Schizophrenia is a severe and chronic illness. 1 The first psychotic breakdown is usually seen in adolescence or early adulthood. The illness has a serious impact on the young person's life through cognitive–perceptual, emotional and behavioural dysfunction that strongly interfere with their social life and work. Reference Melau, Jeppesen, Thorup, Bertelsen, Petersen and Gluud2 Also, many people with schizophrenia struggle with substance misuse and depression, and the disorder is often associated with high rates of suicide attempts, violence and criminal problems. The importance of focusing on first-episode psychosis arises because delayed detection and treatment of the illness predicts a poor outcome. Reference Birchwood, Todd and Jackson3–Reference Marshall, Lewis, Lockwood, Drake, Jones and Croudace5 Furthermore, longer periods of untreated psychosis are associated with poorer outcomes. Reference Marshall, Lewis, Lockwood, Drake, Jones and Croudace5 Therefore, it is important to identify opportunities for prevention and treatment of the illness. Early-intervention psychiatric services in psychosis seek to help people who are in the early stages of their illness and are experiencing their first episode of psychosis. The treatment combines enriched assertive community treatment with psychoeducation and family intervention. Evidence for the effectiveness of early-intervention services targeting people in the earlier phases of a diagnosed psychosis has been demonstrated in various studies. Reference McGorry, Johanessen, Lewis, Birchwood, Malla and Nordentoft6 In particular, randomised controlled trials (RCTs) such as the OPUS study in Denmark and the LEO (Lambeth Early Onset) study in the UK have demonstrated a better outcome in the short term (less than 2 years) for specialised early-intervention programmes over standard treatment on a broad range of outcomes including psychotic and negative symptoms, vocational outcome, social functioning, reduced number of bed days in psychiatric in-patient units and improved treatment adherence. Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7–Reference Petersen, Jeppesen, Thorup, Abel, Ohlenschlaeger and Christensen9 In spite of the rapid development of early-intervention services during the past decade, the evidence base for the health economics of these services is still limited with only a few published studies. Reference Cullberg, Mattsson, Levander, Holmqvist, Tomsmark and Elingfors10–Reference Mihalopoulos, Harris, Henry, Harrigan and McGorry14 The current evidence on cost-effectiveness is based on studies with historical controls or studies without enough power in terms of patient numbers to answer health economic questions definitively. Reference Cullberg, Mattsson, Levander, Holmqvist, Tomsmark and Elingfors10,Reference Malla and Pelosi15 In particular, the question of whether early-intervention services are cost-effective in the long term has not been addressed. Reference Malla and Pelosi15 In this study, we aimed to analyse the cost-effectiveness of an intensive early-intervention programme, using data from the largest trial to date, and comparing it with standard community treatment.
Method
Setting and participants
Our cost-effectiveness analysis was based on a single-blind, randomised controlled clinical study comparing an intensive early-intervention programme (called OPUS) with standard treatment (in community mental health centres) in Copenhagen and Aarhus in Denmark. A total of 547 patients in contact with in-patient or out-patient mental health services for the first time were consecutively included in the study from January 1998 to December 2000. The 5-year follow-up rates were 56% (151 patients) in OPUS and 57% (150 patients) in the control group. In order to assess the influence of missing data on the 5-year patient results, outcome measures were subject to further analysis and no statistically significant differences were found between patients attending the 5-year follow-up and those who had dropped out. Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7
At the time of inclusion, patients were between 18 and 45 years of age and had a clinical diagnosis within the schizophrenia spectrum (ICD-10 codes in the F2 category 16 ). None of the patients had previously received antipsychotic medication for more than 12 continuous weeks.
Full details of the study design and intervention are described in the papers presenting the clinical results of the OPUS trial. Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7,Reference Petersen, Jeppesen, Thorup, Abel, Ohlenschlaeger and Christensen9,Reference Jorgensen, Nordentoft, Abel, Gouliaev, Jeppesen and Kassow17,Reference Petersen, Nordentoft, Jeppesen, Øhlenschaeger, Thorup and Christensen18
OPUS
The intensive early-intervention programme consisted of an enriched assertive community treatment inspired by Stein & Test, Reference Stein and Test19 psychoeducational family treatment modelled on McFarlane et al's manual for multifamily groups, Reference McFarlane, Lukens, Link, Dushay, Deakins and Newmark20,Reference McFarlane21 social skills training, Reference Liberman, Wallace, Blackwell, Kopelowicz, Vaccaro and Mintz22 and low-dose medication. Each patient was offered treatment for 2 years (followed by transition to standard treatment) by a multidisciplinary team providing the integrated treatment. The case-load was planned as 1 staff member for each 10 patients. A primary team member was designated for each patient and they were responsible for maintaining contact and coordinating treatment within the team and across different treatment and support facilities. Patients were visited in their homes or other places in their community or at their primary team member's office according to the patient's preference. A crisis plan was developed for each patient.
