The prevalence of diabetes mellitus in developing countries and throughout the Westernised world is increasing dramatically, with estimates suggesting a doubling in prevalence over the next 10 years. In 1997, an estimated 124 million people worldwide had diabetes, of whom 97% had type 2 diabetes (Reference Amos, McCarty and ZimmetAmos et al, 1997). In the UK alone at least 1.4 million people have been diagnosed with type 2 diabetes (Diabetes UK, 2002), and it has been estimated that 221 million people around the world will have the condition by 2010 (Reference Amos, McCarty and ZimmetAmos et al, 1997). The reasons for this substantial and deepening burden of type 2 diabetes in the general population are complex, but include the increasing prevalence of adult and childhood obesity, unhealthy diets, sedentary lifestyles and a steadily ageing population.
The prevalence of diabetes among people with schizophrenia may be two to four times higher than in the general population (Reference Keskiner, El Toumi and BousquetKeskiner et al, 1973; Reference Mukherjee, Decina and BocolaMukherjee et al, 1996; Reference Dixon, Weiden and DelahantyDixon et al, 2000; Reference Sernyak, Leslie and AlarconSernyak et al, 2002; Reference Lindenmayer, Czobor and VolavkaLindenmayer et al, 2003; Reference Subramaniam, Chong and PekSubramaniam et al, 2003). Schizophrenia appears to be an independent risk factor for diabetes (Reference HendersonHenderson, 2002; Reference Ryan and ThakoreRyan & Thakore, 2002), and many individuals with schizophrenia have a family history of the condition (Reference DynesDynes, 1969; Reference Mukherjee, Schnur and ReddyMukherjee et al, 1989; Reference Cheta, Dumitrescu and GeorgescuCheta et al, 1990). A genetic predisposition to diabetes appears to be unmasked by the many poor health behaviours associated with schizophrenia, including an unhealthy diet, a lack of exercise and a tendency to smoke (Reference Brown, Birtwistle and RoeBrown et al, 1999).
The part played by antipsychotic medications in the development of diabetes is controversial. Recent data from placebo-controlled studies in patients with schizophrenia have shown that the incidence of diabetes is similar in placebo and drug cohorts (Reference Cunningham, Lambert and DalackCunningham et al, 2003; Reference Sowell, Mukhopadhyay and CavazzoniSowell et al, 2003). Some commentators suggest that the risk of blood glucose abnormalities may be higher with the atypical antipsychotic medications than the typical antipsychotic medications (Reference Sernyak, Leslie and AlarconSernyak et al, 2002; Reference Cunningham, Lambert and DalackCunningham et al, 2003), but such conclusions seem to be premature. To date, it has not been possible within studies to adjust for levels of adherence to medication or to control for important confounders such as age, ethnicity or family history. In addition, none of the studies provides data on the number of patients in each cohort who were screened for glycaemic abnormalities, which is another important confounder when attempting to assess the prevalence of diabetes within populations.
At present, therefore, any guidelines for the screening and management of type 2 diabetes in individuals with schizophrenia should be based on the premise that type 2 diabetes (and most probably impaired glucose tolerance) is more common in patients with schizophrenia, and that all antipsychotic medications may have an effect on glucose tolerance.
METHOD
In order to develop pragmatic solutions for the screening and management of diabetes in patients with schizophrenia, a staged review process was adopted, using the evidence currently available in the literature, the standards set out in the National Service Framework (NSF) for Diabetes (Department of Health, 2002), the National Institute for Clinical Excellence (NICE) guidance on the management of diabetes (National Institute for Clinical Excellence, 2002), and recommendations from Diabetes UK (2000, 2002). The first stage of the review involved an overview of the prevalence of diabetes mellitus in both the general population and in people with schizophrenia, and assessed the numbers of patients likely to be undiagnosed and who might benefit from a screening strategy. Stage two reviewed the screening process in terms of who should be screened, how screening should be undertaken, and who should take responsibility for any screening. Finally, recommendations were developed for pragmatic pathways of care in individuals with schizophrenia at risk of developing diabetes.
