Neurobiological models of obsessive-compulsive disorder (OCD), a disabling neuropsychiatric disease characterised by intrusive, ego-dystonic obsessive thoughts and compulsive and/or ritualistic behaviours, Reference Salkovskis1 traditionally emphasise corticostriatal brain abnormalities. Reference Saxena, Brody, Schwartz and Baxter2 In support of this assumption, recent resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) studies have identified corticostriatal connectivity abnormalities in patients with OCD. Reference Harrison, Pujol, Cardoner, Deus, Alonso and Lopez-Sola3,Reference Beucke, Sepulcre, Talukdar, Linnman, Zschenderlein and Endrass4 However, consistent evidence of additional alterations in non-corticostriatal regions Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore5 has led to doubts about whether the existing models can fully explain the clinical phenotype of OCD. Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore5 At the same time, clinical applications of rs-fcMRI, testing the integrity of large-scale brain systems such as the default mode, Reference Greicius, Krasnow, Reiss and Menon6 executive control, Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna7 dorsal attention Reference Fox, Corbetta, Snyder, Vincent and Raichle8 and salience Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna7 networks in neurological and psychiatric disorders, have received increasing interest, and recent network models of psychiatric disorders hypothesise abnormalities in default mode, salience and executive control network functioning. Reference Sylvester, Corbetta, Raichle, Rodebaugh, Schlaggar and Sheline9,Reference Menon10 Accordingly, potential abnormalities in the default mode, salience and the executive control networks represent an emerging topic of interest in studies of patients with OCD. Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor and Welsh11-Reference Li, Li, Dong, Luo, Han and Xiong15
Whereas elevated neural responses to errors Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor and Welsh11,Reference Fitzgerald, Welsh, Gehring, Abelson, Himle and Liberzon16 or conflict Reference Marsh, Horga, Parashar, Wang and Peterson17 in components of the salience network such as the cingulate cortex and/or the anterior insula are consistent with the view that the salience network is hyperactive in OCD, Reference Sylvester, Corbetta, Raichle, Rodebaugh, Schlaggar and Sheline9 the extant investigations of default mode network integrity have been conflicting. More precisely, these analyses have either identified connectivity abnormalities within the default mode network, or aberrant connectivity between the default mode network and components of other functional networks. Studies reporting abnormalities within the default mode network include seed-based rs-fcMRI examinations of core components in this network, such as the posterior cingulate cortex and/or anterior medial prefrontal cortex (aMPFC), as well as independent component analysis of experimental fMRI data Reference Cocchi, Harrison, Pujol, Harding, Fornito and Pantelis12 revealing significant connectivity reductions Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor and Welsh11,Reference Cocchi, Harrison, Pujol, Harding, Fornito and Pantelis12 and trends for lower connectivity Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 in this network in patients with OCD. Studies reporting no connectivity abnormalities within the default mode network, but aberrant connectivity between this network and other functional networks include seed-based rs-fcMRI examinations of the posterior cingulate cortex Reference Jang, Kim, Jung, Choi, Jung and Lee13 and the anterior insula Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 revealing reduced connectivity between posterior cingulate cortex and corticostriatal areas, Reference Jang, Kim, Jung, Choi, Jung and Lee13 and greater connectivity between the anterior insula and several default mode network components Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 in patients with OCD. In addition to inter-network abnormalities involving the default mode network, greater connectivity between the anterior prefrontal cortex and insular and midcingulate areas, indicative of stronger connections between the executive control and the salience networks, has been found in rs-fcMRI studies of people with OCD. Reference Li, Li, Dong, Luo, Han and Xiong15
The present study sought to potentially resolve the disparities among existing default mode network findings in OCD through consideration of recent evidence that this network comprises a midline core constituted by hub regions in the anterior medial prefrontal and posterior cingulate cortices Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner18 and further two functionally dissociable subsystems, one engaging in self-referential processes and the other involved in episodic memory. Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner18 In contrast to previous rs-fcMRI studies in OCD, which exclusively tested for connectivity alterations of the midline core, Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor and Welsh11,Reference Jang, Kim, Jung, Choi, Jung and Lee13,Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 the present study sought to study the default mode network as a whole, thus additionally testing the integrity of the dorsomedial prefrontal cortex (dMPFC) self subsystem and medial temporal lobe memory subsystem in comparably large samples of patients with OCD (the OCD group) and demographically matched healthy controls (the control group). Based on previous findings of reduced default mode network connectivity in OCD, it was hypothesised that the OCD group would exhibit reduced connectivity in subsystems of the default mode network as compared with the control group. No specific subsystem hypothesis (i.e. reduced connectivity in one subsystem but not another) was formulated. Additionally, altered interconnectivity between the default mode network and other functional networks was explored, but given the inconsistency of previous findings, no hypotheses were formulated with respect to inter-network connectivity abnormalities.