Standard treatment
Standard treatment consisted of the standard routine care offered by the mental health services in Copenhagen and Aarhus at that time and patients with first-episode psychosis were mixed in with people with a spectrum of other mental disorders. Appointments were usually held at the local community mental health centres and the patients were in contact with a physician, a psychiatric nurse and, in some cases, a social worker. Case-load varied between 20 and 30 patients per member of staff. Administration of antipsychotic medication was based on the same principles as in the OPUS treatment.
Type of evaluation, perspective and length of study
The objective of the economic analysis was to assess the relative cost-effectiveness of OPUS in comparison with standard treatment. The evaluation adopted a public sector perspective when considering the costs associated with early-intervention services (including those for healthcare and the supported housing facility). Cost-effectiveness was assessed by calculating the incremental cost-effectiveness ratio (ICER). Reference Glick, Doshi, Sonnad and Polsky23 The uncertainty surrounding the estimates of expected costs and expected outcomes was estimated by non-parametric bootstrapping of 2000 replicates of the ICER. The cost-effectiveness acceptability curve (CEAC) was estimated as the probability that the OPUS intervention was cost-effective compared with standard treatment, given observed data, for a range of monetary values that a decision-maker might be willing to pay for a unit increase in health outcome measure. Reference Fenwick and Byford24 The study period was 5 years.
Resource use
We extracted all resource data covering 1998 to 2007 for all patients in the study by their personal identification number registered in the Danish Civil Registration System. Since the patients were consecutively enrolled into the study, we classified the use of services in 1-year intervals from 1 to 5 years after the inclusion date.
Data on psychiatric in-patient and out-patient treatment, and contacts with psychiatric emergency departments were extracted from the Danish Psychiatric Central Register. Reference Mors, Perto and Mortensen25 Out-patient data were collected by interviewers during the trial. These data were supplemented by out-patient data extracted from the Danish Psychiatric Central Register for the 3 years following the intervention period.
Data on somatic in-patient and out-patient treatment and visits to hospital accident and emergency rooms were extracted from the National Patient Registry, which is a central registry of all discharges of individual patients from publicly owned hospitals including out-patient visits and accident and emergency department visits. 26 Data on the use of services from general practitioners (GPs) and other medical specialists, dentists, physiotherapists, chiropractors, chiropodists, and psychological counsellors were obtained from the National Health Insurance Service Register, which is a central registry of healthcare services that are reimbursed by National Health Insurance. 27 Data on all patients' use of prescription drugs were collected from the Register of Medicinal Product Statistics, which is a central registry based on transaction reports from the dispensing pharmacies. Reference Kildemoes, Sørensen and Hallas28 It is administered by the Danish Medicines Agency. We collected information on number of days living in supported houses for patients with mental health problems by combining a database with addresses for all supported housing facilities in Denmark with address information in the Civil Status Register.
Costs
The resource volumes were combined with unit costs to obtain a cost per person over their time in the study. All costs in the study were calculated in 2009 values of Danish Kroners and converted to Euros (1 e = 7.44 DKK) both undiscounted and in present values by discounting costs by the annual rate of 3%. (In health economic evaluations, future costs (and sometimes health gains) are commonly weighted in relation to the time at which they occur. Future costs receiving less weight than present ones.)
Since diagnosis-related group (DRG) charges for psychiatric services were not developed in Denmark for this time period, we estimated the cost of bed days in psychiatric hospitals, out-patient contacts and contacts in emergency wards by multiplying the number of bed days and contacts with fixed charges obtained from the Danish National Board of Health. 26 These charges are based on historical cost data, and were considered to approximate public sector opportunity cost. We used DRG charges as unit costs for somatic hospital treatment. The DRG charges were provided by the Danish National Board of Health and reflect the average cost of treating patients with similar conditions in a Danish hospital. For patients who stayed beyond the number of days covered by the DRG charge, an additional charge per day in excess of the number of days covered was added to the hospital costs in accordance with the Danish DRG charge guidelines. In Denmark, hospital treatment is provided free of charge to the patient. National DRG charges are used for reimbursements between regional healthcare authorities providing hospital treatment if a patient from one region is treated at a hospital in another region. These estimates are based on detailed accounts of resource use per patient group in prior years, and are therefore deemed to represent a good approximation of opportunity costs.
The unit costs of GP services and services from other healthcare providers were based on the prevailing National Health Insurance fee schedules. 26 We estimated the costs of living in supported housing facilities by multiplying the number of days in supported housing by a mean charge that represents the reimbursement that supported houses receive from local authorities. This charge varies between the different supported houses depending on the size and support facilities. Since we have no information about which of the supported houses in the area the patients had been assigned to, we used information from the National Board of Social Services on charges of all housing facilities to estimate a mean charge regarding reimbursement that the housing facilities in the Copenhagen and Aarhus area receive per bed day.
