Antisocial behaviours are complex phenotypes whose variance is influenced by genetic and environmental factors. Twin and adoption studies consistently show that about 50% of individual differences in antisocial behaviours can be attributed to genetics, with the remaining 50% being due to the environment.Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven and Visscher1 Whereas the proximal and distal environmental risk factors of antisocial behaviour are relatively well identified, the genes associated with these phenotypes remain elusive. Genes within the serotonergic system – such as tryptophan hydroxylase (TPH-1 and TPH-2), monoamine oxidase A (MAOA) and B (MAOB), serotonin transporter (SLC6A4) and serotonin receptor (e.g. 5-HTR 1A, 5-HTR 2A, 5-HTR 2C, 5-HTR 5A, 5-HTR 6 and 5-HTR 7) genes – have been targeted because of their prior associations with antisocial behaviour.Reference Ficks and Waldman2, Reference Tielbeek, Karlsson Linnér, Beers, Posthuma, Popma and Polderman3 However, the findings from these studies are inconsistent and of small magnitude.
The gap between the heritability estimates drawn from twin studies and the variance accounted for by measured genes, often referred to as ‘missing’ heritability, is expected to stem from factors such as measurement bias; phenotypic complexity; penetrance; epigenetics; epistasis; gene-by-environment interplay; rare variants (<1%) with potentially larger effects; incomplete linkage disequilibrium between causal variants and genotyped single nucleotide polymorphisms (SNPs); small effects of the genetic variants distributed across the genome; and the presence of more than one model connecting all the candidate genes and their corresponding proteins at the molecular levels, leading to several aetiological pathways of neuronal migration and neurite outgrowth contributing to complex traits.Reference Poelmans, Buitelaar, Pauls and Franke4, Reference van der Sluis, Verhage, Posthuma and Dolan5 Recently, polygenic risk scores (PRSs; derived from genome-wide association studies) and multilocus genetic profile scores (MGPSs; derived from candidate genes studies) have been used to operationalise genetic liability through the additive consideration of numerous variants of small effect sizes.Reference Plomin, Haworth and Davis6, Reference Bogdan, Hyde and Hariri7 Previous investigations relying on either strategy indicate that participants carrying a higher number of genetic risk variants were more likely to experience depressive symptoms,Reference Holmes, Lee, Hollinshead, Bakst, Roffman and Smoller8, Reference Brezo, Bureau, Mérette, Jomphe, Barker and Vitaro9 schizophreniaReference Ruderfer, Fanous, Ripke, McQuillin and Amdur10 and high body mass.Reference Walter, Mejia-Guevara, Estrada, Liu and Glymour11 Because they result from the addition of multiple genetic variants, PRSs and MGPSs are expected to reduce the missing heritability gap.Reference Walter, Mejia-Guevara, Estrada, Liu and Glymour11, Reference Selzam, Krapohl, von Stumm, O'Reilly, Rimfeld and Kovas12 Growing evidence emerging from genome-wide association studies support this assertion, but the overall variance accounted for by the individual SNPs remains low. Specifically, whereas individual SNPs only explained 0.01–0.34% of body mass index,Reference Walter, Mejia-Guevara, Estrada, Liu and Glymour11 0.02% of educational attainmentReference Selzam, Krapohl, von Stumm, O'Reilly, Rimfeld and Kovas12 and 1% of antisocial behaviour,Reference Tielbeek, Medland, Benyamin, Byrne, Heath and Madden13 PRSs accounted for 0.99–1.37% of body mass index,Reference Walter, Mejia-Guevara, Estrada, Liu and Glymour11 approximately 9% of educational attainmentReference Selzam, Krapohl, von Stumm, O'Reilly, Rimfeld and Kovas12 and 5.2% of adult antisocial behaviours.Reference Tielbeek, Johansson, Polderman, Rautiainen, Jansen and Taylor14
To our knowledge, only one study investigated the cumulative contribution of multiple serotonergic genes to antisocial-related outcomes using an MGPS approach. Belsky and BeaverReference Belsky and Beaver15 examined the influence of carrying five risk alleles located in candidate serotonergic (5-HTTLPR, MAOA) and dopaminergic (DAT1, DRD2 and DRD4) genes on adolescent self-regulation in a sample of 1586 adolescents (754 males). No significant associations were found. However, the target phenotype was assessed only at one point in time (i.e. adolescence), the study relied solely on a self-report correlate of antisocial behaviour and only one genetic polymorphism was considered per gene instead of relying on multiple variants to characterise each gene.
Another strategy to increase the explanatory value of multiple genetic polymorphisms for antisocial behaviours could be to consider a combination of nearby SNPs based on linkage disequilibrium (i.e. haplotypes) as a unit of analysis. Because haplotypes maximise information gathered from multiple genetic variants, they should be more strongly associated with the targeted phenotypes and thus contribute to clarifying the genetic aetiology of antisocial behaviours.Reference Morris and Kaplan16, Reference Clark17 From a statistical perspective, haplotypes reduce the burden of multiple testing, thereby reducing the risk of false-positive findings.Reference Clark17 This haplotype-based strategy has been used successfully for complex phenotypes such as schizophreniaReference Shifman, Bronstein, Sternfeld, Pisanté-Shalom, Lev-Lehman and Weizman18 and diabetes.Reference Williams, Jacobs, Moreno-Macias, Huerta-Chagoya and Churchhouse19 A genome-wide association investigation of Finnish people who were violent recidivists further showed that investigating CDH13 variants using a haplotype-based approach provided stronger power to detect associations with violent offending,Reference Tiihonen, Rautiainen, Ollila, Repo-Tiihonen, Virkkunen and Palotie20 thus providing initial indications of the potential value of this strategy to examine the cumulative effect of multiple genetic polymorphisms on antisocial behaviours, especially in smaller samples.
