Bipolar affective disorder is an ICD–10 mental disorder characterised by at least two episodes involving clinically significant disturbed mood, energy and activity (World Health Organization, 1992). Population-based studies using similar methods in ten countries have estimated prevalence rates ranging from 0.3% in Taiwan to 1.5% in New Zealand (Reference Weissman, Bland and CaninoWeissman et al, 1996). Bipolar disorder has been ranked seventh among the worldwide causes of non-fatal disease burden (World Health Organization, 2001). Wyatt & Henter (Reference Wyatt and Henter1995) estimated the cost of bipolar disorder in 1991 in the USA to be $45 billion; a more recent estimate for the UK amounted to £2 billion (Reference Das Gupta and GuestDas Gupta & Guest, 2002). Internationally, little is known about the relative cost-effectiveness of treatments for bipolar affective disorder, particularly at the level of total (rather than clinical) populations. This study examined the cost-effectiveness of key clinical interventions (mood-stabilising drugs, with or without psychosocial treatment) at the global level.
METHOD
Cost-effectiveness framework
The World Health Organization (WHO) currently has a programme entitled CHOosing Interventions that are Cost-Effective (WHO–CHOICE). Using uniform methodology, the project has generated cost-effectiveness data in 14 sub-regions of the world for key interventions capable of reducing leading contributors to disease burden (http://www.who.int/evidence/cea). A standardised approach for cost-effectiveness analysis has been developed (Reference Tan Torres, Baltussen and AdamTan Torres et al, 2003). A core feature of the approach is that costs and effects of strategies are compared with a starting point of no intervention, which enhances the generalisability of findings (since ‘usual care’ varies between settings).
Setting
The 192 member states of the WHO were divided into five mortality strata based on child and adult mortality rates (World Health Organization, 2001). When these strata were applied to the six WHO regions, they gave rise to 14 epidemiologically defined sub-regions (Table 1). Intervention costs and effects were modelled at the total population level in each sub-region and have been derived in a way that allows for contextualised analyses at the country level.
Table 1 Prevalence of bipolar disorder according to World Health Organization epidemiological sub-region

| Region | Sub-region1 | Mortality | Gender | Prevalence (per 1000; by adult age group, years)2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Child | Adult | 15–29 | 30–44 | 45–59 | 60–69 | 70–79 | 80+ | |||
| Africa | AfrD (e.g. Nigeria, Senegal) | High | High | Male | 5.9 | 9.7 | 5.9 | 3.6 | 2.1 | 0.8 |
| Female | 5.1 | 9.3 | 5.8 | 3.6 | 2.4 | 1.1 | ||||
| AfrE (e.g. Botswana, Kenya) | High | Very high | Male | 5.9 | 9.7 | 5.9 | 3.6 | 2.1 | 0.8 | |
| Female | 5.1 | 9.3 | 5.8 | 3.6 | 2.4 | 1.1 | ||||
| The Americas | AmrA (e.g. Canada, USA) | Very low | Very low | Male | 5.6 | 9.6 | 6.0 | 3.5 | 2.1 | 0.8 |
| Female | 5.6 | 9.6 | 6.1 | 3.7 | 2.3 | 1.0 | ||||
| AmrB (e.g. Brazil, Mexico) | Low | Low | Male | 5.4 | 9.5 | 5.7 | 3.2 | 1.8 | 0.7 | |
| Female | 5.5 | 9.8 | 6.0 | 3.6 | 2.2 | 0.9 | ||||
| Amr D (e.g. Ecuador, Peru) | High | High | Male | 5.4 | 9.5 | 5.7 | 3.2 | 1.8 | 0.7 | |
| Female | 5.5 | 9.8 | 6.0 | 3.6 | 2.2 | 0.9 | ||||
| Eastern Mediterranean | EmrB (e.g. Iran, Jordan, UAE) | Low | Low | Male | 5.2 | 9.7 | 6.0 | 3.5 | 1.9 | 0.7 |
| Female | 5.2 | 9.9 | 6.1 | 3.6 | 2.0 | 0.7 | ||||
| EmrD (e.g. Egypt, Iraq, Pakistan) | High | High | Male | 5.2 | 9.7 | 6.0 | 3.5 | 1.9 | 0.7 | |
| Female | 5.2 | 9.9 | 6.1 | 3.6 | 2.0 | 0.7 | ||||
| Europe | EurA (e.g. France, Norway) | Very low | Very low | Male | 5.9 | 9.7 | 5.9 | 3.6 | 2.1 | 0.8 |
| Female | 5.1 | 9.3 | 5.8 | 3.6 | 2.4 | 1.1 | ||||
| EurB (e.g. Armenia, Poland) | Low | Low | Male | 5.6 | 9.7 | 5.9 | 3.3 | 1.8 | 0.6 | |
| Female | 5.5 | 9.6 | 6.0 | 3.6 | 2.2 | 0.9 | ||||
| EurC (e.g. Estonia, Russia) | Low | Low | Male | 5.6 | 9.7 | 5.9 | 3.3 | 1.8 | 0.6 | |
| Female | 5.5 | 9.6 | 6.0 | 3.6 | 2.2 | 0.9 | ||||
| South-East Asia | SearB (e.g. Indonesia, Thailand) | Low | Low | Male | 4.6 | 9.0 | 5.4 | 3.1 | 1.8 | 0.7 |
| Female | 5.5 | 9.7 | 5.9 | 3.4 | 2.0 | 0.8 | ||||
| SearD (e.g. India, Nepal) | High | High | Male | 5.4 | 9.7 | 5.7 | 3.1 | 1.5 | 0.5 | |
| Female | 5.3 | 9.6 | 5.8 | 3.3 | 1.8 | 0.6 | ||||
| Western Pacific | WprA (e.g. Australia, Japan) | Very low | Very low | Male | 5.2 | 9.2 | 5.7 | 3.5 | 2.2 | 0.9 |
| Female | 6.0 | 9.8 | 6.0 | 3.9 | 2.6 | 1.3 | ||||
| WprB | Low | Low | Male | 5.9 | 9.7 | 6.0 | 3.4 | 1.9 | 0.6 | |
| Female | 6.0 | 9.9 | 6.1 | 3.6 | 2.1 | 0.8 | ||||
Population model for bipolar disorder
Intervention effectiveness was determined via a state transition population model (PopMod; Reference Tan Torres, Baltussen and AdamTan Torres et al, 2003). Key transition rates include the incidence of bipolar disorder in the population, case fatality and remission (defined as full recovery of a case). In addition, a disability weight is specified (on a 0–1 scale, where 0 equals no disability) for time spent in different mood states.