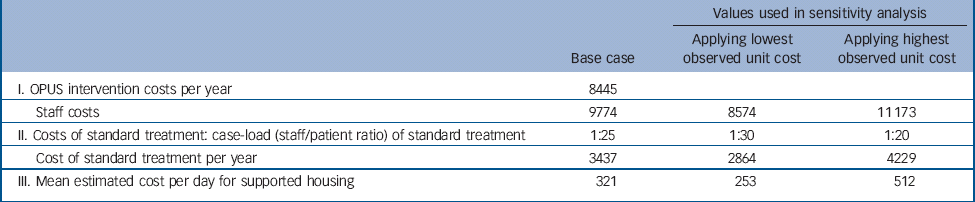
Table 1 Main unit costs and assumptions used in costs analysis and interval for sensitivity analysis, 2009 €
| Values used in sensitivity analysis | |||
|---|---|---|---|
| Base case | Applying lowest observed unit cost |
Applying highest observed unit cost |
|
| I. OPUS intervention costs per year | 8445 | ||
| Staff costs | 9774 | 8574 | 11 173 |
| II. Costs of standard treatment: case-load (staff/patient ratio) of standard treatment | 1:25 | 1:30 | 1:20 |
| Cost of standard treatment per year | 3437 | 2864 | 4229 |
| III. Mean estimated cost per day for supported housing | 321 | 253 | 512 |
In relation to cost assessment, the number of staff per patient treated was the most important difference between OPUS and standard treatment. Whereas OPUS had a case-load of 10 patients per member of staff, the case-load for standard treatment varied between 20 and 30 patients per member of staff. Hence we based our calculation on this information and used a top–down approach to assess the intervention costs and costs of standard treatment.
We estimated the staff cost of one OPUS team as being made up of one full-time: psychiatrist, psychiatric nurse, psychologist, social worker, occupational therapist and secretary. Further, one full-time labour market/educational guide was recruited to the Copenhagen teams in 2000. We added variable costs for transportation, medical drugs and educational and supervision costs of the staff. Fixed costs/operating costs (i.e. rent, electricity, heating and water) were also included. The costs relating to standard treatment were assessed by estimating staff costs of a physician, a psychiatric nurse and a social worker and we included variable and fixed costs.
For the standard treatment we assumed a case-load of 1 member of staff to 25 patients. In sensitivity analysis, we analysed the ways in which the staff costs and charges for supported living facilities affected the overall costs. Further, we examined how different case-loads for standard treatment (1:20 and 1:30) affected the overall costs. Table 1 shows the unit costs that were used for estimation of costs.
Outcome
The primary clinical outcome measure was assessment of overall mental health functioning using the Global Assessment of Functioning (GAF) scale. Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7 The GAF is on a 1–100 scale divided into ten deciles, each of which provides a description of functioning level. A higher score on GAF denotes a better functional level.
Statistical analysis
Data were analysed on an intention-to-treat basis. No statistically significant differences were found between treatment groups at baseline in terms of either clinical or sociodemographic characteristics. Reference Petersen, Nordentoft, Jeppesen, Øhlenschaeger, Thorup and Christensen18 Patients who died during the observation period (7 in OPUS, 10 in the control group) were excluded from the analysis. Resource use and cost data for complete cases were analysed in accordance with the 5-year follow-up of the clinical trial. Mean differences between both groups are presented with their 95% confidence intervals. We estimated confidence intervals by non-parametric bootstrapping as a check of the robustness of the standard parametric t-test. Since we found only minor differences, which did not influence the results, the non-parametric tests are not reported here. Cost-effectiveness was evaluated by relating differences in total costs per patient to differences in effectiveness (GAF) across treatment groups. The ICER was calculated as the difference in mean cost divided by the difference in mean GAF scores at 5-year follow-up. Reference Glick, Doshi, Sonnad and Polsky23 To reflect the uncertainty in the estimates of mean costs and effects, a scatter plot of the 2000 bootstrapped incremental costs and effect is presented on the incremental cost-effectiveness plane shown in Fig. 1. The CEAC was generated by calculating the proportion of 2000 iterations where the incremental cost-effectiveness ratio was below a given threshold. Reference Drummond, Sculpher, Torrance, O'Brien and Stoddart29 All data were analysed using Stata SE 11.0 for Windows at Statistics Denmark's server via remote access.