The aim of this study was to investigate, in a population-based sample of males, the genetic contributions of 11 candidate serotonergic genes to a variety of antisocial behaviours in adolescence and early adulthood. There were three objectives: (a) to assess the association between each SNP and antisocial behaviour to highlight the value of considering haplotype-based superalleles over individual SNPs when investigating a small number of candidate genes; (b) to estimate haplotypes within each serotonergic candidate gene and test their association with antisocial behaviours; and (c) to derive MGPSs depicting higher or lower (i.e. protection) risk of antisocial behaviourReference Rutter, Dodge and Rutter21 and test if they are associated with antisocial behaviours in a cumulative manner.
Method
Participants
The Quebec Longitudinal Study of Kindergarten Children is a representative sample of children attending kindergarten in French-speaking state schools in the province of Quebec, Canada.Reference Rouquette, Cote, Pryor, Carbonneau, Vitaro and Tremblay22 The total sample comprised 3017 children drawn from two initial subsamples. The first subsample comprised 2000 children (1001 boys) selected randomly. The second subsample included 1017 children (593 boys) who scored in the 80th percentile or higher on disruptive behaviours at the end of kindergarten (age 6 years) with gender-specific cut-offs; this subsample was added to ensure a sufficient prevalence of antisocial cases. Mothers and teachers rated disruptive behaviours using 13 items drawn from the Social Behaviour Questionnaire,Reference Tremblay, Loeber, Gagnon, Charlebois, Larivee and LeBlanc23 which covers physical aggression, opposition, hyperactivity and antisocial behaviour (e.g. lying, stealing). Factor analysis suggested that these items belonged to a single factor (Cronbach's α from 0.86 to 0.90 for mothers and 0.82 to 0.89 for teachers). Participants were evaluated on multiple individual and familial characteristics by their mothers from age 6 to 12, via parental reports and self-reports at age 13 and 15 and via self-reports between ages 21 and 23. We focused on adolescence and adulthood in this study.
Similarly to comparable cohorts followed up longitudinally, non-random attrition was noted.Reference Ouellet-Morin, Côté, Vitaro, Hébert, Carbonneau and Lacourse24 Between the ages of 20 and 23 years, 1241 participants took part in the DNA collection (33% males; n = 412). A total of 12 male participants were later excluded due to population stratification,Reference Ouellet-Morin, Côté, Vitaro, Hébert, Carbonneau and Lacourse24 resulting in a homogeneous sample of 410 genotyped Caucasian males for whom antisocial behaviour was assessed in adolescence and adulthood.25 On average, male participants for whom DNA was not collected in early adulthood (20–23 years) exhibited higher levels of disruptive behaviours in kindergarten (t(1, 528.25) = −3.70, P = 0.001) and were from lower socioeconomic backgrounds (t(1, 469.76) = −6.40, P = 0.001). All statistical analyses were thus weighted for this selective attrition. This study focused on males as 5-HTR 2C, MAOA and MAOB genes are linked to the X chromosome, which contributes to differences between the sexes at a molecular level, including sex-specific impact of genetic variations.Reference Kukurba, Parsana, Balliu, Smith, Zappala and Knowles26 Written informed consent was obtained at each data collection. The study was approved by the research ethics board of Sainte-Justine Hospital and its affiliated universities (ethics approvals 2828/2831).
Measures
Genotyping
A total of 11 serotonergic candidate genes (5-HTR 1A, 5-HTR 2A, 5-HTR 2C, 5-HTR 5A, 5-HTR 6, 5-HTR 7, SLC6A4, MAOA, MAOB, TPH-1 and TPH-2) were selected on the basis of existing knowledge about their physiological role, previous associations with antisocial behaviour and availability of suitable and informative genetic markers.Reference Muller and Jacobs27 Common SNPs (minor allele frequency >5%) located 5 kbp upstream of the transcription sites were selected. Additionally, 44 anonymous markers spread across the genome and located outside of gene-coding regions were genotyped to detect population stratification. We used a high-throughput, 768-SNP Illumina platform and GoldenGate panel based on BeadArray technology.Reference Oliphant, Barker, Stuelpnagel and Chee28 The initial genotyping success rate for the SNPs was 95.4%. SNPs less than 60 bp apart were eliminated, and 33 SNPs were eliminated because of low call rate (<0.90). A genotype call rate of 100% was achieved in the remaining sample. Allele frequencies and Hardy–Weinberg equilibrium analyses were completed using Haploview version 4.0.Reference Barrett, Fry, Maller and Daly29 Hardy–Weinberg equilibrium was rejected for ten SNPs, which were eliminated from further analyses.
Antisocial behaviours
General delinquency was assessed at age 13 by trained research assistants using a semi-structured interview based on the Self-Reported Delinquency Questionnaire.Reference Le Blanc and Frechette30 Participants reported whether they had perpetrated violent offences (e.g. threatened to use violence, carried a weapon), drug-related crimes (e.g. sold drugs), theft (e.g. stole something worth $10, worth $100) and vandalism (e.g. voluntarily damaged a vehicle) over the previous 12 months according to a Likert scale varying from ‘0’ for ‘never’ to ‘4’ for ‘frequently’. The general delinquency scale sums 22 items (range 0–29, mean [s.d.] 4.99 [5.41]) and the internal consistency was good (Cronbach's α from 0.82 to 0.90; 0.75 in our sample).