RESULTS
Screening for diabetes
Studies in the UK suggest that in addition to the 1.4 million people who know they have diabetes, there may be a further million people who are unaware that they have the condition (Reference Forrest, Jackson and YudkinForrest et al, 1986; Reference Simmons, Williams and PowellSimmons et al, 1991). Evidence from the UK Prospective Diabetes Study suggests that type 2 diabetes may begin, on average, 9–12 years before the condition is diagnosed (UK Prospective Diabetes Study Group, 1995), which puts people at great risk of developing both macrovascular disease (cardiovascular, cerebrovascular and peripheral vascular disease) and microvascular complications (retinopathy and nephropathy).
The reasons why so many people with diabetes are unrecognised in the general population are hard to define. Type 2 diabetes has an insidious onset and is associated with non-specific symptoms (polyuria, polydipsia, nocturia, unexplained weight loss, loss of energy, recurrent infections such as candidiasis, and blurred vision) that could easily be attributed to other causes. This, coupled with the cost and complexity associated with widespread screening, probably accounts for the ‘missing million’ people with diabetes.
The pre-diabetic state of impaired glucose tolerance is even more difficult to identify, but is of equal if not more concern. A number of studies have evaluated the progression of this condition to overt type 2 diabetes, and these studies indicate that as many as 45% of patients with impaired glucose tolerance will develop diabetes over a 10-year period (Reference AlbertiAlberti, 1996; Reference Warram, Sigal and MartinWarram et al, 1996; Reference Meigs, Muller and NathanMeigs et al, 2003). The risk of developing type 2 diabetes in a high-risk pre-diabetic population has been reported to be as high as 35% over 4 years (Reference Knowler, Barrett-Connor and FowlerKnowler et al, 2002).
The hidden nature of diabetes in individuals with a serious mental illness such as schizophrenia is of major concern, with studies suggesting that the prevalence of known diabetes in people with schizophrenia has been grossly underestimated (Reference Subramaniam, Chong and PekSubramaniam et al, 2003). Active screening for impaired glucose tolerance and diabetes has found that over 30% of individuals with schizophrenia may have either one of these conditions, and that the majority of individuals are unaware that they have these problems (Reference Cohn, Wolever and ZipurskyCohn et al, 2002). The risks associated with undiagnosed diabetes and the pre-diabetic state are clear, with estimates suggesting that half of diabetic patients initially present with a diabetic or vascular complication (UK, Prospective Diabetes Study Group, 1991).
Large-scale clinical trials suggest that the complications associated with type 2 diabetes can be prevented or reduced with effective treatment. The UK Prospective Diabetes Study demonstrated that a reduction of 1% in glycosylated haemoglobin (HbA1c) resulted in a 21% reduction in diabetic complications, a 21% reduction in deaths, a 31% reduction in microvascular complications and a 14% reduction in macrovascular events (UK Prospective Diabetes Study Group, 1998).
Screening strategies for early detection of diabetes
Although type 2 diabetes is a progressive disease and current treatments rarely lead to normoglycaemic profiles, intensive management aimed at improving glycaemic control is undoubtedly a worthwhile aim (UK Prospective Diabetes Study Group, 1998). Identifying patients as early as possible in the course of their condition – ideally at the pre-diabetic stage – would allow for early intervention, a slowing of disease progression, and possibly even a reversal of some of the underlying pathological changes. However, as can be seen from the epidemiological studies, the silent nature of the condition means that the only way to confidently identify all patients at risk of developing diabetes is to use active population-based screening strategies. Questions remain, however, about who should be screened, how screening should be undertaken, and who should take responsibility for screening, both in the general population and among those with schizophrenia.
