Recent studies (Reference Bassuk, Berkman and WypijBassuk et al, 1998; Reference Chen, Ganguli and MulsantChen et al, 1999; Reference Yaffe, Blackwell and GoreYaffe et al, 1999) demonstrate the growing interest in whether depression in dementia may be a cause or a consequence of the dementia process. In Reference Devanand, Sano and Tang1996, Devanand et al reported that depressed mood increased the risk of Alzheimer's disease; in this study, depressed mood was more common in subjects with greater cognitive impairment at baseline, making them more likely to develop Alzheimer's disease. Results of other prospective studies are inconsistent. Dufouil et al (Reference Dufouil, Fuhrer and Dartigues1996) and Henderson et al (Reference Henderson, Korten and Jacomb1997) did not find a relationship between depression and subsequent cognitive decline, whereas Bassuk et al (Reference Bassuk, Berkman and Wypij1998) and Chen et al (Reference Chen, Ganguli and Mulsant1999) did find such a relationship, but only in subjects in whom cognitive impairment was already apparent. In order to study the nature of the relationship between depression and cognitive decline/dementia further, we investigated whether depressed elderly individuals with normal baseline cognition were at increased risk of cognitive decline and Alzheimer's disease. We used data from two cohorts of elderly people living in the community.
METHODS
Baseline samples
Data on incident Alzheimer's disease were obtained from the Amsterdam Study of the Elderly (AMSTEL), whereas data on cognitive decline were obtained from the Longitudinal Aging Study Amsterdam (LASA). Approval was obtained from the Medical Ethics Committee for both studies. The sampling procedures have been described elsewhere (Reference Launer, Dinkgreve and JonkerLauner et al, 1993; Reference Deeg, Knipscheer and Van TilburgDeeg et al, 1993; Reference Beekman, Deeg and Van TilburgBeekman et al, 1995). In brief, for the AMSTEL study 5666 non-institutionalised elderly people, aged 65-84 years, were selected from 30 general practices across Amsterdam. Within each practice a fixed proportion of people was randomly selected from each of four 5-year age strata (65-69 years to 80-84 years) to obtain equal-sized strata; 4051 people (71.5%) gave their written consent and participated in the study. The LASA cohort is based on random, gender- and agestratified (six 5-year age strata from 55 to 84 years) samples drawn from 11 municipal population registries (both urban and rural) in three regions of The Netherlands. The baseline sample of the LASA cohort consists of 3107 people.
Baseline measurements and study samples
AMSTEL
In 1990-1991 trained lay people interviewed all 4051 participants at home. The interview comprised the Dutch version of the Geriatric Mental State Schedule (GMSS) (Reference Copeland, Kelleher and KellettCopeland et al, 1976; Reference Gurland, Fleiss and GoldbergGurland et al, 1976; Reference Hooijer, Jonker and DeweyHooijer et al, 1991), questions on socio-demographic characteristics, current health status, medical history, and four mental status tests, including the Mini-Mental State Examination (MMSE) (Reference Folstein, Folstein and McHughFolstein et al, 1975). Depression was measured using the GMSS, in conjunction with its computerised diagnostic system AGECAT (Reference Dewey and CopelandDewey & Copeland, 1986). The GMSS is a structured interview aimed at identifying various psychiatric disorders, which has been specifically designed for use with elderly individuals. AGECAT consists of the application of hierarchical rules to the items of the GMSS in order to reach a diagnosis for various psychiatric disorders (e.g. depression and dementia syndromes) with different levels of confidence. The presence and absence of depression were indicated by GMS-AGECAT depression syndrome levels 3-6 and 0-2, respectively.
To select a study sample with normal cognition, a cohort without dementia was first selected by excluding from the baseline sample all subjects (n=273) with a DSM-III-R dementia diagnosis (American Psychiatric Association, 1987) (for a more detailed description see Reference Jonker, Schmand and LindeboomJonker et al, 1998) or a GMS-AGECAT dementia diagnosis (i.e. organic illness syndrome levels of 3-5). Second, from this cohort without dementia all subjects with sub-threshold levels of dementia were excluded: that is, with GMS-AGECAT dementia levels of 1-2 or MMSE scores of 25 and below (n=631). In all, 904 subjects were excluded, resulting in a study sample of 3147 non-demented subjects with normal cognition (i.e. MMSE scores of 26-30 and GMS-AGECAT organic illness score 0).
LASA
In 1992-1993, trained lay people interviewed all 3107 participants at home. Cognitive functioning was measured with the MMSE. Depressive symptoms were measured with the Dutch version of the Center for Epidemiologic Studies Depression Scale (CES-D) (Reference Beekman, Deeg and Van LimbeekBeekman et al, 1997). This is a 20-item self-report scale developed to measure current depressive symptoms in the community. The CES-D generates a total score which can range from 0 to 60. In order to identify respondents with clinically relevant levels of depression, the generally used cut-off score of ≥16 was also used. To minimise overlap between affective symptoms and symptoms possibly due to physical illness, analyses were also performed using a sub-scale constructed of seven items of the CES-D reflecting negative affect (bothered, blues, depressed, fearful, lonely, cried and sad) (Reference Radloff and TeriRadloff & Teri, 1986). Scores on the negative affect subscale range from 0 to 20 points, with higher scores indicating greater severity.