Results
Outcome data
The primary outcome measure (GAF) demonstrated an effect for OPUS at the 2-year follow-up. The mean GAF score in the OPUS group was statistically significantly higher than in the control group (difference 3.12, 95% CI 0.37–5.88). Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7 The mean GAF scores at 2- and 5-year follow-up are presented in Table 2. A statistically significant difference between OPUS and the control group was not present at the 5-year follow-up (difference 1.19, 95% CI −2.65 to 5.34). Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7
Resource use
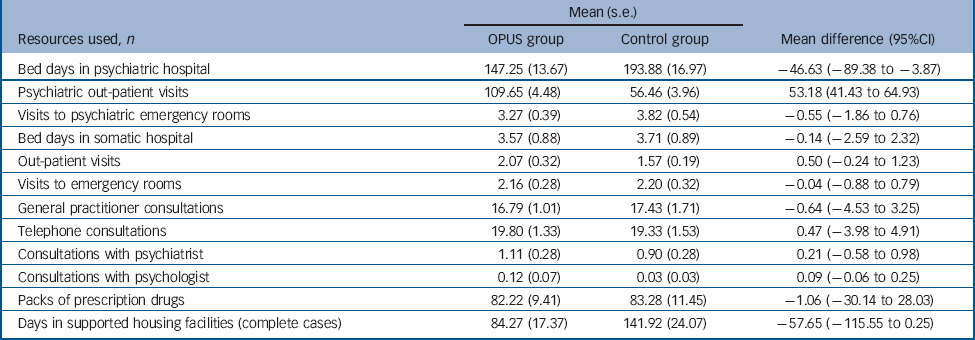
The mean number of psychiatric bed days over 5 years was 46 days lower in the OPUS group than in the control group (95% CI −89.38 to −3.87) (Table 3). As expected, the intervention increased the number of psychiatric out-patient visits compared with standard treatment. Hence, the mean number of psychiatric out-patient visits was 53 days higher in the OPUS than the control group (95% CI 41.43–64.93). The number of days in supported housing facilities was 58 days lower in the OPUS group over 5 years (95% CI 0.25 to −115.55). No other differences in resource use between the two groups were identified.
Costs
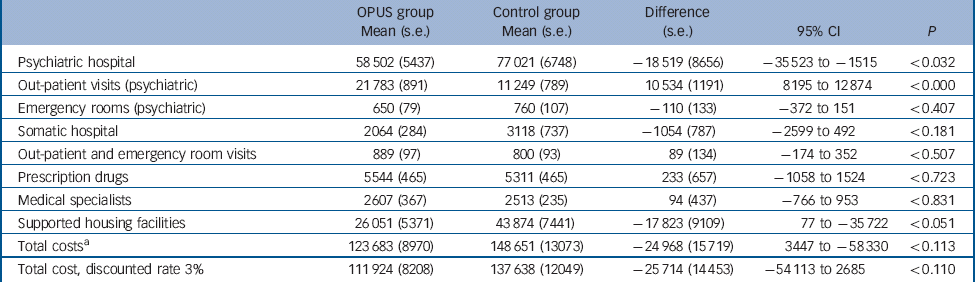
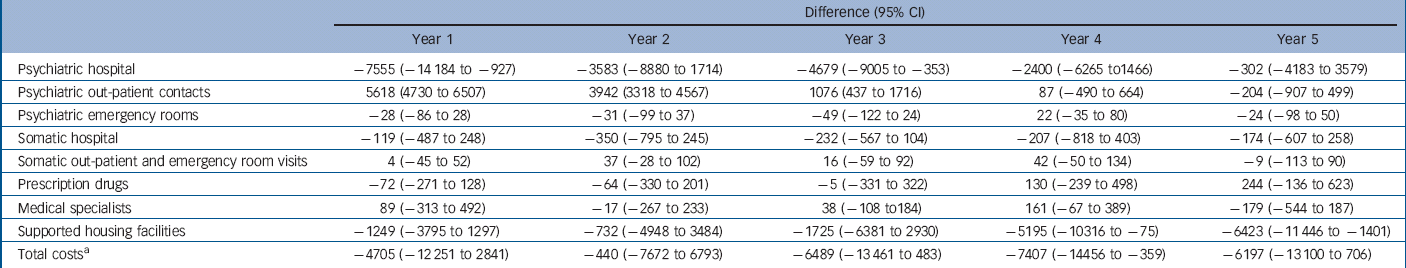
Over 5 years, the mean total costs of OPUS were €123 683 (s.e. = 8970), whereas the mean total costs of standard treatment were €148 651(s.e. = 13073) (Table 4). Table 5 shows that the total costs of OPUS were statistically significantly lower in year 4 (difference 7407, 95% CI −14456 to −359). The psychiatric hospital costs were statistically significantly lower in OPUS in the first and third year after inclusion, whereas the psychiatric out-patient costs were higher in OPUS during the first 3 years. There is no difference in costs of supported housing facilities during the first 3 years, but at 4 and 5 years after inclusion, the costs were lower in OPUS. We found no differences in costs for somatic hospital treatment, medical specialists or prescription drugs over the 5 years.