Who should be screened? Screening the entire population of any country for impaired glucose tolerance or diabetes would be costly and impractical, and it has therefore been suggested that screening should be focused on high-risk individuals, including those over the age of 40 years and people from Black, Asian and minority ethnic groups over 25 years of age with other risk factors (see Appendix). The relatively recent recognition that people with severe mental illnesses such as schizophrenia are at increased risk of developing diabetes suggests that these individuals should now also be considered to be in a high-risk group, and should be included in all national or local diabetes screening strategies.
How should screening be undertaken? Although the identification of suitable individuals for screening is not entirely straightforward, the choice of screening technique is even more of an issue. The gold standard test for diabetes, as endorsed by the World Health Organization (WHO), is the oral glucose tolerance test (OGTT), which requires an overnight fast followed by a 75 g glucose drink, with blood samples taken for glucose levels before the drink and 2 h afterwards (World Health Organization, 1999). A 2 h glucose value of > 11.1 mmol/l is diagnostic of diabetes, and a value between 7.8 mmol/l and 11.1 mmol/l indicates impaired glucose tolerance (Table 1).
Table 1 Making a diagnosis of diabetes

| Test | Impaired glucose tolerance or impaired fasting glycaemia (mmol/l) | Diabetes (mmol/l) |
|---|---|---|
| Oral glucose tolerance test | 7.8-11.1 (IGT) | > 11.1 |
| Fasting plasma glucose test | > 6.1, < 7.0 (IFG) | > 7.0 |
| Random plasma glucose test | 7.0-11.1 (IGT or IFG) | > 11.1 |
Although the OGTT is a relatively simple and inexpensive procedure, it is not suitable for use as a high-throughput screening test in most out-patient clinics or primary care surgeries. Following the initial recommendation of the American Diabetes Association (1997), the World Health Organization (1999) and Diabetes UK (2000) endorsed the use of a fasting plasma glucose test for screening purposes. Using this test, a diagnosis of diabetes can be made if symptoms are present and a glucose value of more than 7.0 mmol/l is found (Table 1). The test must be performed in an accredited laboratory using a venous blood sample. In the absence of symptoms, a second confirmatory blood test is required. A value of more than 6.1 mmol/l from a fasting sample suggests impaired glucose tolerance or impaired fasting glycaemia (Table 1), and requires an OGTT to exclude the diagnosis of diabetes.
The fasting plasma glucose test is more convenient and more suitable as a screening test than the OGTT, but it lacks sensitivity and may miss a large number of individuals with diabetes. A comprehensive review of screening tests for type 2 diabetes has demonstrated that the sensitivity of the fasting sample may be as low as 40%. The specificity of the test is relatively good, at between 84% and 99% (Diabetes UK, 2002).
Although a random plasma glucose measurement would seem on the surface to be unreliable as a screening test, it does have certain merits. It is easy to perform, and if it shows a value of more than 11.1 mmol/l the patient has diabetes (Table 1). The sensitivity of this test is reported to be between 50% and 69%, with a specificity of between 92% and 98% (Diabetes UK, 2002). A glucose value of 7.0–11.0 mmol/l requires follow-up, ideally with an OGTT. In ‘unreliable’ patients, fasting plasma glucose levels may be elevated by a failure to fast; a random plasma glucose test is, therefore, less likely to provide an overestimate, and this reduces the chances of a false positive result.
The HbA1c test provides an ‘average glucose value’ for the preceding 6–8 weeks and is an excellent measure of glycaemic control. Unfortunately, its value as a screening test is unproved. Estimates suggest that test results above the ‘normal’ range provide sensitivities of between 35% and 98%, and specificities of between 79% and 100% when used for the purposes of screening (Diabetes UK, 2002). The upper limit of normal is, however, very dependent upon the assay used. Although the NICE guidelines (National Institute for Clinical Excellence, 2002) recommend a target HbA1c value for individual patients with diabetes of between 6.5% and 7.5%, they do not suggest a normal range.