For the present study, a sample with normal cognition was selected by excluding all subjects of the baseline sample with MMSE scores below 26, resulting in a study sample of 2399 people.
Follow-up measurements
AMSTEL
At follow-up in 1994, all the subjects who were available were interviewed again by trained lay people, using the same interview procedure as in 1990-91. A subsample of subjects who were suspected of having developed dementia were invited for diagnostic evaluation. The screening procedure and diagnostic evaluation have been described elsewhere (Reference Geerlings, Jonker and BouterGeerlings et al, 1999). In brief, all subjects with MMSE scores of 23 or less, or with impairment in orientation in time, recent memory or learning, were invited for diagnostic evaluation. During home visits, physicians specifically trained for this purpose administered the Cambridge Examination for Mental Disorders in the Elderly (including a structured psychiatric interview, the Cambridge Cognitive Examination, and a physical examination) (Reference Roth, Huppert and TymRoth et al, 1988). Clinical diagnoses of Alzheimer's disease were made according to DSM-IV criteria (American Psychiatric Association, 1994). Diagnoses were determined during weekly meetings with the senior neurologist (C.J.) and the neuropsychologist (M.I.G.).
LASA
At follow-up in 1995-1996, all the respondents who were available were interviewed again during home visits, using the same interview procedure as was used at baseline.
Statistical analyses
AMSTEL
Multiple logistic regression analyses were performed to assess the effect of depression on incident Alzheimer's disease. Covariates used in the analyses were age, gender, level of education, memory complaints and psychiatric history. Level of education was expressed as the number of full-time years of education needed to obtain the highest grade of education completed. In the analyses, it was used both as a continuous and as a dichotomous variable (dichotomised at the median of the study sample into ≤8 years v. > 8 years of education; 8 years of education is comparable with 2 years of secondary education after having completed primary school). Memory complaints were assessed by the question: “Do you have complaints about your memory?” Answers were coded “yes” or “no”. Psychiatric history was assessed by the question: “Have you ever had emotional or nervous illness requiring treatment?” If answered positively, age of onset was assessed. Psychiatric history was categorised as no history, or first onset at age 60 or above, v. a psychiatric history before age 60.
First, the analyses were performed, with adjustments for the potential confounders. Second, since the strength of the association between depression and incident Alzheimer's disease may be different for people with different demographic characteristics and for those with or without memory complaints or psychiatric history, we also tested possible interactions between depression and age, gender, education, memory complaints and psychiatric history, respectively.
LASA
Multiple logistic regression analyses were performed to assess the effect of depressive symptoms on cognitive decline. Cognitive decline was defined as a drop of 3 or more points (>1 standard deviation) in the MMSE at follow-up. Depressive symptoms were used in the analyses as a continuous variable (total CES-D score, and negative affect score), as a dichotomised variable (CES-D ≥16 v. <16), and as a categorical variable (the core depression item from the CES-D (“during the past week I felt depressed”) was used to examine the association between mild depressed mood (“some of the time” v. “no”) and incident cognitive decline and a severe depressed mood (“often/always” v. “no”) and incident cognitive decline, respectively. The same covariates as in the AMSTEL sample were used. However, in LASA, data on psychiatric history were not collected and were not therefore used in the analyses of the LASA data. Interactions between the different definitions of depression and the covariates were also tested. Finally, to correct for possible differences in baseline MMSE scores between the two groups with different educational levels, we also performed the analyses with additional adjustments for baseline MMSE score (range 26-30).
RESULTS
AMSTEL
Table 1 shows the baseline characteristics of the AMSTEL study sample (n=3147), according to the presence or absence of depression. The follow-up lasted 3.2 years, on average. Figure 1 shows the numbers of people who were available for follow-up and who were lost to follow-up. At follow-up, 53 people received a diagnosis of Alzheimer's disease.