Conduct disorder symptoms were assessed at age 15 using a semi-structured interview based on the Diagnostic Interview Schedule (DIS) for Children.Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone31 The test-retest reliability and internal consistency of the French version of the DIS for Children were satisfactory.Reference Breton, Bergeron, Valla, Berthiaume and St-Georges32 A total was created by summing the symptoms assessed as being present (range 0–6, mean [s.d.] 0.77 [1.20]). In our population-based sample, the Cronbach's α was 0.61 due to low variability and base rate of some items.
Antisocial personality disorder symptoms were measured at age 21 using the DIS for adults, a semi-structured interview based on the DSM-III-R (1987) criteria (e.g. illegal activities, impulsivity, remorselessness).Reference Robins, Helzer, Cottler and Goldring33 The reliability of the French version of the DIS was satisfactory.Reference Lepage, Jolicoeur, Gheysen, Moulton, Caillard and Diener34 We derived a total score representing the sum of the antisocial personality disorder symptoms indicated as present (range 0–6, mean [s.d.] 1.07 [1.38]). The Cronbach's α was low (0.68) in our sample because of the low base rate of some items in this cohort.
Property/violent crimes were assessed at 21 years of age. Property crimes (e.g. stealing, fraud, burglary) were measured using the Life History Calendar,Reference Freedman, Thornton, Camburn, Alwin and Young-DeMarco35 whereas violent crimes (e.g. assault, possession of a weapon) were assessed using the DIS and the Dimensional Assessment of Personality Pathology–Basic Questionnaire,Reference Livesley and Jackson36 which has good psychometric properties (Cronbach's α from 0.89 to 0.91).Reference Livesley, Jang, Jackson and Vernon37 Each reported behaviour was scored as present or absent in the past year. A total of 78 participants (20.7%) committed at least one property or violent crime.
Physical partner violence was measured at age 21 using 15 items drawn from the French version of the Conflict Tactics Scale,Reference Cyr, Fortin and Chénier38 including violent behaviours against the partner such as pushed/grabbed/shoved, choked/strangled and threatened with a knife/gun. The internal consistency of this instrument was satisfactory.Reference Fortin, Chamberland and Lachance39 Participants who reported at least one instance of physical violence against their partner were identified (n = 40 participants; 10.1%).
Statistical analyses
We investigated the added value of considering haplotype-based superalleles cumulatively instead of relying on individual SNPs when studying candidate genes. To do so, statistical analyses were conducted in four steps. First, we tested the bivariate associations between 116 SNPs mapping to 11 candidate serotonergic genes and the antisocial behaviour using chi-square tests (dichotomous outcomes) and t-tests (continuous outcomes) according to the allelic model using SPSS 24.0 software for Windows. Second, we assessed the linkage disequilibrium between the SNPs located within each gene and identified haplotypes with a frequency of at least 10% using Haploview software.Reference Barrett, Fry, Maller and Daly29 Third, the associations between the haplotype-based superalleles and the antisocial behaviour were tested using PLINK 1.7 software.Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira and Bender40 Nominal and empirical P-values computed from 10 000 Monte Carlo permutations were estimated.Reference Camargo, Azuaje, Wang and Zheng41, Reference Neale, Medland, Neale, Ferreira, Medland and Posthuma42 Fourth, we examined whether participants carrying higher numbers of haplotype-based superalleles conferring risk (or protection) to each antisocial behaviour exhibited more (or less) antisocial behaviours.Reference Rutter, Dodge and Rutter21 To test this possibility, we created a multilocus genetic profile risk score (MGPRS) (using haplotype-based superalleles conferring risk) and a multilocus genetic profile protection score (MGPPS) (using haplotype-based superalleles conferring protection) for each antisocial behaviour based on the observed haplotype associations. Similarly to other studies and given our focus on the cumulative (versus unique) contribution of these haplotype-based superalleles, the candidate gene approach and the relatively small size of our sample, we used an empirical (10 000 permutations) P-value threshold of ≤0.10 for including each superallele in the MGPS.Reference Camargo, Azuaje, Wang and Zheng41–Reference Krapohl, Euesden, Zabaneh, Pingault, Rimfeld and Von Stumm43 In the case where superalleles were respectively positively and negatively associated with antisocial behaviours within the same haplotype block, only the superallele conferring risk was considered to avoid redundancy between the MGPRS and MGPPS. There were no a priori assumptions regarding the genetic model (i.e. allelic, dominant, recessive) and the MGPS was derived according to the best fitting model. Omnibus tests were performed.Reference Knight, Sham, Purcell, Neale, Neale, Ferreira, Medland and Posthuma44 Because antisocial behaviours were not normally distributed, we used negative binominal with log link regression analyses (with robust estimators) to examine the associations between the MGPS, weighting for non-random attrition (see the second paragraph under Participants in the Methods section).
Results
We tested the associations between the 116 SNPs mapping to 11 candidate serotonergic genes and antisocial behaviour (Supplementary Table 1 available at https://10.1192/bjp.2018.251). Several SNPs within the 5-HTR 6, 5-HTR 7, TPH-1 and TPH-2 genes were associated with antisocial behaviours assessed during adolescence and adulthood; 5-HTR 2A, 5-HTR 2C and 5-HTR 5A genes appeared of greater relevance in adulthood. Furthermore, 5-HTR 1A and MAOB genes were not associated with any antisocial behaviour and were thus eliminated from subsequent analyses.
