Elucidation of the role of specific serotonin (5-HT) receptor subtypes in the pathophysiology of depression has been hampered by the limited availability of selective 5-HT receptor probes. The triptan drugs, recently introduced for the treatment of acute migraine, have a high affinity for 5-HT1D receptors which are located both pre- and post-synaptically on 5-HT neurons (Reference Johnson, Schaus and DurkinJohnson et al, 1997; Reference Barnes and SharpBarnes & Sharp, 1999). Triptans increase plasma growth hormone levels, and the growth hormone response to zolmitriptan appears to be mediated via activation of post-synaptic 5-HT1D receptors (Reference Whale, Bhagwagar and CowenWhale et al, 1999). The aim of the present study was to use this response to assess the sensitivity of 5-HT1D receptors in patients with depression before and following treatment with selective serotonin reuptake inhibitors (SSRIs). We predicted that 5-HT1D receptor function would be decreased in patients with depression, particularly in those with melancholic symptoms, and would be further diminished by SSRI treatment.
METHOD
Subjects
We recruited 26 patients (10 men, 16 women) from primary care who on the basis of the Structured Clinical Interview for DSM-IV (SCID) met criteria for major depression. Their mean was age 37.9 years (range 21-54) and mean weight 70.0 kg (range 51-96). Fourteen subjects had never received psychotropic medication; the remainder had not received psychotropic medication for at least 2 weeks (mean 75 weeks). One woman was taking an oral contraceptive pill, two were post-menopausal, and one was taking hormone replacement therapy. Subjects with depression had a mean score on the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960) of 24.9 (range 16-39). Ten subjects met DSM-IV (American Psychiatric Association, 1994) criteria for major depression with melancholia.
A group of 25 healthy controls were selected from a volunteer register, and matched to the patient group for gender, including menstrual status (stage of the menstrual cycle or postmenopausal), age, weight and hormonal medication. This group consisted of nine men and 16 women with mean age 39.9 years (range 23-54) and mean weight 71.5 kg (range 56-102 kg). Controls had been free of psychotropic medication for at least 3 months. The SCID interview was used to ensure that none met criteria for any Axis 1 disorder on DSM-IV. All subjects gave informed consent to the study, which was approved by the local ethics committee.
Neuroendocrine testing and antidepressant treatment
Subjects were brought to the laboratory in the morning, having fasted for at least 4 hours. An indwelling venous cannula was inserted and a 30-minute rest period allowed to elapse before zolmitriptan (5 mg orally) was administered. Blood samples were then removed at 15-minute intervals for a further 180 minutes. Subjects remained at rest throughout the test procedure. Following the neuroendocrine test, antidepressant medication was prescribed as clinically appropriate by the patient's treating clinician. A subgroup of 12 patients (three men, nine women) were re-challenged with zolmitriptan (5 mg orally) following a trial of SSRI treatment of mean duration 33.9 days (range 27-76 days). Of this group, seven patients received fluoxetine (20 mg daily except one subject who took 20 mg on alternate days), three paroxetine (20 mg), one venlafaxine (150 mg daily) and one citalopram (20 mg). Six healthy controls (four men, two women) were also challenged twice with zolmitriptan (5 mg orally). The mean inverval between these two tests was 28.2 days (range 23-35 days). In both these studies female subjects received the second zolmitriptan challenge at the same stage of the menstrual cycle as the first.
Biochemical measurements
Following blood collection, plasma was separated by centrifugation and stored at ‒20°C. Plasma growth hormone was measured using standard immunoradiometric assays (reagents provided by Netria, London). The inter- and intra-assay coefficients of variation of the growth hormone assays over the range encompassed by the standard curve were 4.1% and 2.6%. Because growth hormone can inhibit its own secretion, we excluded subjects whose tests demonstrated elevated baseline growth hormone secretion (>15 mIU/l) at Time 0. Plasma cortisol was determined by radio-immunoassay. The intra- and inter-assay coefficients of variation over the range encompassed by the standard curve were 4.3% and 5.8%. Plasma zolmitriptan levels were measured by a high-performance liquid chromatography (HPLC) procedure which utilised coulometric end-point detection, and solid phase extraction.
Statistical analysis
Analyses were carried out using SSPS for Windows (version 9.0). Growth hormone responses were analysed as peak change from baseline and also area under the curve (AUC), measured by the trapezoid method with extrapolation of baseline secretion measured from Time 0. These two measures were highly correlated (r=0.94, P<0.001). Baseline cortisol was measured as mean concentration from the three baseline sample levels. Plasma zolmitriptan levels were calculated as AUC. Differences between patients and controls were examined with Student's unpaired t-test (two-tailed). Within-group comparisons were made using Student's paired t-test (two-tailed). Correlations were carried out using Pearson's product moment.