Method
Participants
A total of 46 adult out-patients with a DSM-IV 19 diagnosis of OCD were recruited from the OCD out-patient clinic at Humboldt-Universität zu Berlin and we also recruited 46 healthy controls. Pair-wise matching was performed with respect to demographic variables (age, gender, handedness and education). All participants were included in a previous analysis of this data-set. Reference Beucke, Sepulcre, Talukdar, Linnman, Zschenderlein and Endrass4 The OCD group were interviewed by a licensed clinical psychologist and diagnosed using the German version of the Structured Clinical Interview for DSM-IV (SCID). Reference First, Spitzer, Gibbon and Williams20 Comorbid lifetime diagnoses included major depression (n = 24), specific phobia (n = 3), hypochondriasis (n = 3), substance misuse (n = 2), social phobia (n = 7), dysthymia (n = 2), bulimia (n = 2), anorexia (n = 2), generalised anxiety disorder (n = 5), panic disorder (n = 4), somatoform disorder (n = 1), agoraphobia (n = 1) and adjustment disorder (n = 1). Nine patients had OCD as their single diagnosis. In the OCD group, 23 participants were treated with antidepressants (20 receiving selective serotonin reuptake inhibitors (SSRI), 2 receiving selective noradrenalin reuptake inhibitors (SNRI) and 1 treated with a tricyclic antidepressant). Unmedicated participants in the OCD group received no medication for OCD symptoms for at least 6 weeks prior to study participation. Table 1 displays demographic data for both the OCD and control groups. Exclusion criteria for participants included cardiac pacemakers or other metallic implants or artefacts and pregnancy. In the OCD group, there was no significant neurological illness, no prior neurosurgery, dementia, delirium, schizophrenia, delusional disorder or other psychotic disorder. None of the OCD group received benzodiazepines within 4 weeks prior to the imaging study. In the control group, participants did not fulfil criteria of any DSM-IV diagnosis as indicated by negative SCID screening questionnaires. All participants received a complete description of the study and written informed consent was obtained. The study was approved by the local ethics committee.
Clinical scales and questionnaires
Severity of OCD symptoms was evaluated using the German versions of the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) Reference Goodman, Price, Rasmussen, Mazure, Fleischmann and Hill21 and the Obsessive-Compulsive Inventory Revised (OCI-R). Reference Foa, Huppert, Leiberg, Langner, Kichic and Hajcak22 In addition, the State and Trait Anxiety Inventory-Revised (STAI-R), Reference Spielberger, Gorsuch and Lushene23 the Beck Depression Inventory (BDI), Reference Beck, Ward, Mendelson, Mock and Erbaugh24 and the Montgomery-Åsberg Depression Rating Scale (MADRS) Reference Montgomery and Asberg25 were administered prior to the fMRI study. The Edinburgh Handedness Inventory Reference Oldfield26 was used to classify handedness. In each group, there were 42 right-handed and three left-handed participants, and 1 person was ambidextrous. All participants completed a German vocabulary test Reference Schmidt and Metzler27 as a measure of verbal intelligence.
Table 1 Demographic dataa

| Obsessive-compulsive disorder group (n = 46) |
Control group (n = 46) |
|
|---|---|---|
| Gender, female/male: n | 26/20 | 26/20 |
| Age, years: mean (s.d.) | 30.7 (9.4) | 30.3 (8.8) |
| Education, years: mean (s.d.) | 12.0 (1.6) | 12.1 (1.5) |
| IQ (verbal), mean (s.d.) | 106.2 (9.6) | 107.1 (9.8) |
| State Trait Anxiety Inventory - revised version (STAI-R), mean (s.d.) | ||
| STAI-X1 (state) | 40.8 (8.2) | 29.8 (5.1) |
| STAI-X2 (trait) | 49.3 (10.3) | 30.6 (7.2) |
| Obsessive-Compulsive Inventory - revised version, mean (s.d.) | 23.9 (11.0) | 4.3 (3.8) |
| Beck Depression Inventory, mean (s.d.) | 14.1 (9.6) | 2.7 (3.0) |
| Yale-Brown Obsessive Compulsive Scale, mean (s.d.) | 20.8 (6.5) | - |
| Montgomery-Åsberg Depression Rating Scale, mean (s.d.) | 8.5 (6.8) | - |
| Interscan movement, mean (s.d.) | 0.07 (0.03) | 0.07 (0.03) |
Means and standard deviations of demographic variables from the obsessive-compulsive disorder and control groups indicative of successful pair-wise matching.