People with bipolar disorder are modelled to live in one of three health states: (a) manic episodes, (b) depressive episodes, or (c) relatively euthymic health states during which persons are nonsymptomatic or symptomatic below the threshold of a manic or depressive episode. In our model, treatment has two possible effects: (a) a change in the distribution of time spent in each state (treated cases spend more time in the intermittent health state and thus experience less disability) and (b) a change in the case fatality rate (reduced suicide). Interventions have no effect on rates of incidence (i.e. onset of bipolar disorder is not prevented) or remission (i.e. the average duration of a case is not reduced).
Using a lifetime analytical horizon, but a 10-year treatment implementation period, population-level effects were derived by comparing number of healthy years lived by the population with and without intervention. The difference between these two simulations represents the population-level health gain (disability-adjusted life years (DALYs) averted) resulting from intervention, relative to the situation of doing nothing. In the base case analysis, nonuniform age weights (which give less weight to years lived at young and older ages) and a 3% discount rate were used, with the impact of these social preferences evaluated via sensitivity analysis.
Natural history of ICD–10 bipolar disorder
The Global Burden of Disease Study (GBD 2000) has generated age- and gender-specific data on the prevalence, incidence and case fatality of persons with bipolar disorder for different regions (http://www.who.int/evidence/bod; Reference Ayuso-MateosAyuso-Mateos, 2001). Prevalence rates for bipolar disorder from the GBD 2000 are shown in Table 1. Since the onset of bipolar disorder is not preventable by health intervention, current incidence coincides with natural (untreated) history. Remission was calculated based on data from Angst & Preisig (Reference Angst and Preisig1995), who reported a 16% remission rate – defined as being episode-free for 5 years – after an average follow-up period of 21 years, equivalent to a yearly rate of less than 1%. Case fatality rates were calculated based on a standardised mortality ratio of 2.5, a weighted average from four natural history studies for the pre-lithium treatment era (e.g. Reference HelgasonHelgason, 1964; others listed under Table 13a in Reference Harris and BarracloughHarris & Barraclough, 1998).
For the disability weight of untreated bipolar disorder, similar assumptions to those employed by GBD 2000 were used, namely applying the same Dutch disability weight for a manic episode as for psychosis (0.72, where 0 equals no disability), and likewise for a (severe) depressive episode (0.76). A valuation of 0.14 for the intermittent state of euthymia was taken to be equivalent to mild depression (Reference Ayuso-MateosAyuso-Mateos, 2001). Baldessarini & Tondo (Reference Baldessarini and Tondo2000) found that 360 people with bipolar disorder spent almost 50% of their time manic or depressed before receiving treatment, and Judd et al (Reference Judd, Akiskal and Schettler2002) provided data on the amount of time that people with the disorder spent in depressive v. manic episodes (a ratio of 3:1). The composite disability weight of untreated bipolar disorder was therefore calculated to be 0.445 (Table 2).
Table 2 Effect of interventions on the disability weight (DW) for bipolar disorder
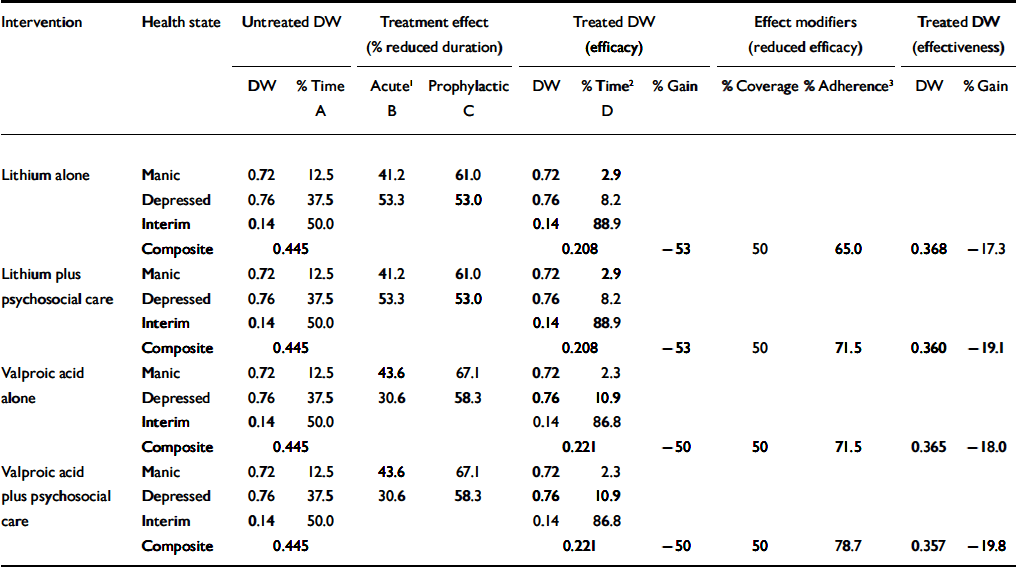
| Intervention | Health state | Untreated DW | Treatment effect (% reduced duration) | Treated DW (efficacy) | Effect modifiers (reduced efficacy) | Treated DW (effectiveness) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DW | % Time A | Acute1 B | Prophylactic C | DW | % Time2 D | % Gain | % Coverage | % Adherence3 | DW | % Gain | ||
| Lithium alone | Manic | 0.72 | 12.5 | 41.2 | 61.0 | 0.72 | 2.9 | |||||
| Depressed | 0.76 | 37.5 | 53.3 | 53.0 | 0.76 | 8.2 | ||||||
| Interim | 0.14 | 50.0 | 0.14 | 88.9 | ||||||||
| Composite | 0.445 | 0.208 | –53 | 50 | 65.0 | 0.368 | –17.3 | |||||
| Lithium plus | Manic | 0.72 | 12.5 | 41.2 | 61.0 | 0.72 | 2.9 | |||||
| psychosocial care | Depressed | 0.76 | 37.5 | 53.3 | 53.0 | 0.76 | 8.2 | |||||
| Interim | 0.14 | 50.0 | 0.14 | 88.9 | ||||||||
| Composite | 0.445 | 0.208 | –53 | 50 | 71.5 | 0.360 | –19.1 | |||||
| Valproic acid | Manic | 0.72 | 12.5 | 43.6 | 67.1 | 0.72 | 2.3 | |||||
| alone | Depressed | 0.76 | 37.5 | 30.6 | 58.3 | 0.76 | 10.9 | |||||
| Interim | 0.14 | 50.0 | 0.14 | 86.8 | ||||||||
| Composite | 0.445 | 0.221 | –50 | 50 | 71.5 | 0.365 | –18.0 | |||||
| Valproic acid | Manic | 0.72 | 12.5 | 43.6 | 67.1 | 0.72 | 2.3 | |||||
| plus psychosocial | Depressed | 0.76 | 37.5 | 30.6 | 58.3 | 0.76 | 10.9 | |||||
| care | Interim | 0.14 | 50.0 | 0.14 | 86.8 | |||||||
| Composite | 0.445 | 0.221 | –50 | 50 | 78.7 | 0.357 | –19.8 | |||||
Estimation of intervention effectiveness