RESULTS
All subjects tolerated zolmitriptan well. High baseline growth hormone levels led to three patients (one with melancholia) and two controls being excluded. In the overall group of patients, mean (s.e.m.) peak growth hormone responses did not differ from those of controls (11.8 (3.4) v. 16.3 (3.4) mIU/l, P=0.36). However, when the patients were divided into those with and those without the melancholic syndrome, the growth hormone responses of patients with melancholic depression were significantly less than controls (see Table 1 and Fig. 1). The mean (s.e.m) peak growth hormone response in the nine patients with melancholic depression was also significantly less when they were compared with nine controls matched for gender, age and weight (4.5 (1.3) v. 20.1 (5.9) mIU/l, P=0.03), as were the AUC responses (-70 (208) v. 1319 (438) mIU × min/l, P=0.015). Neither patient group differed from controls in either baseline cortisol concentration or AUC of plasma zolmitriptan (see Table 1). Zolmitriptan levels, baseline cortisol concentration or HRSD score did not correlate with growth hormone response in either patients or controls (data not shown: further details available from the author upon request).
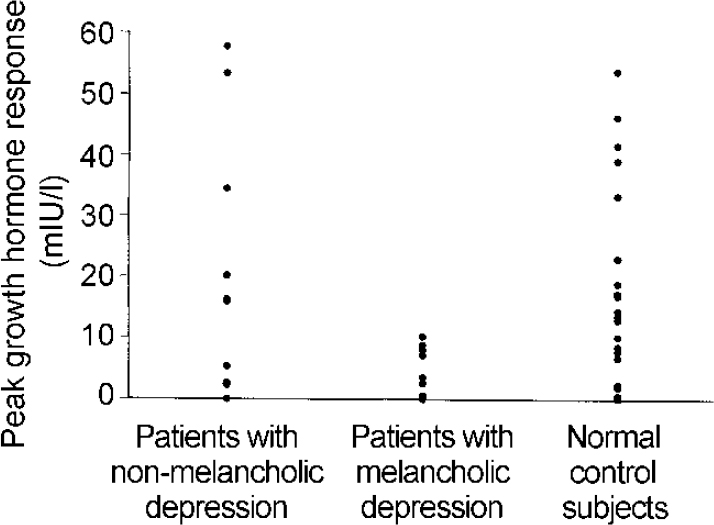
Fig. 1 Peak growth response over baseline following zolmitriptan (5 mg orally) in patients with depression and controls. The responses of the patients with melancholic depression are significantly less than the controls (P<0.005, unpaired t-test).
Table 1 Hormone and zolmitriptan levels in patients with depression and control subjects

| Controls (n=23) Mean (s.e.m.) | Subjects with non-melancholic depression (n=14) Mean (s.e.m.) | Subjects with melancholic depression (n=9) Mean (s.e.m.) | |
|---|---|---|---|
| Baseline cortisol (μg/100ml) | 23.8 (2.5) | 23.8 (2.0) | 20.8 (2.6) |
| Δ Peak growth hormone (mIU/l) | 16.8 (3.4) | 16.5 (5.2) | 4.5 (1.3)*** |
| Area under the curve for growth hormone (mIU × min/l) | 946.0 (237) | 628 (434) | -69.7 (208)*** |
| Zolmitriptan (ng × min/ml) | 619 (89) | 588 (90) | 622 (111) |
| HRSD score | - | 24 (1.3) | 26 (2.4) |
In the 12 patients who were re-challenged with zolmitriptan after SSRI treatment the mean (s.e.m.) peak growth hormone response was significantly attenuated (14.2 (4.8) v. 1.9 (0.7) mIU/l, P=0.024). The AUC of growth hormone secretion after zolmitriptan was also significantly less following SSRI treatment (675 (355) v. ‒368 (173) mIU × min/l, P=0.005) (see Fig. 2). The mean (s.e.m.) AUC of zolmitriptan was not significantly altered by SSRI treatment (588 (83) v. 649 (87), P=0.45). The mean fall in HRSD was 7.9. Five of the patients were responders as judged by a 50% decline in HRSD score. There was no correlation between fall in HRSD and change in peak or AUC growth hormone response to zolmitriptan (r=0.18, P=0.57 and r=-0.10, P=0.98, respectively).