MRI procedures and preprocessing
A total of 160 resting-state volumes (T 2 *-weighted single-shot gradient echo planar imaging sequence) was acquired on a 1.5 T Siemens Sonata system equipped with a circular-polarised headcoil using the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 40 ms; 35 consecutive slices; isotropic 3 mm voxel size; flip angle, 90; field of view (FOV) = 192 mm; 64×64 matrix, aligned parallel to the anterior-posterior commissure line and 176 anatomical slices were acquired using the Modified Driven Equilibrium Fourier Transform (MDEFT) sequence (spatial resolution 1×1×1 mm; TR = 12.24 ms; TE = 3.56 ms; flip angle, 23; 256×224 matrix). Participants were instructed to close their eyes, to relax and not to think of anything specific during this 5 min, 20 s run. Simultaneous electroencephalogram (32 channels with BrainAmp MR, Brain Products) was monitored online, and revealed that none of the participants fell asleep, which was also confirmed by a post-scan questionnaire. In order to reduce head motion, participants’ heads were immobilised by vacuum head cushions. Earplugs were provided to attenuate background noise. Image preprocessing consisted of removal of the first four volumes, slice-time correction, motion correction and linear spatial normalisation to the atlas space of the Montreal Neurological Institute (MNI). Temporal filtering retained frequencies below 0.08 Hz. Spurious or regionally non-specific variance was removed by regression of nuisance variables, including six-parameter rigid body head motion, the signal averaged over the whole brain (to account for respiratory effects), the lateral ventricles and over a region centred in the deep cerebral white matter. The fMRI data was then spatially smoothed using a 4 mm full-width at half maximum kernel.
Default mode network subsystem functional connectivity (seed-to-voxel) analyses
Standard rs-fcMRI functional connectivity analysis was performed for the left-lateralised 11 seed regions that were recently used to identify subsystems of the default mode network Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner18 (online Fig. DS1(a), online Table DS1) using methods that were described in detail previously. Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner18 Briefly, correlation maps for each of the 11 default mode network seeds (spheres of 8 mm in radius) were computed through correlation of regional time courses (averaged over all voxels within the seed region sphere) with every voxel in the brain. Subsequently, correlation maps were transformed to Fisher z-maps. Individual z-maps were then smoothed with a 8 mm kernel using Statistical parametric mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK (http://www.fil.ion.ucl.ac.uk/spm/)) and entered into random effects analyses comparing connectivity maps of each seed between the OCD and control groups (two-sample t-tests). The resulting t-maps indexing differences between the OCD and control groups were corrected for multiple comparisons at the cluster level using the statistical threshold of P<0.0045 (representing a Bonferroni-corrected P-value adjusted for 11 comparisons after cluster-level correction) using Monte Carlo simulations for fMRI data as implemented in the AlphaSim program for Unix (http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html). In order to exclude head motion as a potential confound affecting functional connectivity group differences, Reference Van Dijk, Sabuncu and Buckner28 we calculated each participants mean interscan movement, and compared this measure between groups (Table 1). Detailed information regarding analyses exclusively characterising the averaged connectivity of all nodes constituting each subsystem (i.e. midline core: one connection; dMPFC self system: six connections, medial temporal lobe memory system: ten connections, online Fig. DS2(d)), additional confirmatory rs-fcMRI analyses comparing connectivity of previously identified seed regions for dorsal attention, executive control and salience network connectivity as well as correlational analyses is available in the online supplement DS1.