Table 1 Baseline characteristics of the Amsterdam Study of the Elderly study sample (n=3147), according to the presence or absence of depression

| Depression (n=329) | No depression (n=2818) | P | |
|---|---|---|---|
| Mean age (s.d.), years | 73.6 (5.7) | 73.7 (5.7) | 0.821 |
| Gender | |||
| Male | 62 (18.8%) | 1156 (41.0%) | |
| Female | 267 (81.2%) | 1662 (59.0%) | <0.0012 |
| Mean (s.d.) years of education | 8.0 (2.3) | 8.5 (2.6) | <0.0013 |
| Memory complaints | |||
| No | 263 (80.2%) | 2563 (91.0%) | |
| Yes | 65 (19.8%) | 253 (9.0%) | <0.0012 |
| Psychiatric history | |||
| No/late onset | 261 (79.6%) | 2527 (89.8%) | |
| Early onset | 67 (20.4%) | 286 (10.2%) | <0.0012 |
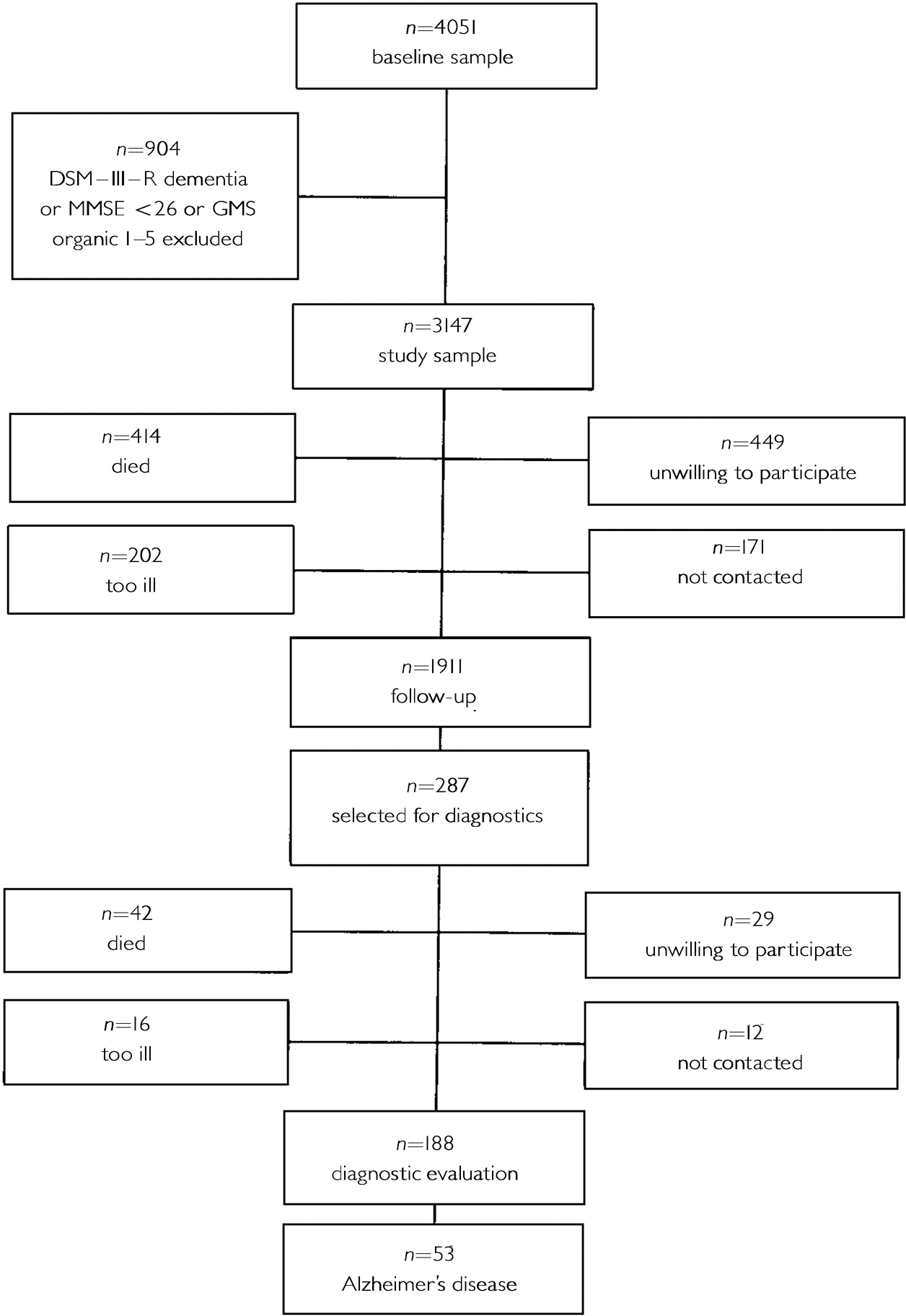
Fig. 1 Amsterdam Study of the Elderly sample; numbers available and not available for follow-up.
Table 2 shows the baseline characteristics for those who were and were not available for follow-up.
Table 2 Baseline characteristics of the Amsterdam Study of the Elderly sample (n=3147) for subjects who were and were not available for follow-up in 1994
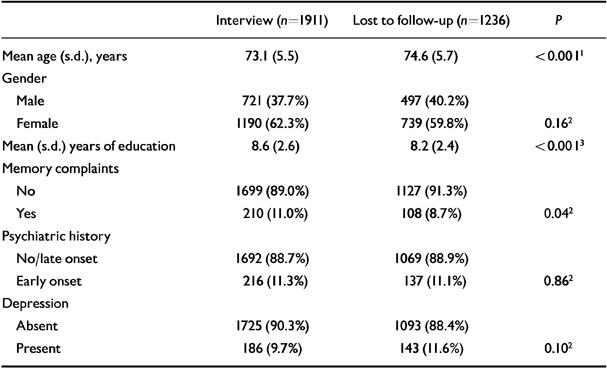
| Interview (n=1911) | Lost to follow-up (n=1236) | P | |
|---|---|---|---|
| Mean age (s.d.), years | 73.1 (5.5) | 74.6 (5.7) | < 0.0011 |
| Gender | |||
| Male | 721 (37.7%) | 497 (40.2%) | |
| Female | 1190 (62.3%) | 739 (59.8%) | 0.162 |
| Mean (s.d.) years of education | 8.6 (2.6) | 8.2 (2.4) | < 0.0013 |
| Memory complaints | |||
| No | 1699 (89.0%) | 1127 (91.3%) | |
| Yes | 210 (11.0%) | 108 (8.7%) | 0.042 |
| Psychiatric history | |||
| No/late onset | 1692 (88.7%) | 1069 (88.9%) | |
| Early onset | 216 (11.3%) | 137 (11.1%) | 0.862 |
| Depression | |||
| Absent | 1725 (90.3%) | 1093 (88.4%) | |
| Present | 186 (9.7%) | 143 (11.6%) | 0.102 |
Table 3 shows the crude and adjusted odds ratios of incident Alzheimer's disease associated with depression, age, gender, education, memory complaints and psychiatric history. Depression moderately increased the risk of Alzheimer's disease. In the multivariate model, with adjustments for all other variables, the odds ratio of Alzheimer's disease associated with depression decreased towards a statistically nonsignificant level.