Table 2 Mean Global Assessment of Functioning – Function (GAF-F) score at 2- and 5-year follow-up

| Treatment group, mean (s.d.) | ||
|---|---|---|
| Follow-up | OPUS | Control |
| 2 years | 55.16 (15.15) | 51.13 (15.92) |
| 5 years | 55.35 (18.28) | 54.16 (18.41) |
Table 3 Resources used, over 5 years, in the OPUS and control groups, selected items
| Mean (s.e.) | |||
|---|---|---|---|
| Resources used, n | OPUS group | Control group | Mean difference (95%CI) |
| Bed days in psychiatric hospital | 147.25 (13.67) | 193.88 (16.97) | –46.63 (–89.38 to −3.87) |
| Psychiatric out-patient visits | 109.65 (4.48) | 56.46 (3.96) | 53.18 (41.43 to 64.93) |
| Visits to psychiatric emergency rooms | 3.27 (0.39) | 3.82 (0.54) | –0.55 (–1.86 to 0.76) |
| Bed days in somatic hospital | 3.57 (0.88) | 3.71 (0.89) | –0.14 (–2.59 to 2.32) |
| Out-patient visits | 2.07 (0.32) | 1.57 (0.19) | 0.50 (–0.24 to 1.23) |
| Visits to emergency rooms | 2.16 (0.28) | 2.20 (0.32) | –0.04 (–0.88 to 0.79) |
| General practitioner consultations | 16.79 (1.01) | 17.43 (1.71) | –0.64 (–4.53 to 3.25) |
| Telephone consultations | 19.80 (1.33) | 19.33 (1.53) | 0.47 (–3.98 to 4.91) |
| Consultations with psychiatrist | 1.11 (0.28) | 0.90 (0.28) | 0.21 (–0.58 to 0.98) |
| Consultations with psychologist | 0.12 (0.07) | 0.03 (0.03) | 0.09 (–0.06 to 0.25) |
| Packs of prescription drugs | 82.22 (9.41) | 83.28 (11.45) | –1.06 (–30.14 to 28.03) |
| Days in supported housing facilities (complete cases) | 84.27 (17.37) | 141.92 (24.07) | –57.65 (–115.55 to 0.25) |
One-way sensitivity analyses were undertaken to explore the impact on the base-case results of changing underlying assumptions of the costing analyses (e.g. changing the staff costs, the case-load for standard treatment, the unit price of supported housing facilities). The results demonstrated that the base-case analysis was robust to different assumptions in the costing analysis. The differences in costs remained statistically insignificant.
Cost-effectiveness analysis
In total, 70% of the points are in the south-eastern quadrant of the cost-effectiveness plane, which represents the position where the intervention is more effective and less costly than the standard treatment (Fig. 1). Figure 1 also shows the point estimate of the ICER.
Figure 2 demonstrates that the probability of the treatment being less costly and more effective than standard treatment is 95.3%, which represents the probability of it being cost-effective when the decision maker is unwilling to incur additional costs for an extra point increase in the GAF. If the decision maker is willing to pay e2000 per point increase in the GAF, the probability rises to 96.5%.
Table 4 Mean cumulative costs (2009 €) per person over 5 years (undiscounted unless stated otherwise), complete cases
| OPUS group Mean (s.e.) |
Control group Mean (s.e.) |
Difference (s.e.) |
95% CI | P | |
|---|---|---|---|---|---|
| Psychiatric hospital | 58 502 (5437) | 77 021 (6748) | –18 519 (8656) | –35 523 to −1515 | <0.032 |
| Out-patient visits (psychiatric) | 21 783 (891) | 11 249 (789) | 10 534 (1191) | 8195 to 12 874 | <0.000 |
| Emergency rooms (psychiatric) | 650 (79) | 760 (107) | –110 (133) | –372 to 151 | <0.407 |
| Somatic hospital | 2064 (284) | 3118 (737) | –1054 (787) | –2599 to 492 | <0.181 |
| Out-patient and emergency room visits | 889(97) | 800 (93) | 89 (134) | –174 to 352 | <0.507 |
| Prescription drugs | 5544 (465) | 5311 (465) | 233 (657) | –1058 to 1524 | <0.723 |
| Medical specialists | 2607 (367) | 2513 (235) | 94 (437) | –766 to 953 | <0.831 |
| Supported housing facilities | 26 051 (5371) | 43 874 (7441) | –17 823 (9109) | 77 to −35 722 | <0.051 |
| Total costsFootnote a | 123 683 (8970) | 148 651 (13073) | –24 968 (15 719) | 3447 to −58 330 | <0.113 |
| Total cost, discounted rate 3% | 111 924 (8208) | 137 638 (12049) | –25 714 (14 453) | –54 113 to 2685 | <0.110 |
a. Total mean costs including intervention costs.