Results
Default mode network subsystem functional connectivity analyses
Seed-to-voxel fcMRI analyses allowed robust identification of connectivity within the subsystems of the default mode network (P FWE<0.05, corrected for multiple comparisons, online Fig. 1(b)-(d), online Table DS3). In the midline core, the posterior cingulate cortex seed revealed significantly (P<0.0045, cluster-corrected) reduced connectivity in the OCD group with the aMPFC and the right posterior cingulate cortex (control group > OCD group, online Fig. DS2(a)), whereas higher (OCD group > control group) connectivity was evident with the fusiform gyrus (online Fig. DS3(a)). In the dMPFC self subsystem, significantly reduced connectivity between lateral temporal cortex seed and dMPFC was evident in the OCD group (control group > OCD group, online Fig. DS2(b)). Further, the temporal pole seed revealed significantly reduced connectivity with anterior cingulate cortex and aMPFC in the OCD group (control group > OCD group, online Fig. DS2(c)) whereas higher connectivity was evident with the superior parietal lobule and the precuneus (OCD group > control group, online Fig. DS3(c)). In addition, the dMPFC seed showed higher connectivity with the anterior insula in the OCD group (OCD group > control group, online Fig. DS3(b)). Additional slices displaying group differences with respect to connectivity of dMPFC self subsystem seeds are available in online Fig. DS4(b). Regarding the medial temporal lobe memory subsystem seeds, no significant group differences were found in components of default mode network subsystems. Reduced connectivity was evident between the parahippocampal cortex seed and superior parietal regions and the precuneus (control group > OCD group, online Fig. DS4(c)), whereas higher connectivity was found between the posterior inferior parietal lobule seed and the lingual gyrus, the retrosplenial cortex seed and the inferior frontal gyrus, and the hippocampal formation seed and superior temporal gyrus (OCD group > control group, online Fig. DS3(d)-(f)). All seed-to-voxel rs-fcMRI group differences are summarised in Table 2.
Table 2 Default mode network (DMN) subsystem functional connectivity differencesFootnote a
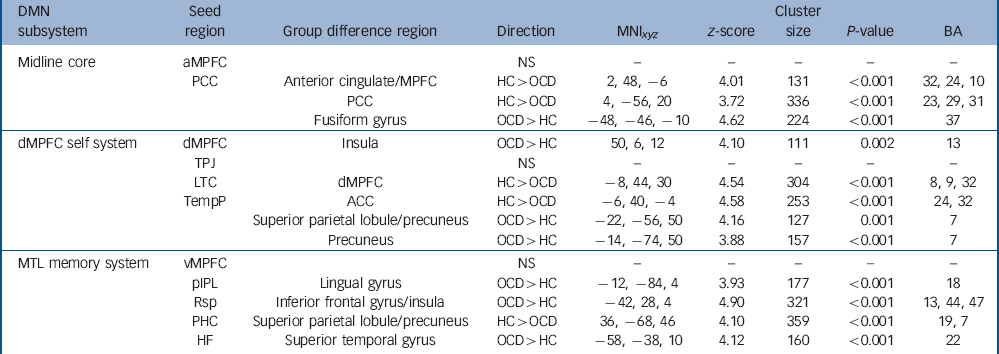
| DMN subsystem |
Seed region |
Group difference region | Direction | MNI xyz | z-score | Cluster size |
P-value | BA |
|---|---|---|---|---|---|---|---|---|
| Midline core | aMPFC | NS | - | - | - | - | - | |
| PCC | Anterior cingulate/MPFC | HC>OCD | 2, 48, –6 | 4.01 | 131 | <0.001 | 32, 24, 10 | |
| PCC | HC>OCD | 4, –56, 20 | 3.72 | 336 | <0.001 | 23, 29, 31 | ||
| Fusiform gyrus | OCD>HC | –48, –46, –10 | 4.62 | 224 | <0.001 | 37 | ||
| dMPFC self system | dMPFC | Insula | OCD>HC | 50, 6, 12 | 4.10 | 111 | 0.002 | 13 |
| TPJ | NS | - | - | - | - | - | ||
| LTC | dMPFC | HC>OCD | –8, 44, 30 | 4.54 | 304 | <0.001 | 8, 9, 32 | |
| TempP | ACC | HC>OCD | –6, 40, –4 | 4.58 | 253 | <0.001 | 24, 32 | |
| Superior parietal lobule/precuneus | OCD>HC | –22, –56, 50 | 4.16 | 127 | 0.001 | 7 | ||
| Precuneus | OCD>HC | –14, –74, 50 | 3.88 | 157 | <0.001 | 7 | ||
| MTL memory system | vMPFC | NS | - | - | - | - | - | |
| pIPL | Lingual gyrus | OCD>HC | –12, –84, 4 | 3.93 | 177 | <0.001 | 18 | |
| Rsp | Inferior frontal gyrus/insula | OCD>HC | –42, 28, 4 | 4.90 | 321 | <0.001 | 13, 44, 47 | |
| PHC | Superior parietal lobule/precuneus | HC>OCD | 36, –68, 46 | 4.10 | 359 | <0.001 | 19, 7 | |
| HF | Superior temporal gyrus | OCD>HC | –58, –38, 10 | 4.12 | 160 | <0.001 | 22 |
ACC, anterior cingulate cortex; HC, healthy controls; OCD, obsessive-compulsive disorder; aMPFC, anterior medial prefrontal cortex; PCC, posterior cingulate cortex; dMPFC, dorsal medial prefrontal cortex; TPJ, temporal parietal junction; LTC, lateral temporal cortex; TempP, temporal pole; vMPFC, ventral medial prefrontal cortex; pIPL, posterior inferior parietal lobule; Rsp, retrosplenial cortex; PHC, parahippocampal cortex; HF, hippocampal formation; MTL, medial temporal lobe; BA, Brodmann area; DMN, Default mode network.