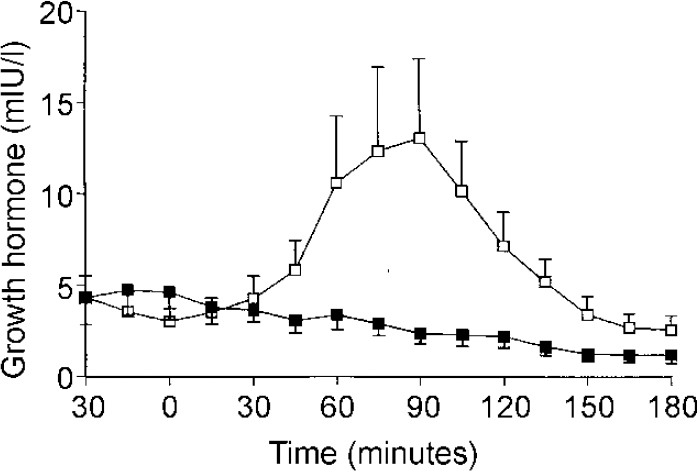
Fig. 2 Mean (s.e.m.) growth hormone concentration in 12 patients with depression who were tested on two occasions, before (□) and after (▪) selective serotonin reuptake inhibitor (SSRI) treatment. Patients received zolmitriptan (5 mg orally) at Time 0). The area under the curve of growth hormone response after zolmitriptan was significantly lowered by SSRI treatment (P=0.005, unpaired t-test).
In the group of healthy controls who were tested on two occasions there was no significant difference between the mean (s.e.m.) peak growth hormone response to the first zolmitriptan challenge and the second (16.9 (7.6) v. 17.3 (8.9) mIU/l, P=0.81). The AUC growth hormone values on the two occasions were also similar (943 (597) and 1189 (854) mIU × min/l, P=0.42).
DISCUSSION
Our findings indicate that the growth hormone response to zolmitriptan is impaired in patients with major depression and melancholia. In patients treated with SSRIs, the growth hormone response to zolmitriptan was markedly attenuated, suggesting an adaptive desensitisation of the 5-HT mechanisms involved in zolmitriptan-induced growth hormone release. In normal subjects rechallenged with zolmitriptan (without SSRI administration), no such desensitisation was observed.
Growth hormone response to zolmitriptan
A number of triptan drugs, including sumatriptan, rizatriptan and zolmitriptan, increase plasma growth hormone in humans (see Reference Whale and CowenWhale & Cowen, 1998). Zolmitriptan is the only one of these agents with good blood—brain barrier permeability and its ability to stimulate growth hormone release appears more robust than that of sumatriptan (Reference Whale, Bhagwagar and CowenWhale et al, 1999).
All the triptans have a high affinity for both the 5-HT1D receptors and the closely related 5-HT1B receptors, and, in the absence of selective antagonists, it is not possible at present to state with certainty which receptor subtype mediates the growth hormone response. However, we recently found that zolmitriptan-induced growth hormone release is attenuated by the 5-HT receptor antagonist, ketanserin, which has some preference for the 5-HT1D receptor over the 5-HT1B receptor (Reference Whale, Bhagwagar and CowenWhale et al, 1999). In addition, because 5-HT pathways stimulate growth hormone secretion, we assume that the 5-HT receptors mediating zolmitriptan-induced growth hormone release are located post-synaptically to 5-HT neurons and do not function as inhibitory 5-HT1D autoreceptors (Reference Barnes and SharpBarnes & Sharp, 1999).
Growth hormone response to zolmitriptan in depression
Our data suggest that patients with melancholic depression have blunted growth hormone responses to zolmitriptan. In this respect our findings are similar to those of challenge studies using 5-HT1A receptor ligands, where blunted endocrine and thermic responses are particularly apparent in subjects with melancholic depression (Reference LeschLesch, 1992; Reference Cowen, Power and WareCowen et al, 1994). Two other studies have reported blunted growth hormone responses to sumatriptan in patients with major depression (Reference Yatham, Athanasios and LamYatham et al, 1997; Reference Cleare, Murray and SherwoodCleare et al, 1998), although results of sumatriptan challenge are difficult to interpret because of the number of healthy subjects who fail to produce useful growth hormone responses. While these data appear to suggest a fairly reliable blunting of growth hormone responses to 5-HT1D receptor challenge in patients with depression, it is worth noting that this abnormality could reflect decreased growth hormone release at pituitary level. However, studies with growth hormone releasing hormone do not consistently support this interpretation (Reference Skare, Dysken and BillingtonSkare et al, 1994).