Many physicians, particularly in primary care, use HbA1c assessment as a complementary test for screening purposes when the random plasma glucose concentration is elevated. This is a relatively pragmatic approach based on the available evidence (Reference Davidson, Schriger and PetersDavidson et al, 1999; Reference Perry, Shankar and FinebergPerry et al, 2001; Reference Wang, Lee and FabsitzWang et al, 2002). This approach has been supported by results from the Early Diabetes Intervention Program, which demonstrated that the sensitivity of the fasting plasma glucose (>7.0 mmol/l) as a screening test for type 2 diabetes could be improved from 45% to 61% by the addition of an HbA1c measurement (>6.1%, or mean +2 standard deviations; Reference Perry, Shankar and FinebergPerry et al, 2001). Simple finger-prick testing to produce a random plasma glucose value may be a valuable initial screening test, although any result would need to be validated in an accredited laboratory on a venous sample.
Unfortunately, there is no universally agreed single test that can be easily employed in large numbers of people being screened for diabetes. All of the tests have their advantages and disadvantages, and in many instances local guidelines will dictate which test to use.
Who should be responsible for screening? The question of who should be responsible for screening is difficult to answer. Ideally, as the management of diabetes is increasingly becoming part of primary care, screening for diabetes should be undertaken within community practice. Unfortunately, primary health care services are already overstretched in many countries, and some primary care practices will be unable or unwilling to commit to offering structured screening programmes, even in high-risk groups.
An increasing number of pharmacists are offering local screening services based upon a random blood glucose test (Diabetes UK, 2002). This may be a matter of concern, not only for the reasons outlined above, but also because pharmacists cannot provide the appropriate clinical advice following a positive result.
Management strategies in diabetes
All patients with type 2 diabetes should be given lifestyle advice, particularly about diet and exercise. Patients should be advised to:
-
(a) decrease the energy and fat content of their diet and increase fibre intake;
-
(b) increase their intake of fruit and vegetables;
-
(c) eat complex rather than simple carbohydrates, e.g. wholewheat bread rather than refined white bread;
-
(d) avoid sugary drinks;
-
(e) exercise for at least 10–15 min per day.
Lifestyle guidance is an integral component of any management plan and ultimately requires active patient participation and empowerment – a principle endorsed by the National Institute for Clinical Excellence (2003). If lifestyle changes alone in newly diagnosed, asymptomatic patients are unsuccessful after 3 months, pharmacological agents are usually given as the next step.
Most diabetologists would consider the biguanide metformin as first-line therapy, particularly in patients who are overweight. The sulphonylureas (e.g. gliclazide, glibenclamide, glipizide, glimepiride) are used as second-line agents, although they may be the first choice for those who are not overweight or are unable to tolerate metformin. The newer, rapid-acting insulin secretagogues (nateglinide and repaglinide) and the thiazolidinediones (rosiglitazone and pioglitazone) are becoming increasingly popular in patients unable to tolerate or failing to benefit from first-line oral therapies. Most patients find themselves on a combination of oral therapies, and at least a quarter of patients will go on to need insulin injections at some stage.
Assessing glycaemic control
There are two main approaches to assessing glycaemic control; home blood glucose monitoring, and the HbA1c test. Home blood glucose monitoring is performed by the patient using a finger-prick spot of blood and a simple home glucose meter, which provides a result in around 20 s. Patients are encouraged to enter the glucose readings into a diary; they are advised on the frequency of tests, and provided with a target blood glucose range by their doctor. This enables patients to closely monitor and detect any changes in their blood glucose values, which should be reported to their primary care physician or diabetes specialist nurse.
The HbA1c test is a complementary approach to home blood glucose monitoring. Most patients with type 2 diabetes are seen in a diabetic clinic every 4–6 months, and would usually have HbA1c tested at, or prior to, each visit.