a. Between-group effects for the obsessive-compulsive disorder (OCD) and control groups from seed-to-voxel functional connectivity analyses for 11 default mode network regions. Peaks of connectivity difference coordinates (x, y, z) are given in Montreal Neurological Institute (MNI) space. Between-group effects are thresholded at P<0.0045 (cluster-corrected for multiple comparisons).
Effect of medication
Potential effects of medication on seed-to-voxel results were addressed by separate comparisons of unmedicated and medicated participants in the OCD group with the control group, and by direct comparisons of unmedicated and medicated participants with OCD (online Table DS4). This set of analyses revealed significantly reduced connectivity in the dMPFC self subsystem in unmedicated participants with OCD (n = 23) as compared with the control group (n = 23) (online Table DS4(a)), no significant differences between medicated participants with OCD and the control group in default mode network regions (online Table DS4(b)) and no differences between unmedicated and medicated participants with OCD in components of the default mode network (online Table DS4(c)).
Effect of history of affective disorders
Seed-to-voxel analyses were repeated after exclusion of participants in the OCD group with a lifetime history of affective disorders and the respective control group, revealing significantly reduced connectivity in the dMPFC self subsystem in the remaining OCD group participants (n = 18) in comparison with those in the control group (n = 18) (online Table DS5).
Confirmatory functional connectivity (seed-to-voxel) analyses for dorsal attention, salience and executive control networks
Additional seed-to-voxel fcMRI analyses comparing connectivity of dorsal attention, executive control and salience network seeds were conducted. The dorsal attention network seed in the left superior parietal lobule revealed higher connectivity with areas of the midline core and dMPFC self system in the OCD group compared with the control group, such as the anterior medial prefrontal cortex, the posterior cingulate cortex and the temporal pole (OCD group > control group, online Fig. DS5(a)), whereas the precentral gyrus showed reduced connectivity with the superior parietal lobule seed (online Table DS2). The salience network seed in the anterior insula exhibited greater connectivity with lateral temporal cortex and parahippocampal cortex in the OCD group compared with the control group (online Fig. DS5(b)), and lower connectivity with the inferior parietal lobule in the reverse contrast (control group > OCD group, online Table DS2). The executive control network seed in the dorsolateral prefrontal cortex revealed greater connectivity with superior temporal areas approaching lateral temporal cortex in the OCD group compared with the control group (online Fig. DS5(c)), whereas dorsolateral prefrontal cortex connectivity with the inferior frontal gyrus was reduced in the OCD group compared with the control group (online Table DS2).
Correlational analyses between dMPFC connectivity and OCD symptom severity
Significant positive relationships were found between global OCD symptom severity as measured by the Y-BOCS (total score) and dMPFC connectivity with the anterior cingulate cortex (MNI xyz = –8, 36, 30, z = 4.61, r = 0.62) and the ventral striatum (MNI xyz = 16, 12, –2, z = 3.98, r = 0.55) (online Fig. DS6(a)) in the entire group with OCD (n = 46). In unmedicated participants with OCD (n = 23) only, significant positive correlations were again found between global OCD symptom severity and connectivity between the dMPFC and the ventral striatum (MNI xyz = –10, 14, –10, z = 4.02, r = 0.74), whereas positive relationships between Y-BOCS scores and dMPFC connectivity with the anterior cingulate cortex were only evident at an uncorrected significance threshold of P<0.001 (online Fig. DS6(b)).