Analyses were limited to first-line interventions. In strict terms, only the conventional mood-stabilising drug lithium meets the criteria for proven efficacy in the acute and prophylactic treatment of both manic and depressive episodes (Reference Bauer and MitchnerBauer & Mitchner, 2004), but these strict criteria were relaxed at least to include a comparator drug for which evidence exists for a prophylactic effect on both manic and depressive episodes (valproic acid; Reference Bowden, Calabrese and McElroyBowden et al, 2000). In addition, the literature indicates that psychosocial approaches enhance adherence to medication (Reference Huxley, Parikh and BaldessariniHuxley et al, 2000; Reference Gonzalez-Pinto, Gonzales and EnjutoGonzalez-Pinto et al, 2004) and potentially affect longer-term improvements in functioning (e.g. Reference Colom, Vieta and Martinez-AranColom et al, 2003). Owing to the restricted level of evidence for these longer-term outcomes (intensive treatment regimens tested within specialist study settings in high-income countries), effects of psychosocial treatment were confined to improved adherence.
Table 2 documents the reduced duration of time spent in a manic or depressed state due to acute and prophylactic treatment (resulting in lower disability), first under optimal conditions (efficacy), then adjusting for adherence and treatment coverage to derive an estimate of population-level effectiveness. An acute treatment effect was calculated as the product of response rate and reduced episode duration. Similar to Goodwin & Jamison (Reference Goodwin and Jamison1990), a slightly higher weighted response rate was found for patients in manic episodes treated with valproic acid than lithium (58.1 v. 55.0%) but a much lower response rate for patients in depressed episodes (38.2 v. 66.7%) (23 source references available from authors on request). The average length of untreated episodes of mania and depression is estimated to be 4 and 5 months, respectively (Reference Angst and SellaroAngst & Sellaro, 2000). Therefore an initial response (within 1 month) will reduce by 75% the time spent in mania and by 80% the time spent depressed. A prophylactic treatment effect was also ascribed: a longitudinal study of 360 people with bipolar disorder adherent to lithium treatment for at least 1 year observed a larger reduction of time spent in mania than depression (61 v. 53%; Reference Tondo, Baldessarini and FlorisTondo et al, 2001a ), and Bowden et al (Reference Bowden, Calabrese and McElroy2000) found a trend favouring a longer time before relapse for valproic acid (median=275 days) compared with lithium (median=189 days). Given a ceiling effect of 1-year follow-up, small sample sizes and exclusion of severe cases, the implied 45% difference in time to relapse is potentially overstated, and accordingly a 10% increased efficacy of valproic acid over lithium in lengthening time to relapse was modelled (half and double this amount were assessed via sensitivity analysis).
A secondary effect of treatment – reduction of the case fatality rate – was also ascribed to lithium (though not to other treatments in the base-case analysis, owing to a current absence of evidence; Reference Goodwin, Fireman and SimonGoodwin et al, 2003). An optimistic estimate comes from a multicentre study by Wolf et al (Reference Wolf, Muller-Oerlinghausen and Ahrens1996), who derived a standardised mortality ratio of 1.1 (natural and unnatural causes of death) for 827 patients treated in lithium clinics over an average period of 7 years. A less optimistic estimate – because it includes studies from the prelithium era – comes from the meta-analysis by Harris & Barraclough (Reference Harris and Barraclough1998), who found a standardised mortality ratio of 2.0 for both natural and unnatural causes (1.5 for natural causes only; 9.2 for unnatural causes only), which is consistent with a review of 22 studies by Tondo et al (Reference Tondo, Hennen and Baldessarini2001b ). A standardised mortality ratio of 1.5 was used in our base-case analysis for people with bipolar disorder treated with lithium, corresponding to a 65% reduction in the instantaneous rate of case fatality. Variations from these estimates were examined via sensitivity analysis.
Changes in disability and case fatality require adjustment for intervention coverage, as well as rates of adherence to intervention. Based on the significant underrecognition and consequent treatment gap for bipolar disorder, a target coverage rate of 50% was set for all sub-regions (Reference Kohn, Saxena and LevavKohn et al, 2004). The review of efficacy studies by Goodwin & Jamison (Reference Goodwin and Jamison1990) found adherence rates for lithium to fall in the range 47–72%. We considered the upper end of this range to reflect rates found in controlled trial settings and the lower end to be a closer estimate of realworld effectiveness. Since our estimates of efficacy already incorporate an element of non-adherence non-adherence (about 30%, see above), we apply a ‘real-world’ adjustment factor (of two-thirds) to obtain a more representative estimate of real-world adherence to lithium (e.g. 70%×66%=47%). The literature suggests that adherence to valproic acid is better than for lithium (e.g. Reference Emilien, Maloteaux and SeghersEmilien et al, 1996; Reference Bowden, Calabrese and McElroyBowden et al, 2000); we therefore set the rate for valproic acid 10% higher than for lithium. Following the reviews of Huxley et al (Reference Huxley, Parikh and Baldessarini2000) and Gonzalez-Pinto et al (Reference Gonzalez-Pinto, Gonzales and Enjuto2004), we also applied a modest improvement of 10% in adherence for combined interventions incorporating a psychosocial component.