Managing impaired glucose tolerance
Patients with impaired glucose tolerance are usually reviewed at least once a year to detect the development of type 2 diabetes and any diabetic complications. Although there is now persuasive evidence for the effectiveness of both lifestyle changes (Reference Tuomilehto, Lindstrom and ErikssonTuomilehto et al, 2001; Reference Knowler, Barrett-Connor and FowlerKnowler et al, 2002) and pharmacological agents (Reference Chiasson, Josse and GomisChiasson et al, 2002; Reference Knowler, Barrett-Connor and FowlerKnowler et al, 2002) as a means of preventing or delaying type 2 diabetes, currently patients with impaired glucose tolerance are usually just given lifestyle advice. Blood glucose monitoring and measurement of HbA1c are also routinely undertaken in most of these patients.
Pragmatic pathways of care in individuals with schizophrenia
All health care professionals should be aware that people with schizophrenia are at high risk of developing diabetes, and timely screening and effective risk management should be a priority for all these individuals. Well-defined responsibilities and good communication between mental health teams, primary care teams and diabetes specialist services will be required to reduce the substantial burden of diabetes in people with schizophrenia.
Mental health teams should take some responsibility for managing all general health issues in their patients. This should include screening for diabetes, providing education about healthy living, and involving other specialist or primary care services when necessary (Fig. 1). Mental health specialists should also be aware of local guidelines for the screening and management of diabetes, and if screening programmes do not exist locally, they should consider establishing their own programmes in consultation with other stakeholders.
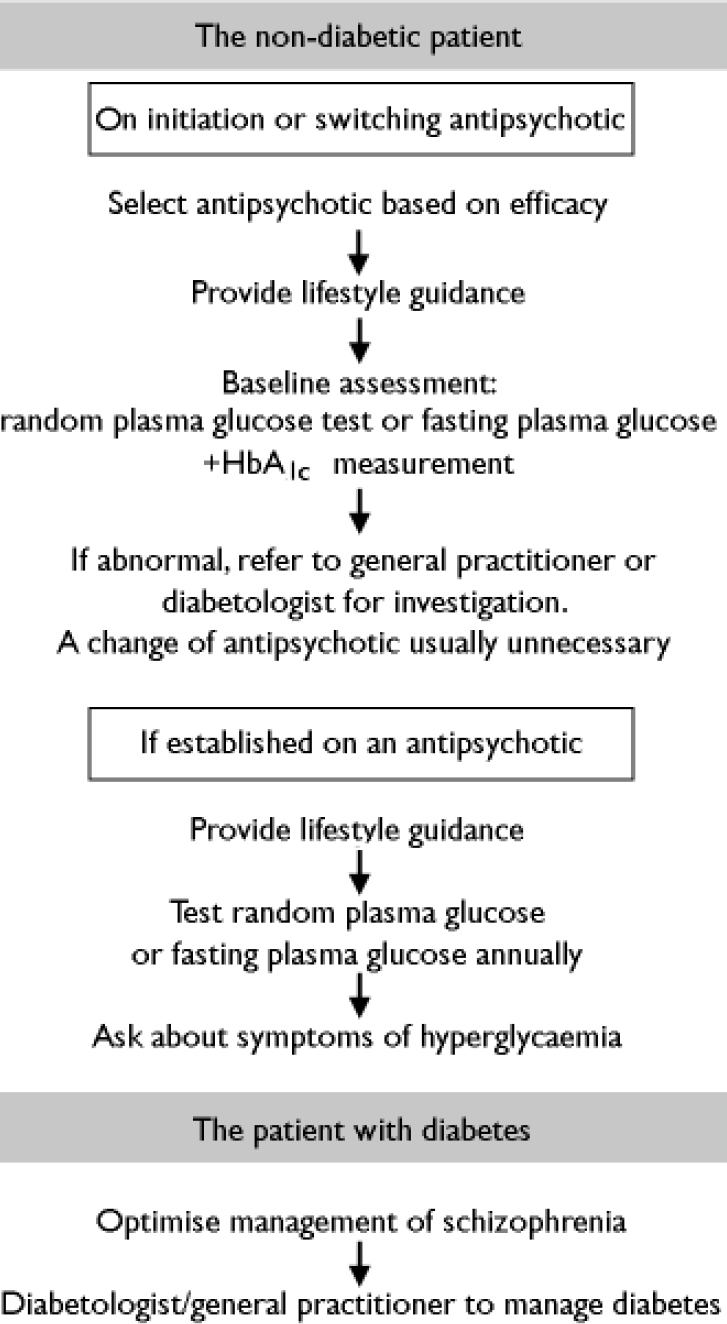
Fig. 1 Pragmatic pathways for managing diabetes risk in people with schizophrenia.