Discussion
Main findings
This study investigated the integrity of the default mode network subsystems in people with OCD using resting-state functional connectivity. Results revealed significantly reduced connectivity within the dMPFC self subsystem in the OCD group compared with a healthy control group, and further similar, but less pronounced effects in the midline core. In addition to abnormalities within the default mode network, the OCD group exhibited higher connectivity between components of the dMPFC self subsystem and the anterior insula and the superior parietal lobule, which represent central nodes of salience and dorsal attention networks, highlighting a general tendency towards higher connectivity with areas involved in active task engagement, which was evident in all three default mode network subsystems. Whereas indication of reduced midline core connectivity and higher interconnectivity among default mode and salience networks are in general agreement with the existing literature, these results provide first evidence of an aberrant default mode network subsystem in patients with OCD, and stronger interconnectivity of this subsystem with key regions of the salience and dorsal attention networks. Consequently, these findings point to multiple large-scale network involvement and suggest there should be careful examination of self-referential processing and activity of dMPFC self system components in patients with OCD in future studies.
Findings from previous studies
Previous studies provided evidence for either reduced connectivity within the default mode network Reference Fitzgerald, Stern, Angstadt, Nicholson-Muth, Maynor and Welsh11,Reference Cocchi, Harrison, Pujol, Harding, Fornito and Pantelis12 or altered interconnectivity with other functional networks in OCD. Reference Jang, Kim, Jung, Choi, Jung and Lee13,Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 Of note, a recent study found stronger connectivity between several default mode network regions and the anterior insula, Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 which is highly consistent with the present confirmatory seed-to-voxel results conducted for a seed in this region (online Fig. DS5(b), online Table DS2). Similarly, trends for reduced posterior cingulate cortex connectivity with the midline core demonstrated by Stern et al Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14 strongly converge with the present pattern of results observed for the midline core. In fact, seed-to-voxel differences for the midline core in the present study were only significant when considering the entire group of participants with OCD (n = 46) as compared with the control group, whereas only trends (P<0.001, uncorrected) were found for reduced midline core connectivity in unmedicated participants with OCD compared with the control group, suggesting that effects in the midline core might, to some extent, depend on sample size rather than medication status. In contrast, reduced connectivity in the dMPFC self system emerged in both seed-to-voxel and voxel-based region of interest (ROI)-to-ROI approaches (online supplement DS2), and remained significant after addressing potential effects of medication (online Table 4(a), online supplemental DS2). Similarly, exclusion of participants with a lifetime history of affective disorders revealed significantly reduced connectivity in seed-to-voxel analyses of the dMPFC self subsystem in the remaining participants with OCD (online Table DS5). This is worth noting because enhanced connectivity of the default mode network has previously been reported in patients with affective disorders. Reference Posner, Hellerstein, Gat, Mechling, Klahr and Wang29 Taken together, the default mode network subsystem approach and the inclusion of a comparably large sample of people with OCD applied in the present study has allowed us to extend the existing literature by demonstrating both abnormal connectivity within the self subsystem of the default mode network and greater interconnectivity with other functional networks in the same sample of participants with OCD.
Connectivity abnormalities in a neural system previously associated with self-referential processes in healthy individuals
The finding of lower connectivity in the dMPFC self subsystem converges with evidence of reduced grey matter volume of the bilateral dMPFC in meta-analyses of structural MRI data of people with OCD. Reference Radua and Mataix-Cols30 Studies in healthy individuals have elucidated a privileged role for the dMPFC subsystem in self-referential and affective processing. Reference Gusnard, Akbudak, Shulman and Raichle31-33 For example, the dMPFC is active when healthy participants judge whether emotional pictures make them feel pleasant or unpleasant, Reference Gusnard, Akbudak, Shulman and Raichle31 when judging whether personality traits accurately describe them Reference Fossati, Hevenor, Graham, Grady, Keightley and Craik32 and during simulation of personal events (in conjunction with midline core areas). Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner18 In light of these findings closely associating activity of dMPFC self system components with self-referential processing, the present findings of reduced dMPFC self system connectivity could be interpreted to reflect altered self-referential processing in people with OCD. However, given the absence of behavioural measures reflective of self-referential thoughts or processes in the present study, this conclusion cannot be drawn from the present data and will require direct examination of people with OCD using experiments that have previously associated activity of dMPFC self system components and self-referential processes in healthy individuals. Reference Andrews-Hanna, Reidler, Sepulcre, Poulin and Buckner18,33