Estimation of intervention costs
Two service models were evaluated, a hospital-based in-patient model and a community-based out-patient model. Patient-level resource inputs for an ‘average’ patient with bipolar disorder were weighted according to time spent in manic, depressed or intermittent states, based on earlier empirical or modelling studies (developed countries; Reference Frye, Altshuler and SzubaFrye et al, 1996; Reference Keck, Nabulsi and TaylorKeck et al, 1996) and on a multinational Delphi consensus panel (developing countries; Reference Ferri, Chisholm and Van OmmerenFerri et al, 2004). Annual expected resource requirements – which did not vary between regions because the same level of effective coverage is being modelled – included daily drug supply (e.g. 1200 mg lithium carbonate), blood monitoring and other tests (monthly for lithium treatment, every 2 months for valproic acid), psychosocial support (eight sessions per year, where applicable), monthly out-patient attendances and primary care attendances (20–30% of cases, with an average of six to eight visits). In-patient hospital and residential care differed according to the service model: for the hospital-based service model, 40% of depressive episodes and 45–50% of manic episodes were estimated to lead to an acute psychiatric admission, average length of stay 21–28 days (10–20% of patients were estimated to reside in longer-term psychiatric facilities); for the community-based model, admission rates to acute psychiatric wards for depression (15%) and mania (25%) were estimated to be lower, as were the numbers of patients expected to require residential care support in community-based housing (5–10%). Finally, a 10% reduction in the expected need for admission for acute in-patient care for mania was estimated for combination treatment v. pharmacology alone, and a 10% reduced length of stay was modelled for valproic acid v. lithium.
Resource items were multiplied by respective sub-regional unit costs (Reference Tan Torres, Baltussen and AdamTan Torres et al, 2003; see WHO–CHOICE website at http://www.who.int/evidence/cea) to give an annual cost per treated case, which was then applied to the 50% of cases in the population that are modelled to be exposed to the intervention strategies. Programme-level costs of central administration (planning, implementation, monitoring) and training (adaptation of guidelines, printing of materials) were also derived for each sub-region. All baseline analysis costs for the 10-year implementation period were discounted at 3% and expressed in international dollars (I$), which adjusts for differences in the purchasing power of countries and thereby facilitates comparison within and across sub-regions (i.e. I$1.00 buys the same quantity of healthcare resources in China or India as it does in the USA).
Uncertainty analysis
One-way sensitivity analyses were performed, first on the impact on final cost-effectiveness analysis of analytical social preferences such as discounting and age-weighting, and second on key drivers of cost (unit price of healthcare services, proportion of patients using secondary services) and treatment effectiveness (changes in mortality, disability and adherence). Best- and worst-case scenarios were also generated; these incorporated the combined impact of upper and lower values.
RESULTS
Intervention effects
Total DALYs averted annually by first-line treatment of bipolar disorder with lithium or valproic acid (with and without psychosocial care) are reported for each sub-region in Table 3. Interventions are estimated to avert between 276 and 443 DALYs per million total population in high-mortality, developing sub-regions (AfrD, AfrE, AmrD, EmrD and SearD; see Table 1 for illustrative countries in these sub-regions) and 375–517 DALYs in the remaining sub-regions (lower health gains in high-mortality, developing sub-regions are due to a higher risk of disablement or premature death from other causes). Corresponding results for ‘disability-free days’ gained per treated case range from 53 to 67 days per year. Implemented at a 50% coverage level, and expressed as a proportion of the current reported burden of bipolar disorder (World Health Organization, 2001), population-level health gain associated with these interventions amounts to 11–19% in high-mortality, developing sub-regions, 15–25% in low-mortality, developing sub-regions (AmrB, EmrB, SearB and WprB) and 26–33% in developed sub-regions (AmrA, EurA, EurB, EurC and WprA). Greater averted burden in developed regions results from higher rates of current effective treatment coverage.
Table 3 Intervention effectiveness and averted burden of bipolar disorder1

| Africa | The Americas | Eastern Mediterranean | Europe | South-East Asia | Western Pacific | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AfrD | AfrE | AmrA | AmrB | AmrD | EmrB | EmrD | EurA | EurB | EurC | SearB | SearD | WprA | WprB | |
| Total population (million) | 294.1 | 345.5 | 325.2 | 430.9 | 71.2 | 139.1 | 342.6 | 411.9 | 218.5 | 243.2 | 293.8 | 1241.8 | 154.4 | 1532.9 |
| Current burden of bipolar disorder (thousand)2 | 767 | 899 | 516 | 1037 | 176 | 360 | 832 | 617 | 473 | 450 | 705 | 2946 | 241 | 3691 |
| Intervention effect3 | ||||||||||||||
| Lithium | 376 | 383 | 442 | 432 | 391 | 389 | 382 | 461 | 458 | 461 | 431 | 408 | 473 | 494 |
| Valproic acid | 301 | 276 | 419 | 400 | 355 | 375 | 360 | 437 | 425 | 412 | 393 | 369 | 454 | 465 |
| Lithium+psychosocial | 405 | 411 | 482 | 470 | 425 | 425 | 416 | 503 | 499 | 501 | 469 | 443 | 517 | 539 |
| Valproic acid+ psychosocial | 331 | 304 | 461 | 440 | 391 | 412 | 396 | 481 | 467 | 453 | 432 | 406 | 499 | 511 |
| Effect per treated case (‘disability-free days’)4 | ||||||||||||||
| Lithium | 61.1 | 62.1 | 54.5 | 56.8 | 59.2 | 60.6 | 58.5 | 54.5 | 56.7 | 54.7 | 59.0 | 58.1 | 55.1 | 56.7 |
| Valproic acid | 56.3 | 53.4 | 53.5 | 55.6 | 57.5 | 60.2 | 57.7 | 53.1 | 55.0 | 51.7 | 57.4 | 56.1 | 53.9 | 55.6 |
| Lithium+psychosocial | 66.6 | 67.3 | 59.7 | 62.2 | 64.8 | 66.4 | 64.1 | 59.6 | 62.0 | 59.8 | 64.5 | 63.6 | 60.3 | 62.1 |
| Valproic acid+ psychosocial | 62.0 | 58.8 | 58.9 | 61.2 | 63.3 | 66.2 | 63.5 | 58.4 | 60.5 | 56.9 | 63.1 | 61.7 | 59.3 | 61.2 |
| % Current burden averted5 | ||||||||||||||
| Lithium | 14.4 | 14.7 | 27.8 | 17.9 | 15.8 | 15.0 | 15.7 | 30.7 | 21.1 | 24.9 | 18.0 | 17.2 | 30.3 | 20.5 |
| Valproic acid | 11.5 | 10.6 | 26.4 | 16.6 | 14.4 | 14.5 | 14.8 | 29.2 | 19.6 | 22.3 | 16.4 | 15.5 | 29.1 | 19.3 |
| Lithium+psychosocial | 15.5 | 15.8 | 30.4 | 19.5 | 17.2 | 16.4 | 17.1 | 33.6 | 23.0 | 27.1 | 19.5 | 18.7 | 33.1 | 22.4 |
| Valproic acid+ psychosocial | 12.7 | 11.7 | 29.1 | 18.3 | 15.8 | 15.9 | 16.3 | 32.1 | 21.6 | 24.5 | 18.0 | 17.1 | 32.0 | 21.2 |
Differences in the effectiveness of the four interventions in the base-case analysis are modest, but strategies using lithium generate marginally greater population-level health gain than those with valproic acid, on account of the additional impact of lithium on case fatality rates. Adjuvant psychosocial treatment provided in tandem with mood stabiliser drugs also improves outcomes by approximately 10%, reflecting the improved adherence modelled.