Table 3 Crude and adjusted odds ratios (ORs), with corresponding 95% confidence intervals (Cls), for the association between depression, age, gender, education, memory complaints, psychiatric history and incident Alzheimer's disease (Amsterdam Study of the Elderly)
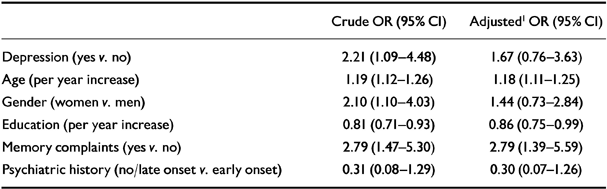
| Crude OR (95% CI) | Adjusted1 OR (95% CI) | |
|---|---|---|
| Depression (yes v. no) | 2.21 (1.09-4.48) | 1.67 (0.76-3.63) |
| Age (per year increase) | 1.19 (1.12-1.26) | 1.18 (1.11-1.25) |
| Gender (women v. men) | 2.10 (1.10-4.03) | 1.44 (0.73-2.84) |
| Education (per year increase) | 0.81 (0.71-0.93) | 0.86 (0.75-0.99) |
| Memory complaints (yes v. no) | 2.79 (1.47-5.30) | 2.79 (1.39-5.59) |
| Psychiatric history (no/late onset v. early onset) | 0.31 (0.08-1.29) | 0.30 (0.07-1.26) |
Interaction terms added to the model containing all covariates showed that the association between depression and Alzheimer's disease was modified by education (centred variable) (likelihood ratio test, P=0.023). The interaction term was also statistically significant when education was used as a dichotomous variable (P=0.004). There were no statistically significant interactions between depression and memory complaints or between depression and psychiatric history, nor were there between depression and age or gender.
To interpret the modifying effect of education on the association between depression and incident Alzheimer's disease, we performed logistic regression analyses within two groups having had education to different levels (dichotomised at the median value of the study sample). Depression greatly increased the risk of Alzheimer's disease among subjects with more than 8 years of education, but not among subjects with 8 years of education or less (Table 4).
Table 4 Odds ratios (95% CI) adjusted for all other variables of the association between depression, age, gender, memory complaints and psychiatric history and incident Alzheimer's disease, for subjects with ≤8 years, and > 8 years of education, respectively (Amsterday Study of the Elderly)
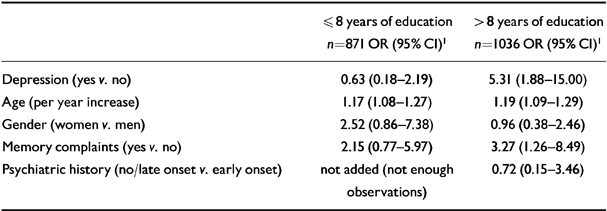
| ≤ 8 years of education n=871 OR (95% CI)1 | > 8 years of education n=1036 OR (95% CI)1 | |
|---|---|---|
| Depression (yes v. no) | 0.63 (0.18-2.19) | 5.31 (1.88-15.00) |
| Age (per year increase) | 1.17 (1.08-1.27) | 1.19 (1.09-1.29) |
| Gender (women v. men) | 2.52 (0.86-7.38) | 0.96 (0.38-2.46) |
| Memory complaints (yes v. no) | 2.15 (0.77-5.97) | 3.27 (1.26-8.49) |
| Psychiatric history (no/late onset v. early onset) | not added (not enough observations) | 0.72 (0.15-3.46) |
LASA
Table 5 shows the baseline characteristics of the LASA study sample (n=2399) according to the presence or absence of depression. The duration of follow-up averaged 3.1 years. Figure 2 shows the numbers of people who were available for follow-up and who were lost to follow-up.