We analysed the patterns of linkage disequilibrium (R 2) within the 5-HTR 2A, 5-HTR 2C, 5-HTR 5A, 5-HTR 6, 5-HTR 7, MAOA, SLC6A4, TPH-1 and TPH-2 genes to estimate haplotypes (Supplementary Fig. 1). Only haplotype-based superalleles with a frequency of at least 10% were considered. Additional associations with antisocial behaviour emerged (see Table 1). Indeed, although only 39% of the SNPs showed at least a trend for significance (empirical P ≤ 0.10) with antisocial behaviour, almost twice as many associations emerged with the haplotypes (78%). For example, whereas SNPs within the 5-HTR 2A gene showed – for the most part – an exclusive pattern of association with physical partner violence, haplotypes AT (block 7, rs2070040, rs9534511) and TA (block 8, rs41142900, rs9534512) now extended the association with general delinquency in adolescence and antisocial personality disorder symptoms in early adulthood. Similarly, we uncovered associations between the TPH-1-TGATCTATG haplotype (block 1, rs10741734, rs1800532, rs10488683, rs10832876, rs685657, rs10488682, rs623580, rs652458, rs546383), conduct disorder (β = 0.27, t(1) = 4.92, P = 0.02) and antisocial personality disorder symptoms (β = −0.29, t(1) = 5.43, P = 0.02).
Table 1 Associations between haplotype-based superalleles within each block and antisocial outcomes
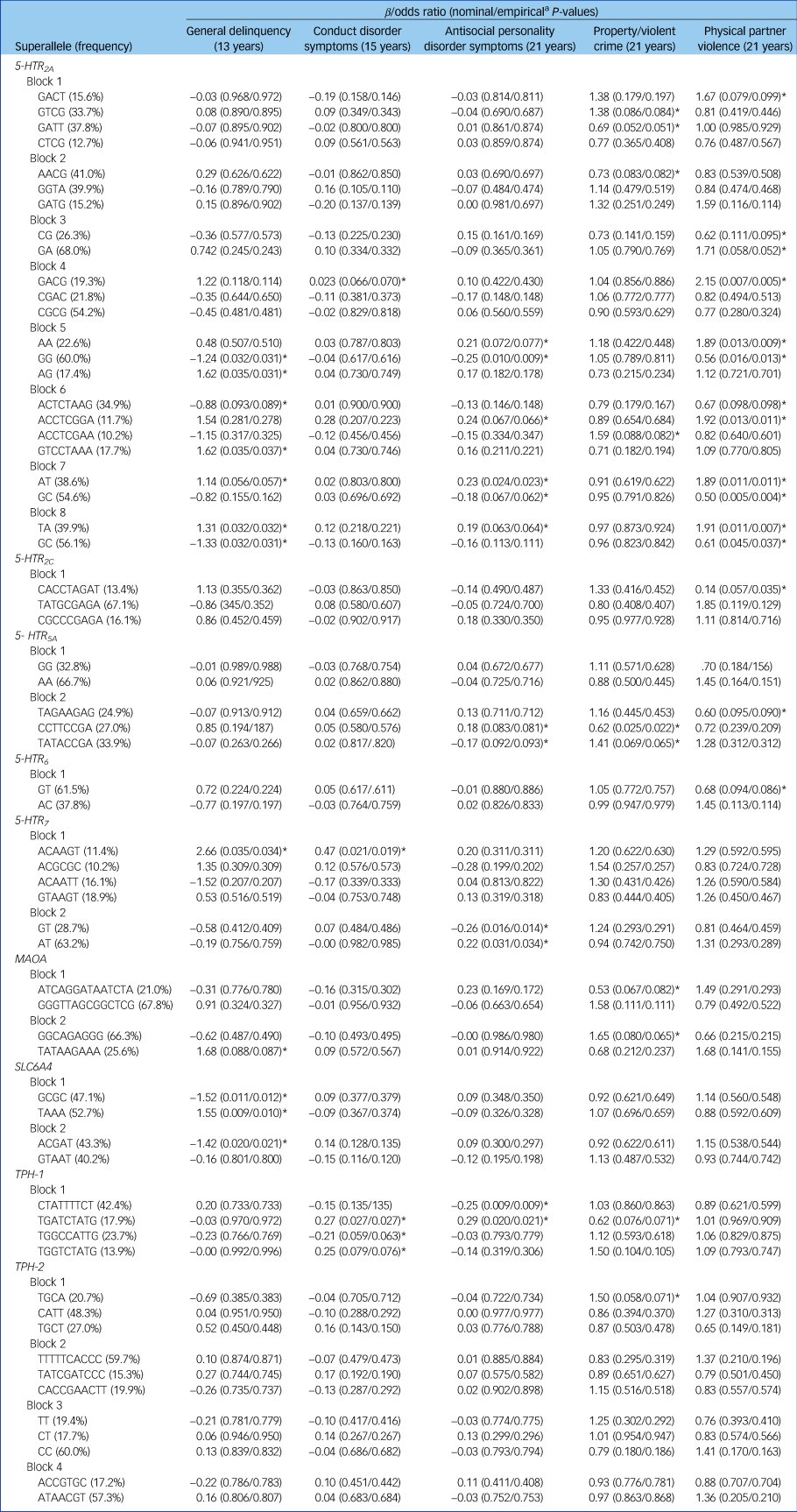
The β statistic is reported for general delinquency, conduct disorder symptoms and antisocial personality disorder symptoms. The odds ratio estimate is reported for property/violent crimes and physical partner violence. Only haplotype-based superalleles with a frequency higher or equal to 10% are reported.
a. Empirical P-values were obtained after 10 000 permutations.