Intervention costs
Intervention costs, both per million total population and per treated case, are presented in Table 4. Hospital-based service models incur notably higher costs than community-based service models (35–50% in very low-income regions to as much as 70% in high-income regions) as a result of differences in the expected use of acute in-patient and longer-term residential facilities. The total programme- and patient-level cost for interventions implemented via a community-based out-patient model, in millions of international dollars per million population (therefore equivalent to cost per capita), ranged from 0.85 to 1.78 in high-mortality, developing sub-regions, 1.77–3.23 in low-mortality, developing sub-regions and 2.74–10.57 in developed sub-regions. Corresponding baseline results per treated case were I$538–998, I$925–1524 and I$1168–4187 per year, respectively.
Table 4 Costs of bipolar disorder treatment (I$2000) at a coverage rate of 50%1

| Africa | The Americas | Eastern Mediterranean | Europe | South-East Asia | Western Pacific | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AfrD | AfrE | AmrA | AmrB | AmrD | EmrB | EmrD | EurA | EurB | EurC | SearB | SearD | WprA | WprB | |
| Total population (million) | 294.1 | 345.5 | 325.2 | 430.9 | 71.2 | 139.1 | 342.6 | 411.9 | 218.5 | 243.2 | 293.8 | 1241.8 | 154.4 | 1532.9 |
| Intervention cost2 | ||||||||||||||
| Hospital-based service model | ||||||||||||||
| Lithium | 1.22 | 1.27 | 16.94 | 5.27 | 2.69 | 2.78 | 2.00 | 14.93 | 4.64 | 5.55 | 3.07 | 1.55 | 17.33 | 3.99 |
| Valproic acid | 1.34 | 1.38 | 16.48 | 5.28 | 2.78 | 2.87 | 2.12 | 14.58 | 4.71 | 5.59 | 3.17 | 1.71 | 16.89 | 4.11 |
| Lithium+psychosocial | 1.24 | 1.29 | 17.17 | 5.17 | 2.67 | 2.77 | 2.00 | 15.26 | 4.58 | 5.46 | 3.04 | 1.58 | 17.64 | 3.95 |
| Vaproic acid+psychosocial | 1.37 | 1.41 | 16.79 | 5.21 | 2.77 | 2.87 | 2.13 | 14.97 | 4.67 | 5.52 | 3.15 | 1.74 | 17.28 | 4.08 |
| Community-based service model | ||||||||||||||
| Lithium | 0.85 | 0.91 | 9.95 | 3.14 | 1.65 | 1.78 | 1.25 | 8.77 | 2.80 | 3.30 | 1.88 | 1.06 | 10.26 | 2.46 |
| Valproic acid | 0.99 | 1.04 | 9.73 | 3.23 | 1.78 | 1.90 | 1.39 | 8.64 | 2.93 | 3.42 | 2.02 | 1.23 | 10.07 | 2.63 |
| Lithium+psychosocial | 0.88 | 0.93 | 10.19 | 3.04 | 1.63 | 1.77 | 1.24 | 9.10 | 2.74 | 3.21 | 1.85 | 1.09 | 10.57 | 2.43 |
| Valproic acid+psychosocial | 1.02 | 1.07 | 10.07 | 3.16 | 1.77 | 1.91 | 1.40 | 9.05 | 2.89 | 3.36 | 2.01 | 1.27 | 10.47 | 2.62 |
| Cost per treated case (1$ per year)3 | ||||||||||||||
| Hospital-based service model | ||||||||||||||
| Lithium | 799 | 864 | 7099 | 2526 | 1532 | 1545 | 1122 | 5774 | 2015 | 2309 | 1654 | 822 | 6417 | 1626 |
| Valproic acid | 883 | 945 | 6906 | 2532 | 1583 | 1596 | 1191 | 5638 | 2045 | 2325 | 1604 | 905 | 6253 | 1672 |
| Lithium+psychosocial | 816 | 880 | 7199 | 2481 | 1519 | 1541 | 1121 | 5900 | 1987 | 2271 | 1540 | 838 | 6531 | 1610 |
| Valproic acid+psychosocial | 903 | 964 | 7036 | 2498 | 1576 | 1598 | 1194 | 5790 | 2025 | 2297 | 1596 | 924 | 6395 | 1663 |
| Community-based service model | ||||||||||||||
| Lithium | 521 | 557 | 4087 | 1483 | 927 | 940 | 646 | 3331 | 1197 | 1361 | 939 | 533 | 3697 | 979 |
| Valproic acid | 614 | 648 | 3996 | 1524 | 998 | 1011 | 730 | 3278 | 1254 | 1409 | 1010 | 626 | 3626 | 1048 |
| Lithium+psychosocial | 538 | 573 | 4187 | 1438 | 914 | 936 | 645 | 3457 | 1168 | 1323 | 925 | 550 | 3812 | 963 |
| Valproic acid+psychosocial | 635 | 668 | 4136 | 1494 | 993 | 1015 | 735 | 3438 | 1236 | 1384 | 1004 | 647 | 3777 | 1040 |
Within sub-regions and service models, variations in intervention costs were very modest, which is attributable to the fact that additional costs of valproic acid over lithium (I$168 per year) and adjuvant psychosocial treatment were expected to slightly reduce the need for in-patient care. This modelled cost-offset effect is more pronounced in high-income sub-regions where the unit cost of an in-patient day is high, resulting in valproic acid becoming a marginally less expensive intervention strategy than lithium. In low-income sub-regions, lithium is estimated to be the cheaper option.
