Fluctuating confusion, accompanied by disturbances of consciousness, is an important clinical symptom, with a frequency of 80-90% in dementia with Lewy bodies (DLB) (Reference Byrne, Lennox and LoweByrne et al, 1989; Reference McKeith, Galasko and KosakaMcKeith et al, 1996), 40% in vascular dementia and 20% in Alzheimer's disease (Reference Kolbeinsson and JonssonKolbeinsson & Jonsson, 1993). Accurate assessment has depended upon clinical judgement, with poor levels of interrater reliability (Reference Mega, Masterman and BensonMega et al, 1996; Reference Litvan, Macintyre and GoetzLitvan et al, 1998). The electroencephalogram (EEG) has been used as a standard tool for staging alterations in consciousness from pathological (e.g. delirium) and physiological causes, while numerous reports describe flurries of slow-wave activity in DLB patients experiencing episodic confusion (Reference Yamamoto and ImaiYamamoto & Imai, 1988). McKeith et al (Reference McKeith, Galasko and Kosaka1996) highlight the importance of attentional impairments in fluctuating confusion, while pronounced deficits of attention are evident in DLB sufferers (Reference Ayre, Ballard and PincockAyre et al, 2000). This present study investigates the value of two new scales to measure fluctuating confusion, by determining the agreement between a clinician's assessment and a scale completed by a non-clinician; and by evaluating correlations with neuropsychological and electrophysiological markers.
METHOD
Clinician Assessment of Fluctuation (Appendix I)
This is a short scale, designed for use by experienced clinicians. It consists of a series of screening questions, put to an informant, regarding fluctuating confusion and impaired consciousness during the month prior to the assessment. Fluctuating confusion is rated as present if the informant is able to give a clear-cut example. If present, the frequency and duration of episodes of fluctuating confusion are both rated on a scale of 0-4, and these two scores are multiplied together to produce a severity score from 0-12 (0 representing no fluctuating confusion, 12 representing severe fluctuating confusion; a score of 16 would signify a continuous clouded state, which, by definition, would denote no fluctuation). In the current study, extensive discussions took place between the three clinician raters (I.McK., C.B., J.O'B.), focusing upon illustrations from previously assessed cases, in order to achieve consistency in the way that these criteria were applied. This scale has been used previously in Newcastle as part of the diagnostic approach, and has achieved good diagnostic accuracy for DLB (sensitivity 83%, specificity 91%) against post-mortem diagnosis (Reference McKeith, Ballard and PerryMcKeith et al, 2000).
One Day Fluctuation Assessment Scale (Appendix 2)
This consists of seven items of confusional behaviour (falls, fluctuation, drowsiness, attention, disorganised thinking, altered level of consciousness, communication), scores for which are summed to provide a severity score for fluctuating confusion ranging from 0 to 21. It is based on a validated delirium assessment scale (Reference Inouye, van Dyck and AlessiInouye et al, 1990), with additional items selected from the Barthel index (communication question) (Reference Mahoney, Wood and WadeMahoney et al, 1965) and the ‘fluctuation’ component of the DLB clinical diagnostic criteria (Reference McKeith, Galasko and KosakaMcKeith et al, 1996). In the current study, the scale was completed by a postgraduate researcher (M.W.), blind to the operationalised clinical diagnosis and the Clinician Fluctuation Assessment score. This scale focuses upon fluctuating confusion over the day before the assessment, and takes approximately 15 minutes to administer.
Subjects and assessment
Patients were recruited from a dementia case register of consecutive referrals to old age psychiatry services in Tyneside (UK), and the spouses of the patients are recruited as healthy elderly volunteers. All patients were assessed by a structured psychiatric history (History and Aetiology Schedule; Reference Dewey, Copeland and LoboDewey et al, 1992), the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975), a standardised physical examination which incorporated the Unified Parkinson's Disease Rating Scale (UPDRS; Reference Fahn, Marsden and CalneFahn et al, 1987) and a validated instrument to evaluate psychotic symptoms (Columbia University Scale for psychopathology in Alzheimer's disease; Reference Devanand, Miller and RichardsDevanand et al, 1992), and fluctuating confusion was evaluated by the Clinician Assessment of Fluctuation.
Diagnosis was undertaken separately, several months after the assessments were completed, at a consensus meeting between the three senior clinicians (I.McK., J.O'B., C.B.). Patients with DLB were diagnosed according to the internationally agreed consensus criteria (Reference McKeith, Galasko and KosakaMcKeith et al, 1996), patients with Alzheimer's disease were diagnosed according to the guidelines of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (Reference McKhann, Drachman and FolsteinMcKhann et al, 1984), an operationalised clinical diagnosis of vascular dementia was made using the criteria of the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l'Enseignement en Neurosciences (Reference Roman, Tatemichi and ErkinjunttiRoman et al, 1993).