*Empirical P ≤ 0.10.
The sample distribution of the total number of haplotype-based superalleles conferring risk to each antisocial behaviour (Supplementary Fig. 2) show that, for each antisocial behaviour except conduct disorder symptoms (range 0–3, mean 1, s.d. = 1.1), participants carried on average three risk superalleles (general delinquency: range 0–8, mean 2.7, s.d. = 2.1; antisocial personality disorder symptoms: range 0–9, mean 3.4, s.d. = 2.1; property/violent crimes: range 0–7, mean 3.3, s.d. = 1.6; physical partner violence: range 0–12, mean 4.0, s.d. = 3.5).
Figure 1 shows that as the number of superalleles carried by participants increased, there were higher levels of general delinquency (Wald χ 2 = 8.92(1), P ≤ 0.001) and conduct disorder symptoms (Wald χ 2 = 5.10(1), P ≤ 0.05) in adolescence, as well as higher levels of antisocial personality disorder symptoms (Wald χ 2 = 11.89(1), P ≤ 0.001), property/violent crimes (Wald χ 2 = 15.18(1), P ≤ 0.001) and physical partner violence in adulthood (Wald χ 2 = 11.87(1), P ≤ 0.001). Sensitivity analyses were conducted to explore the impact of using more (or less) liberal thresholds on the reported findings (see Supplementary Fig. 3). Results suggest that a threshold for significance at 0.10 offers the best balance between more and less liberal thresholds. MGPRSs contributed to explaining between 2.0% (conduct disorder) and 5.5% (general delinquency) of the phenotypic variance in adolescence, and from 4.2% (antisocial personality disorder symptoms) to 8.0% (physical partner violence) of the variance in outcomes measured in adulthood (Table 2).

Fig. 1 Associations between the multilocus genetic profile risk scores and their related antisocial outcome. Results are shown for (a) general delinquency, (b) conduct disorder symptoms, (c) antisocial personality disorder symptoms, (d) property/violent crimes and (e) physical partner violence.
Table 2 Associations between each multilocus genetic profile risk and protection score and their related antisocial outcome
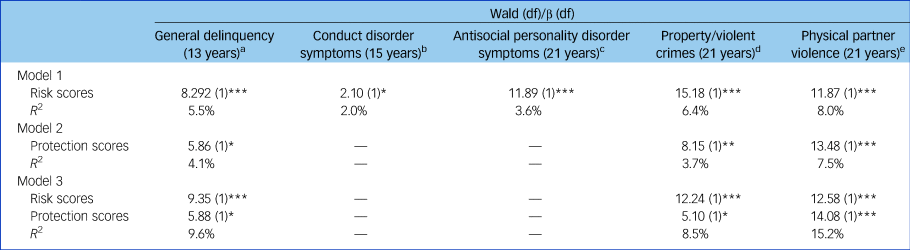
a. MGPRS: HTR2A-AG (rs2770293, rs9316235), HTR2A-GTCCTAAA (rs582385, rs666693, 6561336, rs972979, rs2770304, rs985934, rs927544, rs4941573), HTR2A-AT (rs2070040, rs9534511), HTR2A-TA (rs4142900, rs9534512), HTR7-ACAAGT (rs11599921, rs7904560, rs12261011, rs12259401, rs10785973, rs4520504), MAOA-TATAAGAAA (rs3027400, rs2235186, rs2235185, rs3027405, rs2072744, rs979606, rs979605, rs2239448, rs3027407). MGPPS: SLC6A4-ACGAT (rs3794808, rs4583306, rs2020942, rs6354, rs2020936).
b. MGPRS: HTR2A-GACG (rs9534496, 9526240, rs2224721, rs9316233), HTR7-ACAAGT (rs11599921, rs7904560, rs12261011, rs12259401, rs10785973, rs4520504), TPH1-TGGTCTATG (rs10741734, rs1800532, rs10488683, rs10832876, rs685657, rs10488682, rs623580, rs652458, rs546383).
c. MGPRS: HTR2A-AA (rs2770293, rs9316235), HTR2A-ACCTCGGA (rs582385, rs666693, 6561336, rs972979, rs2770304, rs985934, rs927544, rs4941573), HTR2A-AT (rs2070040, rs9534511; allelic model), HTR2A-TA (rs4142900, rs9534512), HTR5A-CCTTCCGA (rs2873379, rs1017488, rs1881691, rs6320, rs2241859, rs6597455, rs731107, rs1657268), HTR7-AT (rs12259062, rs1891311).
d. MGPRS: HTR2A-GATT (rs3125, rs7322347, rs7997012, rs977003), HTR2A-ACCTCGAA (rs582385, rs666693, 6561336, rs972979, rs2770304, rs985934, rs927544, rs4941573), HTR5A-TATACCGA (rs2873379, rs1017488, rs1881691, rs6320, rs2241859, rs6597455, rs731107, rs1657268), MAOA-GGCAGAGGG (rs3027400, rs2235186, rs2235185, rs3027405, rs2072744, rs979606, rs979605, rs2239448, rs3027407), TPH2-TGCA (rs4448731, rs10748185, rs4565946, rs11179000). MGPPS: HTR2A-AACG (rs6561333, rs1923885, rs1923886, rs7330636), MAOA-ATCAGGATAATCTA (rs3788862, rs5905702, rs5906729, rs3788863, rs6520894, rs5906893, rs1465107, rs1465108, rs5906938, rs5953385, rs5906957, 5906974, rs3027397, rs2283725), TPH1-TGATCTATG (rs10741734, rs1800532, rs10488683, rs10832876, rs685657, rs10488682, rs623580, rs652458, rs546383).