Table 5 Baseline characteristics of the Longitudinal Aging Study Amsterdam study sample (n=2399), according to the presence or absence of depressive symptoms
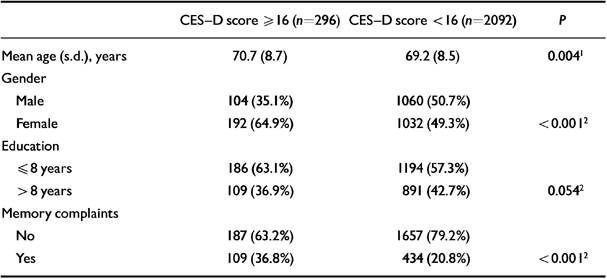
| CES-D score ≥ 16 (n=296) | CES-D score < 16 (n=2092) | P | |
|---|---|---|---|
| Mean age (s.d.), years | 70.7 (8.7) | 69.2 (8.5) | 0.0041 |
| Gender | |||
| Male | 104 (35.1%) | 1060 (50.7%) | |
| Female | 192 (64.9%) | 1032 (49.3%) | < 0.0012 |
| Education | |||
| ≤ 8 years | 186 (63.1%) | 1194 (57.3%) | |
| > 8 years | 109 (36.9%) | 891 (42.7%) | 0.0542 |
| Memory complaints | |||
| No | 187 (63.2%) | 1657 (79.2%) | |
| Yes | 109 (36.8%) | 434 (20.8%) | < 0.0012 |
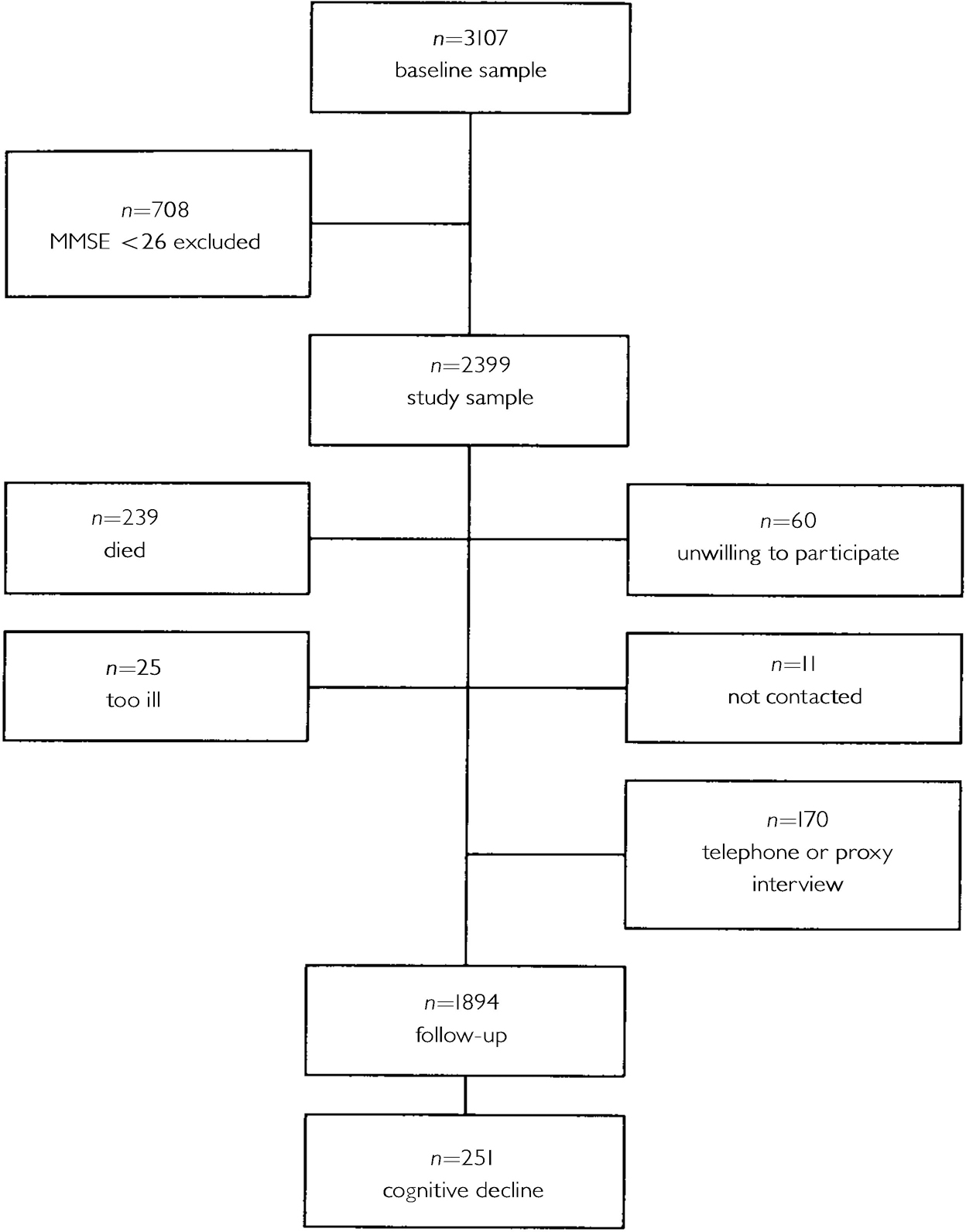
Fig. 2 Longitudinal Aging Study Amsterdam sample; numbers available and not available for follow-up.
Table 6 shows the baseline characteristics for subjects who were and were not available for follow-up. At follow-up, 251 people showed a drop of three or more points on the MMSE.