Intervention cost-effectiveness
When total population-level costs and effects are merged to produce average cost-effectiveness ratios (Table 5), it becomes apparent that a community-based approach represents a more efficient strategy than a hospital-based approach for addressing the current burden of bipolar disorder (cost-effectiveness ratios are estimated to be 25–40% lower). Differences in cost-effectiveness ratio between interventions are modest, but in all sub-regions the single most cost-effective strategy for the base-case analysis is lithium with psychosocial care, delivered within a community-based service framework, each averted DALY costing I$2165–3830 in high-mortality, developing sub-regions, I$3953–6475 in low-mortality, developing sub-regions and I$5487–21123 in developed sub-regions. This is equivalent to averting 47–182 DALYs per I$1 million expenditure in developed sub-regions, 154–253 DALYs in low-mortality, developing sub-regions and 261–462 DALYs in high-mortality, developing sub-regions.
Table 5 Comparative cost-effectiveness of interventions for bipolar disorder1
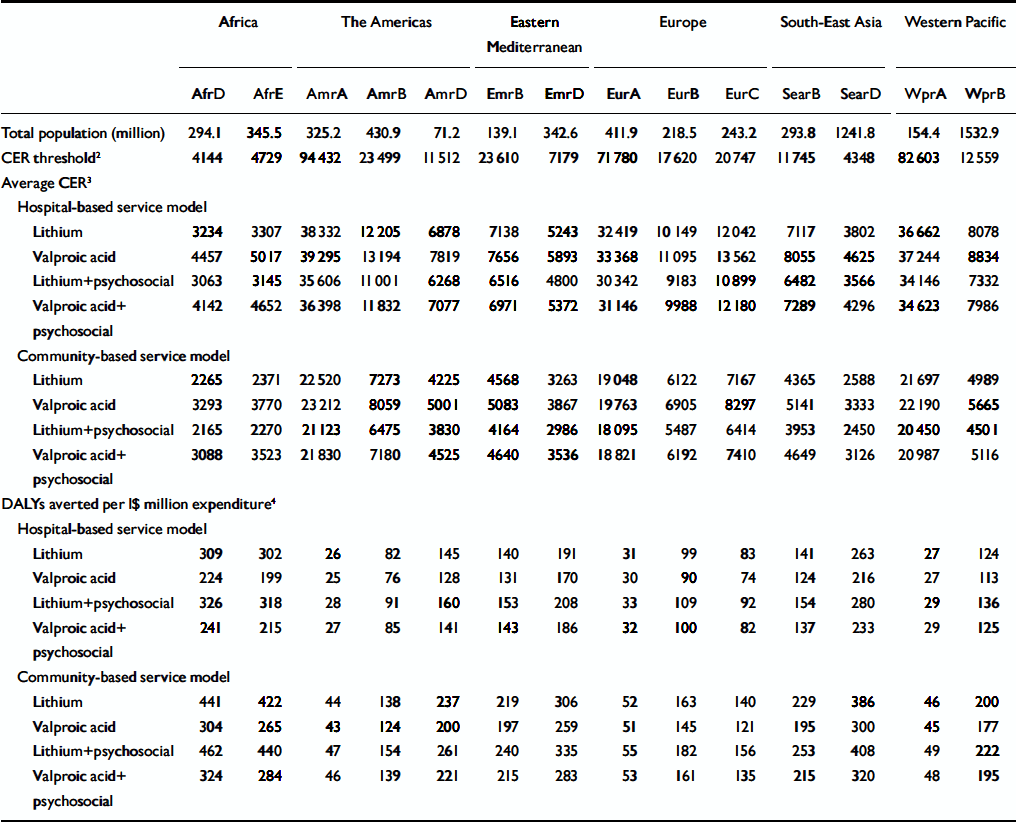
| Africa | The Americas | Eastern Mediterranean | Europe | South-East Asia | Western Pacific | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AfrD | AfrE | AmrA | AmrB | AmrD | EmrB | EmrD | EurA | EurB | EurC | SearB | SearD | WprA | WprB | |
| Total population (million) | 294.1 | 345.5 | 325.2 | 430.9 | 71.2 | 139.1 | 342.6 | 411.9 | 218.5 | 243.2 | 293.8 | 1241.8 | 154.4 | 1532.9 |
| CER threshold2 | 4144 | 4729 | 94 432 | 23 499 | 11 512 | 23 610 | 7179 | 71 780 | 17 620 | 20 747 | 11 745 | 4348 | 82 603 | 12 559 |
| Average CER3 | ||||||||||||||
| Hospital-based service model | ||||||||||||||
| Lithium | 3234 | 3307 | 38 332 | 12 205 | 6878 | 7138 | 5243 | 32 419 | 10 149 | 12 042 | 7117 | 3802 | 36 662 | 8078 |
| Valproic acid | 4457 | 5017 | 39 295 | 13 194 | 7819 | 7656 | 5893 | 33 368 | 11 095 | 13 562 | 8055 | 4625 | 37 244 | 8834 |
| Lithium+psychosocial | 3063 | 3145 | 35 606 | 11 001 | 6268 | 6516 | 4800 | 30 342 | 9183 | 10 899 | 6482 | 3566 | 34 146 | 7332 |
| Valproic acid+psychosocial | 4142 | 4652 | 36 398 | 11 832 | 7077 | 6971 | 5372 | 31 146 | 9988 | 12 180 | 7289 | 4296 | 34 623 | 7986 |
| Community-based service model | ||||||||||||||
| Lithium | 2265 | 2371 | 22 520 | 7273 | 4225 | 4568 | 3263 | 19 048 | 6122 | 7167 | 4365 | 2588 | 21 697 | 4989 |
| Valproic acid | 3293 | 3770 | 23 212 | 8059 | 5001 | 5083 | 3867 | 19 763 | 6905 | 8297 | 5141 | 3333 | 22 190 | 5665 |
| Lithium+psychosocial | 2165 | 2270 | 21 123 | 6475 | 3830 | 4164 | 2986 | 18 095 | 5487 | 6414 | 3953 | 2450 | 20 450 | 4501 |
| Valproic acid+psychosocial | 3088 | 3523 | 21 830 | 7180 | 4525 | 4640 | 3536 | 18 821 | 6192 | 7410 | 4649 | 3126 | 20 987 | 5116 |
| DALYs averted per 1$ million expenditure4 | ||||||||||||||
| Hospital-based service model | ||||||||||||||
| Lithium | 309 | 302 | 26 | 82 | 145 | 140 | 191 | 31 | 99 | 83 | 141 | 263 | 27 | 124 |
| Valproic acid | 224 | 199 | 25 | 76 | 128 | 131 | 170 | 30 | 90 | 74 | 124 | 216 | 27 | 113 |
| Lithium+psychosocial | 326 | 318 | 28 | 91 | 160 | 153 | 208 | 33 | 109 | 92 | 154 | 280 | 29 | 136 |
| Valproic acid+psychosocial | 241 | 215 | 27 | 85 | 141 | 143 | 186 | 32 | 100 | 82 | 137 | 233 | 29 | 125 |
| Community-based service model | ||||||||||||||
| Lithium | 441 | 422 | 44 | 138 | 237 | 219 | 306 | 52 | 163 | 140 | 229 | 386 | 46 | 200 |
| Valproic acid | 304 | 265 | 43 | 124 | 200 | 197 | 259 | 51 | 145 | 121 | 195 | 300 | 45 | 177 |
| Lithium+psychosocial | 462 | 440 | 47 | 154 | 261 | 240 | 335 | 55 | 182 | 156 | 253 | 408 | 49 | 222 |
| Valproic acid+psychosocial | 324 | 284 | 46 | 139 | 221 | 215 | 283 | 53 | 161 | 135 | 215 | 320 | 48 | 195 |
Uncertainty analysis