e. MGPRS: HTR2A-GACT (rs3125, rs7322347, rs7997012, rs977003), HTR2A-GA (rs9567739, rs655888; allelic), HTR2A-GACG (rs9534496, 9526240, rs2224721, rs9316233), HTR2A-AA (rs2770293, rs9316235), HTR2A-ACCTCGGA (rs582385, rs666693, 6561336, rs972979, rs2770304, rs985934, rs927544, rs4941573), HTR2A-AT (rs2070040, rs9534511; allelic), HTR2A-TA (block 8, rs4142900, rs9534512). MGPPS: HTR2C-CACCTAGAT (rs508865, rs3795182, rs498208, rs518147, rs2497551, rs2497530, rs2428706, rs6579511, rs4911874), HTR5A-TAGAAGAG (rs2873379, rs1017488, rs1881691, rs6320, rs2241859, rs6597455, rs731107, rs1657268 HTR6-GT (rs6693503, rs9659997).
* P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
In addition to the cumulative contributions noted for the MGPRSs, we also identified haplotypes uniquely associated with lower antisocial behaviour (i.e. general delinquency, property/violent crimes, physical partner violence). On average, participants carried one protective superallele (general delinquency: range 1–2, mean 1.3, s.d. = 0.9; property/violent crimes: range 0–5, mean 1.6, s.d. = 1.2; physical partner violence: range 0–5, mean 1.4, s.d. = 1.2; Supplementary Fig. 4). Each MGPPS had a cumulative effect on antisocial behaviour (see Table 2 and Fig. 2): the more protective superalleles the participants carried, the less likely they were to manifest antisocial behaviours in adolescence (i.e. general delinquency: Wald χ 2 = 5.86(1), P ≤ 0.05) and in early adulthood (i.e. property/violent crimes: Wald χ 2 = 8.15(1), P ≤ 0.01; physical partner violence: Wald χ 2 = 13.48(1), P ≤ 0.001). MGPPSs contributed to explaining from 4.1 to 7.5% of the variance for antisocial behaviour measured in adolescence and adulthood, respectively. When included in the same regression models, both the MGPRS and MGPPS remained significant, thus denoting their unique, additive contribution to antisocial behaviours. Cumulatively, MGPRSs and MGPPSs contributed to 9.6, 8.5, and 15.2% of the variance in general delinquency, property/violent crimes, and physical partner violence, respectively.
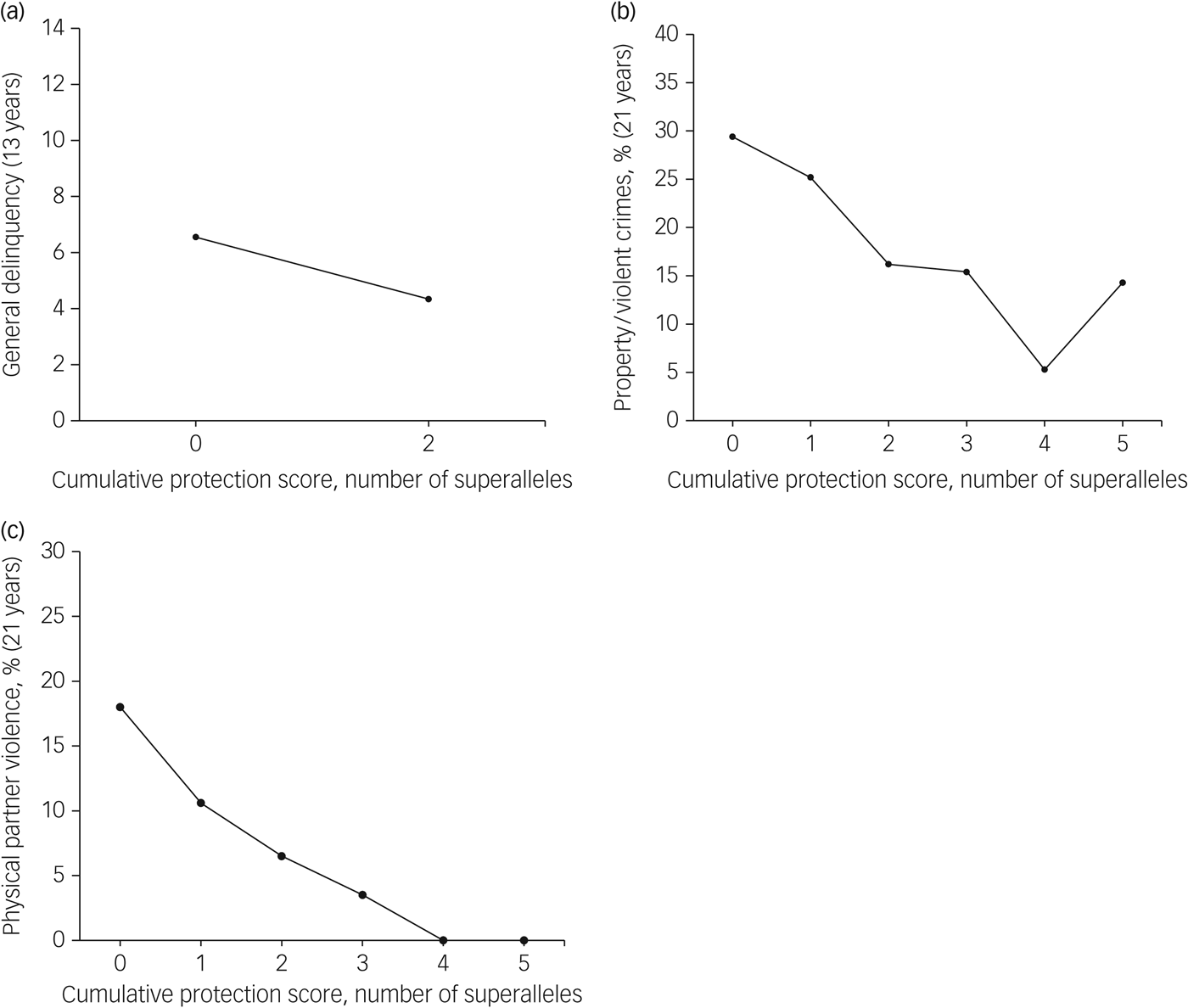
Fig. 2 Associations between the multilocus genetic profile protection scores and their related antisocial outcome. Results are shown for (a) general delinquency, (b) property/violent crimes, and (c) physical partner violence.