Table 6 Baseline characteristics of the Longitudinal Aging Study Amsterdam sample (n=2399) for subjects who were and were not available for follow-up
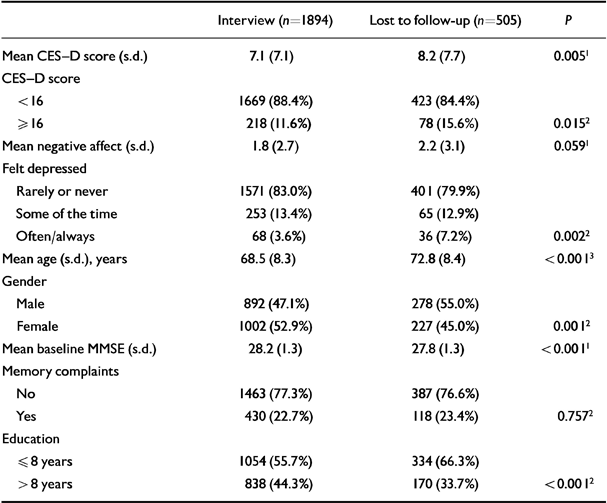
| Interview (n=1894) | Lost to follow-up (n=505) | P | |
|---|---|---|---|
| Mean CES-D score (s.d.) | 7.1 (7.1) | 8.2 (7.7) | 0.0051 |
| CES-D score | |||
| < 16 | 1669 (88.4%) | 423 (84.4%) | |
| ≥ 16 | 218 (11.6%) | 78 (15.6%) | 0.0152 |
| Mean negative affect (s.d.) | 1.8 (2.7) | 2.2 (3.1) | 0.0591 |
| Felt depressed | |||
| Rarely or never | 1571 (83.0%) | 401 (79.9%) | |
| Some of the time | 253 (13.4%) | 65 (12.9%) | |
| Often/always | 68 (3.6%) | 36 (7.2%) | 0.0022 |
| Mean age (s.d.), years | 68.5 (8.3) | 72.8 (8.4) | < 0.0013 |
| Gender | |||
| Male | 892 (47.1%) | 278 (55.0%) | |
| Female | 1002 (52.9%) | 227 (45.0%) | 0.0012 |
| Mean baseline MMSE (s.d.) | 28.2 (1.3) | 27.8 (1.3) | < 0.0011 |
| Memory complaints | |||
| No | 1463 (77.3%) | 387 (76.6%) | |
| Yes | 430 (22.7%) | 118 (23.4%) | 0.7572 |
| Education | |||
| ≤ 8 years | 1054 (55.7%) | 334 (66.3%) | |
| > 8 years | 838 (44.3%) | 170 (33.7%) | < 0.0012 |
Multiple regression analyses adjusting for age, gender, education and memory complaints showed that neither higher scores on the continuous CES-D nor CES-D scores of ≥16 were associated with subsequent cognitive decline (adjusted odds ratio (OR)=1.01; 95% confidence interval (CI)=0.99-1.03 and OR=1.07; 95% CI=0.70-1.62, respectively). However, negative affect increased the risk of cognitive decline (adjusted OR per point increase=1.05; 95% CI=1.00-1.09). A severe depressed mood was also associated with subsequent cognitive decline (adjusted OR=1.97; 95% CI=1.09-3.56), but a mild depressed mood was not (OR=1.05; 95% CI=0.71-1.57).
When the interaction between CES-D (centred variable) and education (dichotomous variable) was entered into the model containing CES-D (as a continuous variable), age, gender, education and memory complaints, the association between depressive symptoms and cognitive decline was modified by education (P=0.012). A similar result was found when the dichotomised variable was used in the interaction term (P=0.047) and when negative affect was used in the interaction term (P=0.017). Finally, the interaction between a severe depressed mood and education was of borderline significance (P=0.076), but the interaction between mild depressed mood and education was not (P=0.74). No significant interactions between depressive symptoms and one of the other covariates was found.
Logistic regression analyses stratified by level of education showed that depressive symptoms were associated with cognitive decline among subjects with more than 8 years of education, but not among those with 8 years of education or less (Table 7). Although the association between CES-D scores of ≥16 and cognitive decline was attenuated after adjusting for the possible confounders, higher scores on the continuous CES-D, higher negative affect scores and a severe depressed mood remained predictive of subsequent cognitive decline (Table 7). Among less-educated subjects, no association between depressive symptoms and cognitive decline was found, regardless of the definition of depressive symptoms. When the multivariate analyses were performed with additional adjustments for baseline MMSE score, the results were very similar.