From a clinical perspective, abnormal cognitive processing in relation to the self is frequently observed in OCD. Reference Salkovskis1,Reference Purdon and Clark34 More precisely, obsessions are usually ego-dystonic Reference Salkovskis1 in the sense that their content is perceived as inconsistent with the patient’s sense of self. Reference Purdon and Clark34 Considering that the mental representation of the self and the degree of certainty with which a self-view is held are specifically mediated by the dMPFC, 33 a compelling conclusion is that reduced connectivity in the dMPFC self system is related to impaired dissociation from obsessional content, because impaired recruitment of this system providing certainty of a self-view might facilitate the occurrence of obsessive, ego-dystonic thoughts. This interpretation receives support from the observation that relatively higher connectivity of the dMPFC with corticostriatal areas thought to mediate obsessions Reference Saxena, Brody, Schwartz and Baxter2 was associated with higher severity of obsessive-compulsive symptoms (online Fig. DS6). However, the assumptions made regarding this potential mechanism require study of the dynamic interactions between the dMPFC self subsystem regions and corticostriatal areas that are involved in the generation of obsessions, and determination of whether reduced dMPFC self subsystem connectivity at rest leads to aberrant activity in this subsystem in response to self-reflective stimuli in people with OCD. This appears plausible since spontaneous resting activity and task-related responses are strongly related. Reference Fox, Snyder, Zacks and Raichle35
As briefly indicated above, connectivity of the dMPFC with striatal and cingulate areas positively correlated with global OCD symptom severity. Despite the fact that these regions play important roles in the functional neuroanatomy of OCD, Reference Saxena, Brody, Schwartz and Baxter2 and even though these results emerged in the entire OCD group as well as in medicated and unmedicated participants alone, based on voxel-wise analyses using corrections for multiple comparisons, it has to be emphasised that these post hoc analyses aimed to explore the clinical validity of the finding of reduced default mode network subsystem connectivity, and thus did not test a priori hypotheses. Further, the interpretation of these correlational findings in the context of the present study is complicated by the fact that the identified regions are outside the default mode network. The present findings indicate lower dMPFC self system connectivity and associate higher dMPFC connectivity with corticostriatal areas with more severe symptoms. The existing OCD neuroimaging literature points to corticostriatal hyperactivity and hyperconnectivity. Thus, one possibility could be that a stronger development of corticostriatal networks at the expense of deficient development of the default mode network occurs in people with OCD, and therefore, higher connectivity between the corticostriatal and default mode networks, or impaired segregation between the two, might relate to symptoms. However, this idea remains entirely speculative at this point, since testing it would require longitudinal rs-fcMRI assessments of, for example, individuals at risk of developing OCD.
Abnormal default mode network interconnectivity with other large-scale brain networks
In addition to the observation of reduced connectivity (control group > OCD group) within the dMPFC self system and the midline core, default mode network whole-brain seed-to-voxel analyses also revealed predominantly stronger connectivity (OCD group > control group) of the default mode network components with cortical areas known to actively participate in cognitive task execution, and which also represent components of previously identified rs-fcMRI networks. Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna7-Reference Menon10 For example, the temporal pole was more connected to the superior parietal lobule of the dorsal attention network, and further the dMPFC showed greater connectivity with the anterior insula of the salience network, whereas the retrosplenial cortex was more connected to the inferior frontal gyrus of the executive control network, respectively. This notion is supported by confirmatory seed-to-voxel analyses using dorsal attention, executive control and salience network seed coordinates that have been previously described in the literature (see online supplement DS1, online Table DS2, online Fig. DS5). Thus, in addition to the identification of reduced connectivity in the dMPFC self system, our data support previous rs-fcMRI findings of abnormal interconnectivity between brain networks in OCD, Reference Stern, Fitzgerald, Welsh, Abelson and Taylor14,Reference Li, Li, Dong, Luo, Han and Xiong15 as well as the recently formulated hypothesis that psychiatric abnormalities might be associated not only with alterations in a single system, but rather involve aberrant interconnectivity among multiple networks. Reference Menon10 More precisely, the triple network model formulated by Menon suggests a central role for the anterior insula of the salience network, which is thought to assign salience to external stimuli or internal mental events, thus disengaging (for example self-referential processing or stimulus-independent mental activity of) the default mode network, while initiating stimulus-dependent processing of the executive control network. Reference Menon10 Given the present pattern of results, it is plausible to consider that greater connectivity of the anterior insula with the dMPFC subsystem and other default mode network areas might lead to an abnormally high or even constant disengagement and reduced connectivity of the dMPFC self system, resulting in insufficient recruitment of self-referential processes when dissociation from obsessional impulses is demanded.