Substitution of the baseline discount rate of 3% with values of 0% and 6% altered total costs and average cost-effectiveness ratios for all interventions by +14% and 711%, respectively. The removal of age-weighting had a larger impact on results, reducing health gain estimates by 11–23% across sub-regions (resulting in an increase of 13–30% in average cost-effectiveness ratios).
One-way sensitivity analysis showed that cutting the impact of lithium on case fatality rates by half (from 65% to 32.5%, equivalent to a revised standardised mortality ratio of 2.0 compared with 1.5 for the base case) reduced DALYs averted by approximately 8%; attribution of a small anti-suicide effect for valproic acid (a reduction of 16%, standardised mortality ratio 2.25) increased total health gain by 4%. Either change is enough to remove the small baseline effectiveness advantage of lithium over valproic acid. Use of alternative disability weights for mania, depression and euthymia had no bearing on the relative effectiveness of different interventions (and only a small impact on absolute levels of health gain). Higher and lower values for the assumed prophylactic advantage of valproic acid over lithium likewise had a negligible impact. A more sensitive variable is adherence, where plausible variations in both the level of adherence to lithium (10%) as well as the expected size of adherence differentials between mood stabilisers and between monotherapy v. combination treatments (a lower value of 5% and an upper value of 15%, compared with a baseline difference of 10%) changed baseline effectiveness results by 10–20%.
Best- and worst-case scenarios were derived for cost-effectiveness by according lower and upper 95% CIs to the unit costs of health services, the proportion of cases using secondary care hospital services (relative changes of 20–50%, for example an admission rate of 30% rather than 20%) and number of psychosocial treatment sessions, in addition to the upper and lower values reported above for treatment response and adherence. Under the best-case scenario, total costs were 31–47% lower for the hospital service model and 20–37% lower for the community service model, total effects were 18–39% higher (including a potential impact of valproic acid on suicide rates), thereby lowering the overall cost per DALY averted by approximately half. Results for the worst-case scenario were in the same range; in this case increases of close to 45–75% in costs and 23–30% less health gain led to average cost-effectiveness ratios 120–150% higher than their baseline values. To illustrate, the expected cost per DALY for community-based lithium treatment in the Western Pacific sub-region WprB (baseline value I$4989) ranged from I$2771 to I$10 952. The principal finding from these multiway sensitivity analyses was that lithium-based treatments remain the cost-effective choice in high-mortality developing sub-regions, whereas in the three high-income sub-regions of America, Europe and the Western Pacific valproic acid – alone or in combination with psychosocial treatment – now produces the lowest cost per DALY averted (full details in spreadsheet format available from the authors on request).
DISCUSSION
Health system uses of sectoral cost-effectiveness analysis
This study examined the cost-effectiveness of interventions capable of reducing the burden of bipolar disorder. The purpose of this exercise is to locate the relative position of effective and applicable interventions for this disorder within a wider cost-effectiveness and priority-setting framework in the healthcare sector. Such information is of particular use in developing regions of the world where there remains a high level of untreated disease burden attributable to bipolar disorder, with very limited resources for appropriate management. More useful still is the contextualisation of sub-regional results at the national level, a process now underway in a small number of countries through which default values for key model parameters can be substituted with local estimates of psychiatric epidemiology, clinical effectiveness, resource use profiles and unit prices.