All participants also received a computerised neuropsychological assessment using the COGDRAS-D battery (Reference Simpson, Surmon and WesnesSimpson et al, 1991). The main parameters used in the present study were choice reaction time (each time ‘YES’ or ‘NO’ was presented in the centre of the screen the participant was required to press the corresponding ‘YES’ or ‘NO’ button as quickly as possible) and digit vigilance reaction time (a digit was displayed constantly on the right-hand side of the screen and 90 digits were serially presented in the middle of the screen; participants were required to press ‘YES’ every time the central digit matched the right-hand side digit). Within-trial variability (standard deviation) of these measures was assessed in single trials, all lasting approximately 90 seconds. The choice reaction time and vigilance reaction time trials were administered at another visit, separate from the diagnostic assessment, by a different rater (M.W.).
As it was not feasible with the available staff resources to complete a more comprehensive evaluation for all patients, a subgroup of 40 subjects (15 with DLB, 15 with Alzheimer's disease and 10 healthy elderly volunteers) from this sample received a more detailed assessment. This included an additional evaluation of fluctuation with the One Day Fluctuation Assessment Scale and an evaluation of EEG delta-band frequency (0-3.9 Hz) variability over 90 seconds. Seventeen primary channel electrodes were used, placed according to the international 10-20 system, which recorded EEG from Fp1, Fp2, Fz, F3, F4, Cz, C3, C4, Pz, P3, P4, T3, T4, T5, T6, O1 and O2 linked to mastoid electrodes. Eye movement compensation was derived from nasion-linked mastoid electrodes. Signals were amplified using a SynAmps and Neuroscan™ acquisition system. The mean spectral frequency was obtained resting with eyes open and resting with eyes closed. The variability (s.d.) in frequency from this mean was used as a measure of fluctuating electrocortical activity. In these patients the attention tasks, the EEG and the One Day Fluctuation Assessment Scale were all assessed on the same day.
The tests were approved by the Joint Ethics Committee of Newcastle and North Tyneside Health Authority and the University of Newcastle upon Tyne. Following full explanation and discussion of the study, the patients and the healthy volunteers gave their consent to the assessment, with additional assent from the next of kin for all patients with cognitive impairments.
Analysis of data
Non-parametric statistical analysis was applied, since the rating scale yielded ordinal data. A Spearman's rank-order correlation was used in the assessment of associations between the two assessment scales for fluctuation. Cronbach's α was used to assess internal consistency of the Fluctuation Assessment Scale. Optimal cut-off values were obtained following a receiver operating characteristics (ROC) analysis according to the method of Altman (Reference Altman1995), and used to calculate the sensitivity and specificity with which the different groups could be distinguished, both in the overall sample and in the subsample with the more detailed evaluation. All analyses were performed using the SPSS package (SPSS, 1992).
RESULTS
Although all diagnoses were clinical, the patients came from a case register in which the specificity of clinical diagnosis had been established against neuropathological examination (Reference McKeith, Ballard and PerryMcKeith et al, 2000).
One hundred and fifty-five people were studied (61 with Alzheimer's disease, 37 with DLB, 22 with vascular dementia, 35 elderly controls). The age, gender and performance on the MMSE schedule for the different diagnostic groups are shown in Table 1.
Table 1 Clinical and experimental variables
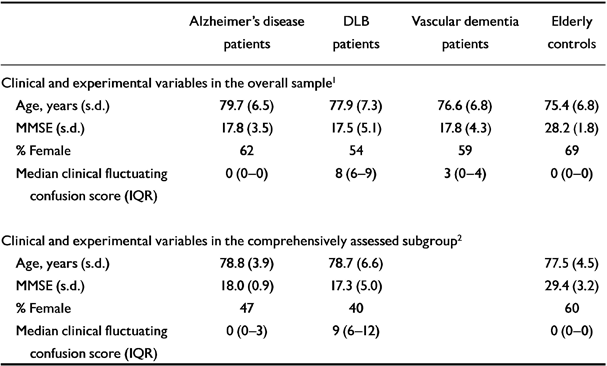
| Alzheimer's disease patients | DLB patients | Vascular dementia patients | Elderly controls | |
|---|---|---|---|---|
| Clinical and experimental variables in the overall sample1 | ||||
| Age, years (s.d.) | 79.7 (6.5) | 77.9 (7.3) | 76.6 (6.8) | 75.4 (6.8) |
| MMSE (s.d.) | 17.8 (3.5) | 17.5 (5.1) | 17.8 (4.3) | 28.2 (1.8) |
| % Female | 62 | 54 | 59 | 69 |
| Median clinical fluctuating confusion score (IQR) | 0 (0-0) | 8 (6-9) | 3 (0-4) | 0 (0-0) |
| Clinical and experimental variables in the comprehensively assessed subgroup2 | ||||
| Age, years (s.d.) | 78.8 (3.9) | 78.7 (6.6) | 77.5 (4.5) | |
| MMSE (s.d.) | 18.0 (0.9) | 17.3 (5.0) | 29.4 (3.2) | |
| % Female | 47 | 40 | 60 | |
| Median clinical fluctuating confusion score (IQR) | 0 (0-3) | 9 (6-12) | 0 (0-0) | |
In the total sample of 155 patients, the Clinician Assessment of Fluctuation score was strongly correlated with the neuropsychological measures of fluctuation (choice reaction time variability and Clinician Assessment of Fluctuation r=0.73, P<0.0001; vigilance reaction time variability and Clinician Assessment of Fluctuation r=0.50, P<0.001). In the subgroup of 40 patients who received a more detailed evaluation, the Clinician Assessment of Fluctuation correlated significantly with the electrophysiological measures of fluctuations in delta-band frequency on EEG (resting, eyes open, r=0.52, P<0.001; resting, eyes closed, r=0.43, P<0.006), and correlated strongly with the independently rated One Day Fluctuation Assessment Scale (r=0.88, P<0.0001).