Table 7 Associations (odds ratios with 95% confidence intervals) between four definitions of depressive symptoms and subsequent cognitive decline, according to level of education (Longitudinal Aging Study Amsterdam)
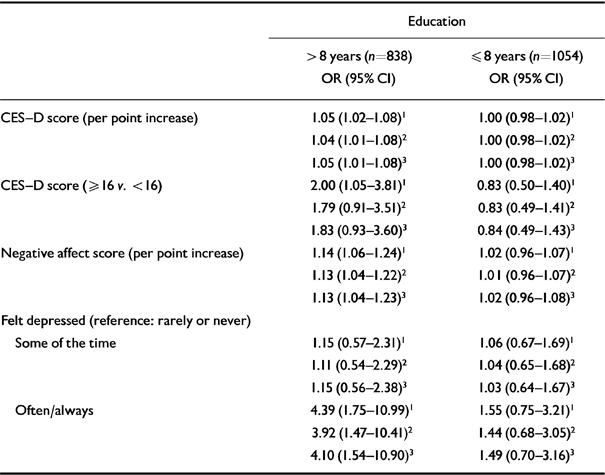
| Education | ||
|---|---|---|
| > 8 years (n=838) OR (95% CI) | ≤ 8 years (n=1054) OR (95% CI) | |
| CES-D score (per point increase) | 1.05 (1.02-1.08)1 | 1.00 (0.98-1.02)1 |
| 1.04 (1.01-1.08)2 | 1.00 (0.98-1.02)2 | |
| 1.05 (1.01-1.08)3 | 1.00 (0.98-1.02)3 | |
| CES-D score (≥ 16 v. < 16) | 2.00 (1.05-3.81)1 | 0.83 (0.50-1.40)1 |
| 1.79 (0.91-3.51)2 | 0.83 (0.49-1.41)2 | |
| 1.83 (0.93-3.60)3 | 0.84 (0.49-1.43)3 | |
| Negative affect score (per point increase) | 1.14 (1.06-1.24)1 | 1.02 (0.96-1.07)1 |
| 1.13 (1.04-1.22)2 | 1.01 (0.96-1.07)2 | |
| 1.13 (1.04-1.23)3 | 1.02 (0.96-1.08)3 | |
| Felt depressed (reference: rarely or never) | ||
| Some of the time | 1.15 (0.57-2.31)1 | 1.06 (0.67-1.69)1 |
| 1.11 (0.54-2.29)2 | 1.04 (0.65-1.68)2 | |
| 1.15 (0.56-2.38)3 | 1.03 (0.64-1.67)3 | |
| Often/always | 4.39 (1.75-10.99)1 | 1.55 (0.75-3.21)1 |
| 3.92 (1.47-10.41)2 | 1.44 (0.68-3.05)2 | |
| 4.10 (1.54-10.90)3 | 1.49 (0.70-3.16)3 | |
DISCUSSION
We investigated whether depression in older people with normal cognition was associated with subsequent cognitive decline and incident Alzheimer's disease. The findings in both AMSTEL and LASA showed that depression increased the risk of Alzheimer's disease and cognitive decline, respectively, but only among people with higher levels of education.
Depression as a psychological reaction
Most commonly, two explanations have been suggested for the frequent occurrence of depression in Alzheimer's disease patients. First, depression may be a psychological reaction to perceived cognitive decline (Reference Migliorelli, Teson and SabeMigliorelli et al, 1995) that may particularly occur in mild stages of Alzheimer's disease, when patients may still be aware of their failing cognitive capacities (Reference Ott and FogelOtt et al, 1992). Although this explanation implies that depression is a consequence of the dementia process, depressive symptoms may become apparent before the diagnosis of Alzheimer's disease is made. The development of the illness is a gradual process that may start long before the diagnostic criteria are met, and patients may notice these changes in cognition at an early stage of the disease, and develop depressive symptoms as a reaction. However, although the prevalence of depression has been found to be inversely related to the severity of dementia (Reference Fischer, Simanyi and DanielczykFischer et al, 1990), others found no association between depression and insight into cognitive deficits (Reference Verhey, Rozendaal and PondsVerhey et al, 1993; Reference Cummings, Ross and AbsherCummings et al, 1995), suggesting that the association is not purely a psychological one. Moreover, if the explanation were psychological, the association between depression and incident Alzheimer's disease would be modified by memory complaints, since these may reflect realistic self-observation of cognitive decline (Reference Geerlings, Jonker and BouterGeerlings et al, 1999). Although memory complaints were more prevalent among depressed subjects than among non-depressed subjects, we did not find that the association between depression and incident Alzheimer's disease was stronger among those with memory complaints. Finally, a psychological mechanism does not explain the observed interaction between depression and education.
Depression as an early symptom of Alzheimer's disease
Our data may agree better with the second explanation, which hypothesises that depression is an early symptom of the neuropathological process of Alzheimer's disease. Studies on neurochemical and neuropathological changes in the brain of people with Alzheimer's disease suggest either that they may have lower thresholds for depressive disorders, or that the dementia process contributes to the development of depression (Reference Zubenko and MoossyZubenko & Moossy, 1988; Reference Zweig, Ross and HedreenZweig et al, 1988; Reference Förstl, Burns and LuthertFörstl et al, 1992). It could be that depression as an early symptom or a subclinical expression of Alzheimer's disease may have become more overt in more highly educated people, who may have greater ‘reserve capacity’. This may be a ‘brain reserve’, reflected in a greater number of large neurons, greater brain weight or increased neocortical synaptic density (Reference KatzmanKatzman, 1993), or a ‘cognitive reserve’, reflected in greater intellectual capacity or coping skills more adequate to deal with the dementia process (Reference Stern, Gurland and TatemichiStern et al, 1994). Several studies suggest that the neuropathological process of Alzheimer's disease may be more advanced in elderly people with higher levels of educational and occupational attainment than in those with lower levels (Stern et al, Reference Stern, Alexander and Prohovnik1995a , Reference Stern, Tang and Denaro b ), despite similar clinical severity. Thus, it is possible that in people with greater (cognitive) reserve the cognitive symptoms of Alzheimer's disease may be delayed, but not the depressive symptoms of the disease.