Comparative cost-effectiveness of interventions for bipolar disorder
The treatments analysed in this sectoral cost-effectiveness analysis of interventions for bipolar disorder enabled three key comparisons: older v. newer mood-stabilising drugs (lithium v. valproic acid); combined pharmacotherapy and psychosocial care v. pharmacotherapy alone; and hospital-based v. community-based service models. Valproic acid was modelled to have a marginally greater prophylactic treatment effect and better adherence than lithium, but a lower acute treatment effect for depressive episodes and no effect on case fatality. It is the expected health gain associated with a demonstrated impact on suicide rates (Reference Tondo, Hennen and BaldessariniTondo et al, 2001b ; Reference Goodwin, Fireman and SimonGoodwin et al, 2003) that suggests lithium is a more beneficial and no more costly population-level treatment compared with valproic acid. Adjuvant psychosocial treatment alongside use of mood-stabilising drugs is expected to improve the cost-effectiveness of treatment for bipolar disorder, as a result of improved adherence (which in effect reduces the proportion of time spent in a manic or depressed state), and because the additional costs of psychosocial treatment are largely offset by a reduced probability of admission to hospital. Finally, and again because of expected reductions in the use of expensive hospital in-patient facilities, treatments provided within a community-based service model offer a more efficient (and because of improved accessibility, more equitable) use of resources than hospital-based services.
At a broader, sectoral level of comparison, interventions for bipolar disorder are not substantially different from each other. Expressed in relation to gross national income, the cost-effectiveness ratios for community-based interventions fall between one and three times the gross national income per capita, a range considered by the Commission on Macroeconomics and Health (2001; p. 103) to be ‘cost-effective’ (as opposed to ‘very cost-effective’, below average gross national income per capita; or ‘not cost-effective’, more than three times gross national income per capita). Comparisons with other studies are limited owing to the aggregate level of analysis employed here; however, our results for the Western Pacific sub-region WprA (which includes Australia) can be compared with the recent study by Sanderson et al (Reference Sanderson, Andrews and Corry2003); our estimated cost of I$20 000–22 000 for each DALY averted by community-based treatment is slightly higher than their estimated cost for current and optimal treatment (Aus$24 000, or I$18 200), which can be attributed to our inclusion of programme-level costs, a higher expected rate of in-patient admission for mania and also greater downward adjustment of efficacy estimates for ‘real-world’ effectiveness at the population level.
Compared with many other strategies analysed to date under the WHO–CHOICE project – including population-based interventions to reduce heavy alcohol use, smoking and cardiovascular disease – bipolar disorder interventions have a relatively high ratio of cost to health outcome, and exceed the average cost per averted DALY for efficient primary-care-based depression interventions by a factor of 4–6 (Reference Chisholm, Sanderson and Ayuso-MateosChisholm et al, 2004). Such a finding is hardly surprising in terms of costs, given the multiple health service needs of persons with a diagnosis of bipolar disorder, but does serve to highlight the rather modest impact of these interventions on the natural course of this disorder. Indeed, even at a population treatment coverage rate of 50%, modelled interventions are only able to avert between 10 and 33% of the burden, which points to a clear need to further develop culturally appropriate psychosocial approaches capable of delivering improved long-term functioning over and above shorter-term considerations such as medication adherence (e.g. Reference Colom, Vieta and Martinez-AranColom et al, 2003).
Limits and limitations of economic modelling
In common with other modelling studies, this analysis is a highly restricted representation of reality. Bipolar disorder has a heterogeneous course, with patients experiencing marked differences in rates of cycling between different mood states, which is not well captured here. Separate sub-analyses for ‘rapid cyclers’ would be expected to reveal higher costs and worse outcomes, for example, as might an analysis that would take into account the full range of possible comorbidities. In modelling the ‘average’ patient with bipolar I disorder, we also make use of best available evidence on sub-regional epidemiology and the expected impact of interventions, which for many developing regions has necessitated extrapolation from neighbouring regions or from the international literature. Although comprehensive literature reviews for the period 1990–2002 were performed for key model parameters, including remission, mortality, functioning, acute/prophylactic treatment effects and resource use patterns (Goodwin & Jamison (Reference Goodwin and Jamison1990) was relied on for pre-1990 data inputs), there are evident limitations inherent to such an approach, which techniques like sensitivity analysis can only partially address. Until such time that there exists robust evidence at a genuinely global level, however, we see this as a valuable way of providing evidence-based guidance to policy makers on broad strategies to reduce leading contributors to current disease burden.
Use of a population-level measure of health gain such as the DALY has advantages – in terms of comparability with other diseases, for instance – but does not encompass the full range of consequences that may follow from intervention. For bipolar disorder, important additional benefits of treatment include reduction of family burden (including informal caregiving time) and reduced absenteeism and unemployment (productivity). A recent cost-of-illness study of bipolar disorder in the UK estimated that no less than 86% of total societal costs were attributable to these indirect costs, mainly due to excess unemployment (Reference Das Gupta and GuestDas Gupta & Guest, 2002). Despite the pursuit of a societal perspective in WHO–CHOICE (Reference Tan Torres, Baltussen and AdamTan Torres et al, 2003), considerable challenges in the measurement of productivity gains, as well as patient and informal carer time spent seeking or providing care, have precluded their valuation in the present analysis.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Assuming a population coverage of 50%, clinical interventions have the potential to reduce the current burden of bipolar disorder by 10–33%.
-
▪ Baseline results showed lithium to be no more costly yet more effective than valproic acid, assuming an anti-suicidal effect for lithium but not for valproic acid.
-
▪ The most cost-effective interventions under best-, worst- and base-case scenarios were combination strategies of a mood stabiliser plus psychosocial treatment delivered within a community-based service framework.
LIMITATIONS
-
▪ Population models are restricted representations of the clinical reality of treating bipolar disorder and require assumptions about the generalisability of clinical research findings beyond the setting in which they were conducted.
-
▪ Analysis was performed at the highly aggregated level of world sub-regions, which may have reduced relevance to the particular healthcare context of individual countries.
-
▪ The analysis restricted itself to the health system; wider economic consequences of bipolar disorder and its treatment, including lost/restored work opportunities, were not measured.
Acknowledgements
We acknowledge the following colleagues who have actively contributed to the conceptual and methodological development of WHO - CHOICE (CHOosing Interventions that are Cost-Effective). Taghreed Adam, Rob Baltussen, Baltussen, David Evans, Raymond Hutubessy, Benjamin Johns, Jeremy Lauer, Stephen Lim, Christopher Murray and Tessa Tan Torres. The views expressed are those of the authors and not necessarily those of the World Health Organization.








eLetters
No eLetters have been published for this article.