Discussion
The economic evaluation revealed that there was no statistically significant difference in total mean costs between OPUS and standard treatment over 5 years. The difference in health outcome had disappeared at the 5-year follow-up. The incremental cost-effectiveness analysis showed that there was a high probability of OPUS being cost-effective compared with standard treatment.
To our knowledge, no other studies have analysed the cost-effectiveness of early intervention in psychosis based on an RCT in such a large patient group during an extended 5-year follow-up period. In the LEO study Reference McCrone, Craig, Power and Garety12 in south London, 114 patients with newly diagnosed psychosis were randomised to an intervention by a multidisciplinary assertive outreach team. The follow-up period in this study was 18 months. The intervention was comparable with the OPUS intervention and consisted of cognitive–behavioural therapy, family therapy, vocational rehabilitation and low-dose medication regimes. Standard treatment was provided by a community mental health team with no training in dealing with first-episode psychosis. As in the OPUS evaluation, the overall costs showed no statistically significant differences between the treatment groups. The LEO study found a reduction of one-third of psychiatric in-patient costs in the early-intervention group compared with the control group over 18 months. The psychiatric in-patient costs in OPUS were also reduced by one-third; this was during the first 3 years after inclusion and compared with standard treatment and after 3 years the costs reached the same level as standard treatment. In an extension of the follow-up period with the same LEO patient group, the gains of early intervention during the first 1–2 years were lost when transferred to standard treatment. Reference Gafoor, Nitsch, McCrone, Craig, Garety and Power30
Table 5 Difference (95% CI) in mean annual costs in the OPUS group compared with the control group for years 1 to 5, 2009 €, undiscounted
| Difference (95% CI) | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Psychiatric hospital | –7555 (–14 184 to −927) | –3583 (–8880 to 1714) | –4679 (–9005 to −353) | –2400 (–6265 to1466) | –302 (–4183 to 3579) |
| Psychiatric out-patient contacts | 5618 (4730 to 6507) | 3942 (3318 to 4567) | 1076 (437 to 1716) | 87 (–490 to 664) | –204 (–907 to 499) |
| Psychiatric emergency rooms | –28 (–86 to 28) | –31 (–99 to 37) | –49 (–122 to 24) | 22 (–35 to 80) | –24 (–98 to 50) |
| Somatic hospital | –119 (–487 to 248) | –350 (–795 to 245) | –232 (–567 to 104) | –207 (–818 to 403) | –174 (–607 to 258) |
| Somatic out-patient and emergency room visits | 4 (–45 to 52) | 37 (–28 to 102) | 16 (–59 to 92) | 42 (–50 to 134) | –9 (–113 to 90) |
| Prescription drugs | –72 (–271 to 128) | –64 (–330 to 201) | –5 (–331 to 322) | 130 (–239 to 498) | 244 (–136 to 623) |
| Medical specialists | 89 (–313 to 492) | –17 (–267 to 233) | 38 (–108 to 184) | 161 (–67 to 389) | –179 (–544 to 187) |
| Supported housing facilities | –1249 (–3795 to 1297) | –732 (–4948 to 3484) | –1725 (–6381 to 2930) | –5195 (–10316 to −75) | –6423 (–11 446 to −1401) |
| Total costsFootnote a | –4705 (–12 251 to 2841) | –440 (–7672 to 6793) | –6489 (–13 461 to 483) | –7407 (–14456 to −359) | –6197 (–13 100 to −06) |
a. Total mean costs including intervention costs.