All patients at risk of developing diabetes – especially those with schizophrenia or other serious mental illness – should receive lifestyle advice, including attention to diet and exercise. Patients should be encouraged to decrease their energy and fat intake, to increase their intake of fruit and vegetables, and to exercise for at least 10–15 min per day. Patients taking antipsychotic medications should be given clear guidance on how to avoid side-effects such as weight gain, and should be screened regularly to detect any glucose abnormalities.
Patient involvement in their own diabetes management is vital, and is supported by Standard 3 of National Service Framework for Diabetes (Department of Health, 2002). However, patients with severe mental illness may require additional support to enable them to change deeply entrenched unhealthy behaviours, and to implement the dietary and lifestyle advice necessary to reduce their risk of diabetes and its complications.
Patients with schizophrenia need to understand the benefits of a healthy lifestyle to their overall well-being and future health. Physicians need to understand how their patients live their lives, and whose help they can enlist to change their patients’ daily habits and to support the patient through any lifestyle adjustment. Motivating patients to take an interest in their own health may be a challenge – especially when dealing with a relatively asymptomatic condition such as diabetes. Pragmatic pathways to care, aimed at reducing the risk of diabetes and preventing disease progression in people with schizophrenia, will depend primarily on the individual's circumstances. Recommended pathways for care in the most commonly encountered circumstances are outlined below.
The non-diabetic patient with schizophrenia
On the basis of guidelines outlined for the general population, it would seem sensible that all individuals with schizophrenia are screened for diabetes on a regular basis. For the many reasons outlined above, it would be impractical to suggest the use of the OGTT or the fasting plasma glucose test to screen for diabetes in the population of people with schizophrenia, and we therefore recommend an annual random plasma glucose test as being the most appropriate screening method in this group.
Enquiring about the symptoms of diabetes is an additional and straightforward way to identify patients requiring further investigation. Simple questions (‘Do you find yourself getting thirsty?’ ‘Have you noticed an increase in the amount you drink?’ ‘Do you get up to pass water at night?’ ‘Do you need to take a drink to bed?’) may be useful to identify some patients with diabetes.
For drug-naïve patients starting an antipsychotic treatment or changing antipsychotic agent, it may be useful to perform a baseline random or fasting plasma glucose test and an HbA1c test. These should be repeated after 4 months of treatment in order to identify any changes resulting from the antipsychotic treatment. If the 4-month tests are normal, a random or fasting plasma glucose test should be repeated annually. The results of all tests not undertaken by the primary care team should be reported back to them.
Patients already established on antipsychotic medication should have annual random or fasting blood glucose levels assessed and should be asked routinely about any symptoms of hyperglycaemia. The results of all tests not undertaken by the primary care team should be reported back to them.
If any of the screening tests in a nondiabetic patient with schizophrenia suggest the presence of diabetes or impaired glucose tolerance, the patient should be referred to their general practitioner or a diabetes specialist for a formal diagnosis. If the diagnosis is positive, the general practitioner and/or diabetologist should coordinate the future management of diabetes and ensure that the patient is reviewed regularly. The decision to continue, stop or change antipsychotic medication at this stage should be taken only after consultation between the psychiatrist and diabetologist or general practitioner, and only after careful consideration of the benefits of the antipsychotic agent. The primary goal is always to ensure best control of the mental illness; a change in antipsychotic medication might have adverse consequences for the mental health of the patient, and would not normally be necessary.