The current results from challenge studies with directly acting 5-HT receptor agonists in patients with depression differ from those obtained with the 5-HT precursor, tryptophan, where endocrine responses are blunted both in subjects with melancholic and non-melancholic depression (Reference Cowen and CharigCowen & Charig, 1987). This suggests that whereas patients with depression in general may have impaired pre-synaptic release of 5-HT, patients with melancholia may additionally exhibit impaired sensitivity of post-synaptic 5-HT receptors. This would be expected to result in a larger decrement in overall 5-HT neurotransmission. This could explain why SSRIs may not be as effective as less selective antidepressants in patients with severe depressive illness (Reference AndersonAnderson, 2000). Where brain 5-HT function is particularly compromised the therapeutic effect of SSRIs could be impaired.
It seems paradoxical that impaired presynaptic 5-HT release should be associated with diminished post-synaptic receptor sensitivity. Normally a compensatory up-regulation in post-synaptic receptor responsiveness would be expected. This makes it likely that in patients with depression some other factor is overriding this normal compensatory response. For example, in the case of the 5-HT1A receptor there is a possible role of cortisol hypersecretion to decrease 5-HT1A receptor expression (see Reference DinanDinan, 1994). Conceivably cortisol could exert a similar effect on the 5-HT1D receptor. In addition we have recently shown that patients who have recovered from depression have impaired up-regulation of post-synaptic 5-HT receptor sensitivity when exposed to dietary-induced tryptophan deficit (Reference Smith, Williams and CowenSmith et al, 2000). It is, therefore, also possible that people prone to depression have a trait abnormality in 5-HT receptor regulation.
The possible contribution of impaired post-synaptic 5-HT1D receptor sensitivity to the depressive syndrome is unclear because there are few known functional correlates of 5-HT1D receptor activation in animals or humans. Higher concentrations of post-synaptic 5-HT1D receptors are found in basal ganglia which might indicate involvement in depression-associated psychomotor changes (Reference Barnes and SharpBarnes & Sharp, 1999). In addition, 5-HT1D receptors in the cortex modulate glutamate release, which might suggest a role in the memory impairments experienced by patients with depression (Reference Maura, Marcoli and TortaroloMaura et al, 1998).
Effect of SSRIs on 5-HT1D receptor sensitivity
In patients treated with SSRIs the growth hormone response to zolmitriptan was markedly decreased. This suggests that SSRIs produce a down-regulation of post-synaptic 5-HT1D receptors. There is also evidence that repeated SSRI treatment can down-regulate post-synaptic 5-HT1A and 5-HT2 receptors, in both healthy subjects and patients with depression (Reference LeschLesch, 1992; Reference Quested, Sargent and CowenQuested et al, 1997; Reference Sargent, Williamson and PearsonSargent et al, 1997).
Despite these effects of SSRIs on post-synaptic 5-HT receptors, it is likely that overall, SSRIs increase 5-HT neurotransmission because procedures such as tryptophan depletion, which lower synaptic 5-HT availability, reverse the antidepressant effects of SSRIs in patients who have recovered from depression (Reference Delgado, Miller and SalomonDelgado et al, 1999). From this point of view the down-regulation of post-synaptic receptors seen during SSRI treatment is presumably an adaptive response which limits, but does not remove, the increase in 5-HT neurotransmission produced by chronic 5-HT reuptake blockade and increased levels of synaptic 5-HT. This may explain the apparently paradoxical finding that some unmedicated patients with major depression have impaired post-synaptic 5-HT1D receptor function which is further diminished by effective treatment.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Patients with melancholic depression have impaired sensitivity of 5-HT1D receptors.
-
▪ Impaired function of 5-HT1D receptors could contribute to psychomotor and cognitive changes seen in depression.
-
▪ Although selective serotonin reuptake inhibitors lower the sensitivity of post-synaptic 5-HT receptors their primary action is to facilitate 5-HT neurotransmission by increasing synaptic 5-HT levels.
LIMITATIONS
-
▪ The number of patients with melancholic depression studied was small.
-
▪ The 5-HT1 receptor subtype that mediates zolmitriptan-induced growth hormone secretion has not been definitely established.
-
▪ Changes in 5-HT1D receptors involved in growth hormone secretion may not necessarily be associated with changes in 5-HT1D receptors in brain regions implicated in the depressive syndrome.
ACKNOWLEDGEMENTS
We thank M. Clements and A. Reed for technical assistance and R. Hockney and G. Pearson for nursing care. E.M.C. and Z.B. are Medical Research Council (MRC) Clinical Training Fellows. P.J.C. is an MRC Clinical Scientist. The study was supported by the MRC.






eLetters
No eLetters have been published for this article.