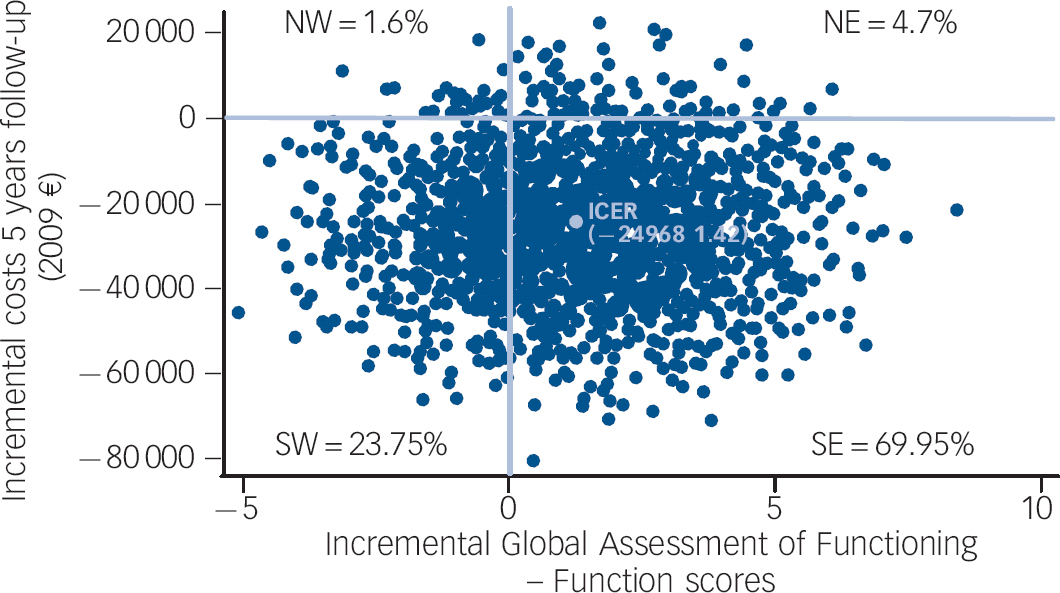
Fig. 1 Cost-effectiveness plane: incremental cost-effectiveness ratio based on 2000 bootstrap replicates, complete cases.
Quadrants: NW, north west; NE, north east; SW, south west; SE, south east.
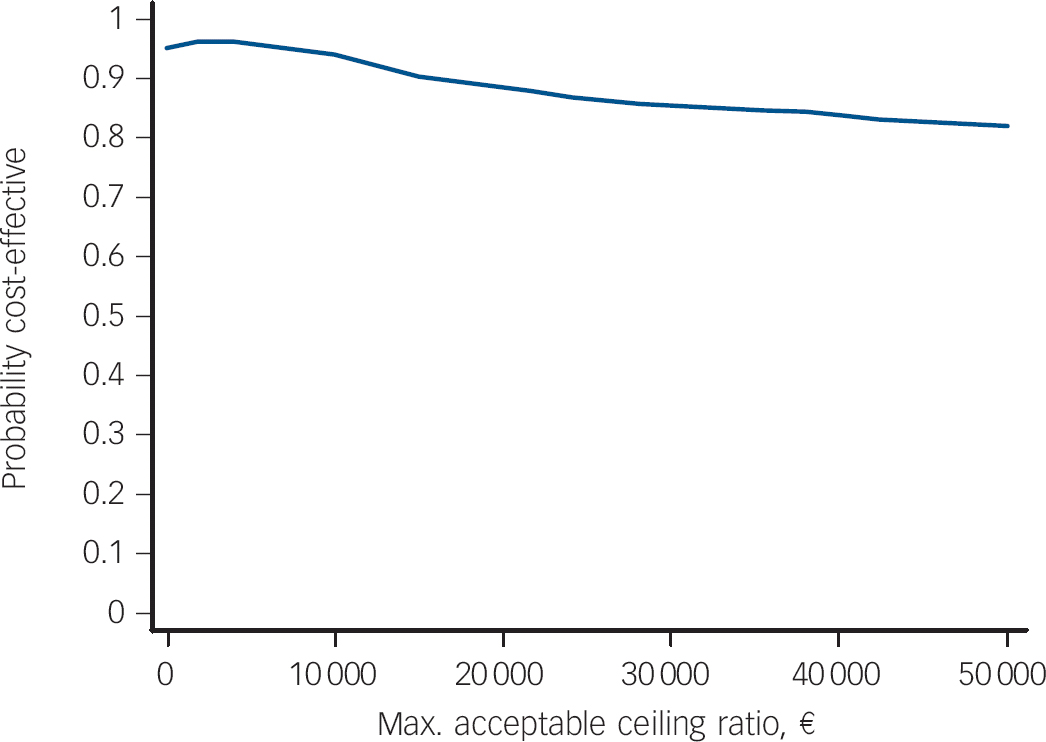
Fig. 2 Cost-effectiveness acceptability curve (Global Assessment of Functioning – Function scores) showing the probability that OPUS is cost-effective over 5 years in comparison with standard treatment, as a function of a decision makers ceiling cost-effectiveness ratio (x-axis), €.