Discussion
The association between serotonergic function and antisocial behaviour is one of the most robust findings in biological psychiatry, and it is replicated and supported by endocrine challenge and brain imaging studies.Reference Holmes, Lee, Hollinshead, Bakst, Roffman and Smoller8 However, the identification of the genetic variants involved in these putative mechanisms has been challenging, and inconsistent findings have been reported. Using a population-based sample of Caucasian males, we examined the cumulative contributions of serotonergic genes to antisocial behaviours in adolescence and adulthood. To our knowledge, this is the first study to investigate the genetic burden of antisocial behaviour using haplotype-based MGPSs in a sizeable sample of males. In line with previous genetic and neuroimaging research,Reference Tielbeek, Medland, Benyamin, Byrne, Heath and Madden13, Reference Tielbeek, Johansson, Polderman, Rautiainen, Jansen and Taylor14, Reference Tiihonen, Rautiainen, Ollila, Repo-Tiihonen, Virkkunen and Palotie20 we found a moderate association between the MGPSs conferring risk and antisocial behaviours in adolescence and adulthood. This is consistent with previous findings with this cohortReference Ouellet-Morin, Côté, Vitaro, Hébert, Carbonneau and Lacourse24 and PRSs (derived from genome-wide association studies) of antisocial behaviour.Reference Tielbeek, Medland, Benyamin, Byrne, Heath and Madden13, Reference Tielbeek, Johansson, Polderman, Rautiainen, Jansen and Taylor14, Reference Tiihonen, Rautiainen, Ollila, Repo-Tiihonen, Virkkunen and Palotie20 More generally, our findings echo results from previous haplotype-based studies of complex phenotypes such as depression, bipolar disorder and schizophrenia.Reference Holmes, Lee, Hollinshead, Bakst, Roffman and Smoller8–Reference Ruderfer, Fanous, Ripke, McQuillin and Amdur10
Our study provides preliminary support for the protectiveReference Rutter, Dodge and Rutter21 role of haplotype-based MGPSs with respect to general delinquency in adolescence, as well as regarding property/violent crimes and physical partner violence in early adulthood. These MGPPSs were associated with a reduction in antisocial behaviours. Clearly, these findings need to be replicated in larger samples, correcting for multiple testing before investigating further the mechanisms conferring possible protection, or resilience, to antisocial behaviour. Nevertheless, the stability of these results at both developmental periods, combined with similar findings for pathologies such as diabetes and heart diseases, suggests that the protective value of genetic variants warrants further investigation.Reference Cohen, Boerwinkle, Mosley and Hobbs45, Reference Todd and Wicker46 Indeed, results from inflammatory type 1 diabetes studies in humans and mice suggest that a number of haplotypes are associated with lower risk for disease over and above the haplotypes conferring risk.Reference Todd and Wicker46 In our study, participants carrying the SLC6A4-ACGAT haplotype were less likely to manifest delinquent behaviours in adolescence than their counterparts. Previous studies that have considered another polymorphism, the 5-HTTLPR long and short alleles, reported higher levels of antisocial behaviours when exposed to adverse environments, but not always.Reference Ficks and Waldman2, Reference Belsky and Beaver15 Our results may not be incompatible with prior findings, as the 5-HTTLPR and the SLC6A4-ACGAT haplotype may not be in high linkage disequilibrium together. The effect of 5-HTTLPR may also be conditional to the environment. Again, replication in larger independent samples, correcting for multiple testing and relying on more stringent threshold is needed.