Divergent default mode network connectivity profiles in OCD v. affective disorders
As briefly indicated above, hyperconnectivity of the default mode network has been detected in affective disorders such as dysthymia Reference Posner, Hellerstein, Gat, Mechling, Klahr and Wang29 and major depression. Reference Greicius, Flores, Menon, Glover, Solvason and Kenna36,Reference Sheline, Barch, Price, Rundle, Vaishnavi and Snyder37 These findings contrast with the present finding of reduced default mode network connectivity identified in the present study. The interpretation that people with OCD and people with affective disorders are characterised by divergent default mode network connectivity profiles receives further support from the observation that the finding of reduced connectivity in the dMPFC self system remained significant when considering only those participants with no history of affective disorders (online Table DS5), despite a substantial reduction in statistical power because of the exclusion. Considering that the default mode network is thought to engage in mental processes such as introspection or mind-wandering, Reference Buckner, Andrews-Hanna and Schacter38 which involves moving away from externally focused thoughts while initiating internal, self-focused thoughts, these potentially diverging default mode network connectivity profiles of the two patient groups might reflect differences in introspective processes. Default mode network hyperconnectivity in affective disorders is thought to relate to excessive introspection and thus internal focus in the form of rumination, Reference Posner, Hellerstein, Gat, Mechling, Klahr and Wang29 whereas externally focused thoughts related to the OCD symptom profile and greater attention to potentially threatening Reference Muller and Roberts39 as well as novel stimuli Reference Ischebeck, Endrass, Simon and Kathmann40 characterise patients with OCD. To further investigate these aspects, default mode network connectivity profiles as well as behavioural measures of self-referential processing should be directly compared between participants with OCD and affective disorders in future studies.
Limitations
This study has several limitations. As briefly indicated above, the question of how the identified dMPFC self subsystem abnormalities during the resting-state contribute to OCD behaviour remains to be tested using approaches that consider both resting-state and experimental fMRI data. Notably, direct associations between dMPFC self system dysconnectivity and potential alterations in self-referential processing in people with OCD remain to be demonstrated, and will require study designs collecting both dMPFC self system connectivity parameters and measures of self-referential processing, and/or fMRI experiments involving manipulation of self-referential processes in people with OCD. Furthermore, connectivity abnormalities were identified using a purely correlative methodological procedure that provides no information about causal influences between brain regions.
In conclusion, the present study conducted a detailed investigation of default network integrity in participants with OCD by testing for abnormalities in subsystems of the default mode network in this patient group. Results identified reduced connectivity within the dMPFC self subsystem, which also showed greater interconnectivity with dorsal attention and salience networks. Furthermore, dMPFC connectivity with striatal and anterior cingulate cortex areas positively correlated with symptom severity. Therefore, the present results show that multiple brain networks contribute to the clinical phenotype of OCD. They further provide first evidence of a default mode network subsystem abnormality that suggests the need for careful examination of potentially altered self-referential processing in this disorder. These aspects require further study in order to advance neurobiological models of OCD.
Funding
This work was supported by a grant from the Federal Ministry of Education and Research of Germany (grant number BMBF-01GW0724 to N.K.). J.C.B. is supported by a PhD scholarship from Evangelisches Studienwerk e.V. Villigst (Schwerte, Germany), and is a ERP scholar of the German National Academic Foundation. J.S. is supported by the Alzheimer's Association (grant number NIRG-11-205690).
Acknowledgements
The authors are grateful to the Cognitive Neuroscience Lab at Harvard University and the Athinoula A. Martinos Center for Biomedical Imaging for providing space and tools for rs-fcMRI analyses and Koene Van Dijk (PhD) for assistance with head motion analyses. Further, we thank Eva Kischkel (PhD) and Rüdiger Spielberg (PhD) for clinical assessments, Susanne Schwab (MS) for patient recruitment, Rosa Gruetzmann (MS) and Katja Zschenderlein (MS) for assistance involving data acquisition, Thomas Pinkpank (MS) for technical assistance and acknowledge scanning facility access provided by Charité Universitätsmedizin Berlin.





eLetters
No eLetters have been published for this article.