In the EPPIC (Early Psychosis Prevention and Intervention Centre) study from Australia, a cohort of 51 participants with first-episode psychosis who received high-quality care were compared at 1-year follow up with a historical matched control group of 51 patients who received community care. Reference Mihalopoulos, McGorry and Carter13 After the first year of treatment the EPPIC group had significantly better functional and symptomatic outcomes. The improved outcome was demonstrated in association with a reduction in mental health service costs due to a halving of the use of psychiatric in-patient services, which more than compensated for a doubling in the intensity and costs of the EPPIC treatment. In an extension of the EPPIC study, the long-term cost-effectiveness was assessed at the follow-up after approximately 7.5 years. Reference Mihalopoulos, Harris, Henry, Harrigan and McGorry14 Complete follow-up data were available for 65 of the original 102 patients. In contrast to the present study, the EPPIC study found an advantage both in terms of clinical outcomes and treatment costs, which was maintained beyond the period over which the intervention was provided. The EPPIC patients had better functional outcome (GAF) and lower levels of positive psychotic symptoms (Brief Psychiatric Rating Scale total) than the historical controls at follow-up. Moreover, the mean total costs per EPPIC patient, including mental healthcare and medication costs, were one-third of the costs of the control group. The authors, however, acknowledge the caveats of the study that among others include a small sample size and large attrition (36%) of the original cohort of 102 patients. In the Swedish ‘Parachute Project’, 61 patients with first-episode psychotic were followed over 3 years and compared with two other treatment groups. Reference Cullberg, Mattsson, Levander, Holmqvist, Tomsmark and Elingfors10 The study assessed mental healthcare costs and found that the psychiatric in-patient costs in the first-episode group were one-third of the control group, whereas the out-patient costs were more than four times higher in the first-episode group. The study found no differences in costs 2 and 3 years after inclusion.
Strengths and weaknesses of the study
A major strength of the present study is the design of the trial, which was conducted as an RCT in a routine psychiatric out-patient setting, with a relatively large patient sample and a long follow-up period. Further, the study relies on data drawn from official Danish registers, which are known to be of high quality and characterised by a high degree of completeness and validity. Reference Andersen, Madsen, Jorgensen, Mellemkjoer and Olsen31–Reference Olivarius, Hollnagel, Krasnik, Pedersen and Thorsen33 The limitations include the fact the study did not include indirect costs derived from contacts with the criminal justice system or care provided by informal caregivers, such as family and friends. Informal caregiving is unpaid but may clearly carry an economic cost since the time used for informal caregiving usually could be used for other purposes. Reference McCrone34 Other studies among patients with schizophrenia have shown that informal caregiving is substantial in this patient group. Reference Awad and Voruganti35
In addition, productivity loss was not included in the study, but we calculated the costs of early retirement pension as an indicator of the patients' ability to be a part of the labour force. There appeared to be no difference between the treatment groups in the costs of early retirement pension in the 5 years after inclusion in the study. Likewise, there was no difference in the proportion of patients who had a job or were in education after 5 years (data not shown here). Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7 Since the economic evaluation was not planned from the beginning of the trial, the trial was powered to measure differences in health outcome but a power analysis was not performed for economic evaluation. Another limitation was that a preference-based quality of life measure was not available as a supplement to GAF, as an outcome measure for the economic evaluation.
A top–down approach was used to assess the intervention costs and standard treatment based on staff costs, variable and fixed costs. An average cost per patient treated in the OPUS and standard treatment group respectively was calculated by dividing the costs by the number of patients. Hence, there may be cost differences, which are not captured by the average cost per patient, for example cost differences across types of members of staff or use of individual v. group therapy. We believe, however, that the calculated average costs reflect the additional costs of the OPUS intervention due to the higher case-load per patient treated compared with standard treatment.
Unit costs were based on tariffs or charges and therefore do not reflect exact estimates of the true opportunity costs. There may be some variations in the costs of psychiatric in-patient services, which are not accurately reflected in the average unit cost applied. Nevertheless, as most of the services that are included in our analysis are provided by the national healthcare service (i.e. non-profit organisations) the reimbursement rates are likely to be reasonable reflections of the costs of services.
Implications for clinical practice and for policy makers
Since the difference in effect on the primary outcome measures disappeared during the period of 3 years following transition to standard treatment, it is has been suggested that the 2-year intervention period is too short, at least for some of the patients. Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7 Currently, there is no evidence regarding the optimal duration of specialised early-intervention treatment to prevent relapse Reference Bertelsen, Jeppesen, Petersen, Thorup, Øhlenschlaeger and le Quach7 or which specific elements of early intervention need to be offered for an extended period to prevent relapse. Birchwood et al hypothesised that there is a critical period up to 5 years after onset of psychosis, which represents a window of opportunity where the long-term course can be influenced. Reference Birchwood, Todd and Jackson3 An RCT, OPUS-II, is being carried out in Denmark in order to test whether an extension of the specialised intervention up to 5 years will allow the beneficial effects to continue beyond the critical phase, through consolidation of improved social and functional outcomes. Reference Melau, Jeppesen, Thorup, Bertelsen, Petersen and Gluud2 In the light of this evaluation, the costs of a 5-year intervention seem to depend on the intensity of the intervention in terms of out-patient treatment costs and on supported housing facility costs, since the psychiatric in-patient costs reached the same low level in both treatment groups 4 years after the intervention.










eLetters
No eLetters have been published for this article.