a nine-point scale from -4 (worse than before) to +4 (better than before).
Other neurocognitive functions
-
(a) Mini-Mental State Examination (Reference Folstein, Folstein and McHughFolstein et al, 1975); German version by Kessler et al (Reference Kessler, Markowitsch and Denzler1990).
-
(b) Trail Making Test A and B (Reference ReitanReitan, 1979).
-
(c) Digit span: Wechsler Adult Intelligence Scale – Revised (Reference WechslerWechsler, 1981).
-
(d) Letter–number span (Reference Gold, Carpenter and RandolphGold et al, 1997).
-
(e) Word fluency: Leistungs-Prüf-System (Reference HornHorn, 1983).
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ In people with treatment-resistant depression, even unilateral electroconvulsive therapy (ECT) is associated with memory deficits 1 week after the last treatment.
-
▪ Repetitive transcranial magnetic stimulation is not accompanied by such memory impairments.
-
▪ Self-reported memory impairments after ECT can be related to objective memory deficits and must not be dismissed as being depressive complaints only.
LIMITATIONS
-
▪ Patient groups were not randomised and no patient control group was assessed, limiting conclusions about clinical efficacy.
-
▪ Sample sizes were small.
-
▪ There was no long-term follow-up.







eLetters
No eLetters have been published for this article.