In the LASA sample, the association between depressive symptoms and cognitive decline became stronger when the analyses were restricted to affective symptoms and to a more severe form of these symptoms. The observation that a severe depressed mood was strongly associated with subsequent cognitive decline may also support the hypothesis that depression is an early manifestation of a dementia process. A severe depressed mood is a more specific indicator of major depression (Reference Meyers and BruceMeyers & Bruce, 1998), and this could indicate some biological relationship rather than a purely psychological one (Reference Migliorelli, Teson and SabeMigliorelli et al, 1995).
Psychiatric history
In the AMSTEL sample, no modifying effect of psychiatric history on the association between depression and Alzheimer's disease was observed, as might have been expected if late-onset depression were associated with organic illness (Reference Alexopoulos, Young and MeyersAlexopoulos et al, 1988). However, there may not have been enough observations in the subgroups for the interaction term to be statistically significant, since at baseline the majority (80%) of depressed people reported no psychiatric history or a late-onset history. This finding in itself suggests that the depression that we observed in our study sample is indicative of a dementia process.
LASA and AMSTEL
The most notable strength of this study was that we were able to analyse data from two independent samples. Both the AMSTEL and the LASA studies are prospective community-based studies in The Netherlands; both cohorts contain elderly people who were interviewed at home, and the follow-up interval between the first and the second assessment was very similar in both studies. An important difference is that the samples were drawn independently of one another and for different purposes. Moreover, depression and cognitive decline or dementia were assessed using different instruments. In the AMSTEL study, depression was assessed with the GMSS, which is an instrument based on the British tradition, while the CES-D, used in the LASA study, is more widely used in the USA. Furthermore, although both instruments measure a broad spectrum of depressive symptoms, the CES-D contains fewer items than the GMSS and does not rely on the clinical judgement of the interviewer. With respect to the outcome variable, the differences between a clinical diagnosis of Alzheimer's disease and cognitive decline based on a subtraction score on the MMSE may be even greater. In view of these differences in both determinant and outcome, it is striking that the results of both the LASA and the AMSTEL studies demonstrated that depression increased the risk of cognitive decline and Alzheimer's disease only among subjects with higher levels of education.
Loss to follow-up
We have to consider that our findings may be biased because of differential loss to follow-up. Particularly in the AMSTEL sample, the proportion of subjects lost to follow-up was considerable. Although depressed people were not more likely to be lost to follow-up in this sample, in the LASA sample depressed subjects did become lost more often. However, we consider it unlikely that in the LASA sample the great difference in odds ratios between the two groups with different levels of education for those with a severe depressed mood is explained by differential loss to follow-up alone.
Highly educated elderly at greater risk
We observed in two independent community-based samples that depressed elderly people with normal cognition and higher levels of education were at increased risk of incident cognitive decline and Alzheimer's disease. The data do not support the hypothesis that depression is primarily a psychological reaction to deteriorating cognitive capacities. Instead, they support the hypothesis that depression is a subclinical expression or an early symptom of an underlying dementia process, which may become apparent in a subgroup of more highly educated older people in whom commonly used mental status tests do not yet detect a decline from a previous level of cognitive functioning. The data do not provide proof of possible pathogenetic mechanisms, however, and it remains to be determined whether treatment of depression would result in a delay of the clinical expression of cognitive decline and dementia.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Depression, and in particular a severe depressed mood, may be an early or subclinical manifestation of cognitive decline and Alzheimer's disease in elderly people with higher levels of education.
-
▪ In these people, commonly used mental status tests such as the Mini-Mental State Examination are inadequate to detect subtle cognitive decline.
-
▪ The subclinical expression of cognitive decline or dementia may be different for different subgroups of elderly people, according to their educational level.
LIMITATIONS
-
▪ Although our data confirm the clinical impression that late-onset depression is associated with organic illness, it is uncertain whether the question about psychiatric history in the Amsterdam Study of the Elderly study indeed assessed a history of depression.
-
▪ The nature of this study does not permit any inferences to be drawn regarding possible pathogenetic mechanisms that may underlie the observed association between depression and incident Alzheimer's disease.
-
▪ Based on the findings of this study, no suggestions can be made as to whether treatment of depression would result in a delay of the clinical expression of cognitive decline and dementia.
ACKNOWLEDGEMENTS
The Amsterdam Study of the Elderly (AMSTEL) was supported by grants from The Netherlands Health Research Promotion Programme (SGO) and The Netherlands Fund of Mental Health (NFGV). The AGECAT computer program was installed with the kind cooperation and final authorisation of Michael E. Dewey, Department of Psychiatry, University of Liverpool. The Longitudinal Aging Study Amsterdam is financed primarily by the Ministry of Welfare, Health and Sports of The Netherlands.












eLetters
No eLetters have been published for this article.