Out of the 11 serotonergic candidate genes investigated in this study, 9 were associated with antisocial behaviours. Pending replications in independent samples, our findings provide additional information regarding the serotonergic basis of individual differences in antisocial behaviours. Serotonin is a widespread neurotransmitter in the central nervous system, and serotonergic neurons are found in numerous brain regions underlying psychopathological and antisocial traits.Reference Strobel, Gutknecht, Rothe, Reif, Mössner and Zeng47, Reference Brown, Peet, Manuck, Williamson, Dahl and Ferrell48 Serotonergic genes such as TPH-1 and TPH-2, which are important in the regulation of serotonin biosynthesis, were shown to be related to smaller amygdala volume and reactivity.Reference Brown, Peet, Manuck, Williamson, Dahl and Ferrell48 A recent meta-analysis of imaging genetic studies also reported an association between the 5-HTTLPR polymorphism and amygdala activation.Reference Robins, Helzer, Cottler and Goldring33 In agreement with prior results,Reference Cuartas Arias, Palacio Acosta, Valencia, Montoya, Arango Viana and Nieto49 the TPH-1 gene rs1800532 increased the risk of antisocial personality disorder symptoms in our study. The TPH-1-TGATCTATG haplotype, which comprised this SNP, was also associated with a greater number of conduct disorder symptoms in adolescence. Similarly to previous studies,Reference Strobel, Gutknecht, Rothe, Reif, Mössner and Zeng47 we found no association between the 5-HTR 1A functional variant rs6295 and antisocial behaviour. We did not, however, replicate the association between the 5-HTR 2A rs7322347A > T SNP and physical aggression.Reference Nomura, Kusumi, Kaneko, Masui, Daiguji and Ueno50 But we did find a significant association between the 5-HTR 2A-GTCG haplotype, which comprised rs6295, and property/violent crimes in adulthood. Of the serotonergic genes implicated in antisocial behaviours, four were linked to more than one antisocial behaviour. The 5-HTR 2A gene, which codes for receptors heavily distributed in the frontal cortex (an area of the brain involved in impulse control), was included in all MGPRSs. For instance, the 5-HTR 2A gene haplotype blocks 7 (AT) and 8 (TA) increased the risk of general delinquency in adolescence, antisocial personality disorder symptoms and physical partner violence in adulthood. Finally, the 5-HTR 2A-TGATCTATG haplotype, which has previously been found to be less prevalent in participants suffering from depression,Reference Brezo, Bureau, Mérette, Jomphe, Barker and Vitaro9 was associated with a greater number of conduct disorder symptoms, antisocial personality disorder symptoms and self-reported property/violent crimes in adulthood. These findings suggest a partly common genetic aetiology of antisocial behaviour in adolescence and early adulthood, and tend to support the model of generalist genes. This also echoes findings from behavioural genetic studies, which identified a shared genetic aetiology across multiple externalised behaviours.Reference Beauchaine and McNulty51
More importantly, our results suggest a cumulative explanatory value of MGPRSs and MGPPSs, which together contribute to better understand the genetically based variance in antisocial behaviours. Our findings fall in line with those reported by others,Reference Shifman, Bronstein, Sternfeld, Pisanté-Shalom, Lev-Lehman and Weizman18, Reference Tiihonen, Rautiainen, Ollila, Repo-Tiihonen, Virkkunen and Palotie20, Reference Knight, Sham, Purcell, Neale, Neale, Ferreira, Medland and Posthuma44 suggesting that the cumulative effect of multiple genetic variants may help, to some extent, in bridging the gap between the heritability estimates derived from twin studies and the variance explained by measured genes. Our results are also consistent with existing literature suggesting that haplotype-based superalleles confer greater statistical power to detect genetic risk than SNPs, especially in smaller samples.Reference Morris and Kaplan16, Reference Clark17 The use of haplotypes may also help to understand the genetic aetiology of antisocial behaviours via independent causal cis-effects of multiple genes. Nonetheless, a large gap remains between the previously reported heritability estimates and our serotonergic haplotype-based MGPSs. To better understand the genetic aetiology of antisocial behaviour, other factors should be considered such as epistasis, epigenetics, gene–environment interactions and the use of intermediate phenotypes.
This study is not without limitations. First, we relied on self-report measures to ascertain several antisocial behaviours, which could be prone to recall bias and memory loss. Importantly, however, the pattern of findings was consistent across both self-reported antisocial behaviour and reports of antisocial behaviours derived from semi-structured interviews (i.e. conduct disorder and antisocial personality disorder symptoms). Second, we used a significance threshold of 0.10 for the inclusion of each haplotype-based superallele in the MGPS. Sensitivity analyses suggested that this threshold offered the best balance between more and less stringent thresholds and that, when more than two haplotype-based superalleles were included, the serotonergic genes’ haplotype-based MGPS had a cumulative effect on antisocial behaviours at two developmental periods and across multimethod assessments. Third, we did not apply corrections for multiple testing. To partially circumvent this issue, we applied several methodological precautions such as selecting haplotype-based superalleles to reduce the number of tests to derive our cumulative scores, focusing on empirical P-values,Reference Camargo, Azuaje, Wang and Zheng41, Reference Neale, Medland, Neale, Ferreira, Medland and Posthuma42 performing an omnibus test when creating our cumulative scoresReference Knight, Sham, Purcell, Neale, Neale, Ferreira, Medland and Posthuma44 and relying on a genetically homogeneous sample.25 Fourth, our results are based exclusively on Caucasian males and they may not be generalisable to females and people of other ethnicities. Finally, few haplotype-based superalleles conferred a protective effect in the absence of risk effect, resulting in more restricted distributions of MGPPSs in comparison to MGPRSs, which could have constrained the statistical power for these indices.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2018.251.
Funding
M. B. was supported by the Canada Research Chair programme from the Canadian Institutes of Health Research. S. L. was supported by the Fonds de Recherche Société et Culture du Québec.
Acknowledgements
We thank the participants and their family members who have given their time to take part in this study.







eLetters
No eLetters have been published for this article.