The patient with diabetes and schizophrenia
For patients with both diabetes and schizophrenia, good control of the mental illness is also the priority. There are strong arguments to support the use of antipsychotic agents in people with diabetes in order to aid compliance with both dietary and lifestyle advice and any drug treatment needed to manage the diabetes. Yu et al (Reference Yu, Ortiz and Chong2002) evaluated 22 patients with diabetes beginning atypical antipsychotic therapy, and found that blood glucose levels actually improved in a significant number. Lindenmayer et al (Reference Lindenmayer, Czobor and Volavka2003) reported similar findings in seven patients, and a recent case report by Green (Reference Green2003) drew similar conclusions. It would therefore seem appropriate that choice of medication for schizophrenia should be based purely upon efficacy, and not be influenced by potential concerns over glucose metabolism (Reference Citrome and VolavkaCitrome & Volavka, 2002).
Patients with diabetes who are already established on antipsychotic medication should be under the care of a diabetologist or general practitioner and, as for all other patients with diabetes, should be receiving annual checks for metabolic control and screening for complications. Patients should be asked to continue with their usual home blood glucose monitoring and should inform their general practitioner/practice nurse or diabetes specialist nurse should there be any change in their glucose readings. Ideally, the mental health team managing the schizophrenia should be kept informed of any issues or concerns relating to glucose control.
DISCUSSION
Patients with schizophrenia are at increased risk of developing type 2 diabetes as a result of an independent interaction between genetic and environmental factors. For this reason, all patients with schizophrenia should be managed as a high-risk group for the development of diabetes or impaired glucose tolerance and regularly screened according to local guidelines. Achieving good control of the mental illness remains the treatment priority, and antipsychotic treatments should be selected on the basis of their efficacy.
Good communication between mental health teams, primary care services and diabetes specialists will be the key to reducing the inherent risk of developing diabetes in individuals with schizophrenia. Although it is important for the psychiatrist to take responsibility for reducing that risk, the diabetologist and/or general practitioner should be responsible for managing diabetes in patients with schizophrenia affected by the condition.
APPENDIX
Risk factors for developing type 2 diabetes (adapted from Diabetes UK, 2002 )
Membership of a high-risk group for the development of diabetes
Membership of a White (>40 years of age) or Black, Asian or other minority ethnic groups (>25 years of age), with one of the following:
-
(a) first-degree relative with diabetes;
-
(b) overweight (body mass index >25 kg/m2) plus sedentary lifestyle;
-
(c) ischaemic heart disease, cerebrovascular disease, peripheral vascular disease or hypertension.
Gestational diabetes
Polycystic ovary syndrome and overweight
Impaired glucose tolerance or impaired fasting glycaemia
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Patients with schizophrenia are at high risk of developing diabetes and should be screened annually.
-
▪ Psychiatrists should be responsible for reducing the risk of diabetes in their patients with schizophrenia by offering advice on healthy living and checking for symptoms of hyperglycaemia.
-
▪ Effective management of schizophrenia remains the priority, but careful diabetes risk management should reduce the burden of diabetes in this high-risk group.
LIMITATIONS
-
▪ Pragmatic pathways of care to reduce the risk of diabetes in people with schizophrenia have not been developed previously and may be hard to implement locally.
-
▪ Cost and workload issues could limit the success of proposed screening programmes in patients with schizophrenia.
-
▪ Poorly defined responsibilities and complex communication channels may preclude the multidisciplinary approach needed to manage diabetes risk successfully in patients with schizophrenia.





eLetters
No eLetters have been published for this article.