In this group of 40 patients, the One Day Fluctuation Assessment Scale also correlated strongly with the neuropsychological and electrophysiological measures of fluctuation (choice reaction time variability and One Day Fluctuation Assessment Scale r=0.70, P<0.0001; vigilance reaction time variability and One Day Fluctuation Assessment Scale r=0.52, P<0.001; fluctuation in delta-band frequency and One Day Fluctuation Assessment Scale: resting, eyes open r=0.60, P<0.0001; resting, eyes closed r=0.38, P<0.01).
A reliability coefficient analysis between the seven items of confusional behaviour on the One Day Fluctuation Assessment Scale indicated good internal consistency (Cronbach's α=0.78).
Distinguishing diagnostic groups
In the subsample of 40 patients, an ROC analysis identified an optimal cut-off value of ≥5 for the Clinician Assessment of Fluctuation (see Fig. 1). Using this threshold, all 15 DLB patients, but none of the controls of Alzheimer's disease sufferers, had significant fluctuation (DLB v. Alzheimer's disease, sensitivity 100%). A further analysis, using the same cut-off point in the total sample of 155 patients, indicated that 30 (81%) of the DLB patients, four (18%) of the vascular dementia patients and five (8%) of the Alzheimer's disease patients had significant fluctuation (DLB v. Alzheimer's disease, sensitivity 81%, 95% CI 73.2-88.8; specificity 92%, 95% CI 86.6-97.4; DLB v. vascular dementia, sensitivity 81%, 95% CI 71-91; specificity 82%, 95% CI 72.2-91.8).
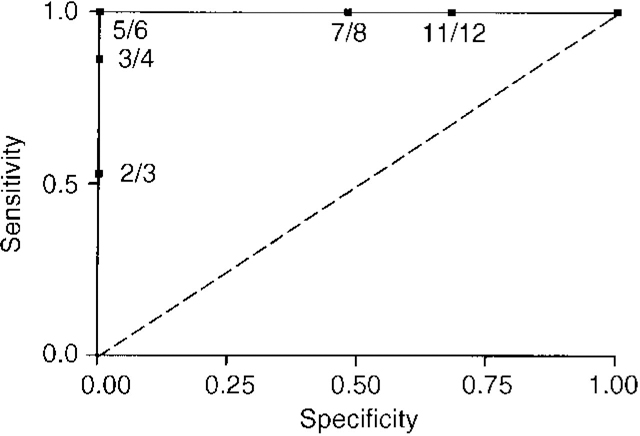
Fig. 1 Receiver operating characteristics analysis for Clinician Assessment of Fluctuation scale score.
In the subgroup of 40, an ROC was also used to determine the optimal threshold for the One Day Fluctuation Assessment Scale (Fig. 2). A score of ≥6 achieved the best discrimination, with 14 of the 15 (93%) DLB patients, but only two (13%) of the Alzheimer's disease sufferers and none of the controls, scoring above this threshold (sensitivity 93%, 95% CI 83.9-100, specificity 87%, 95% CI 75-99).
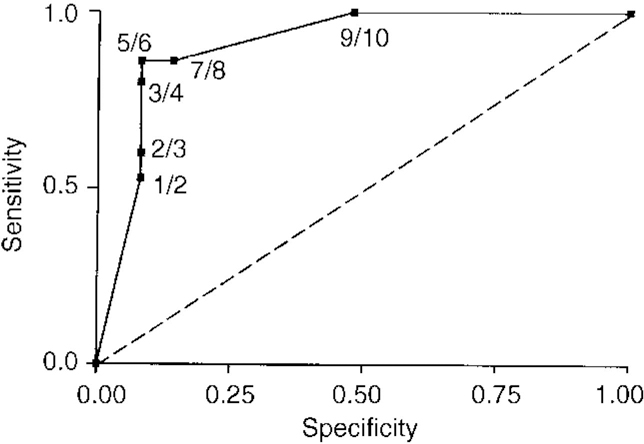
Fig. 2 Receiver operating characteristics analysis for One Day Fluctuation Assessment Scale score.
Direct agreement between scales
A 90% agreement was achieved for the presence of significant fluctuating confusion between the expert clinicianrated scale and the One Day Fluctuation Assessment.
DISCUSSION
Main findings
The current study indicates that structured clinical assessment methods are helpful for the assessment of fluctuating confusion in people with dementia. The two instruments, the Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale, show excellent agreement with each other, have good internal consistency and were significantly associated with neuropsychological and electrophysiological markers of fluctuation. Although there is no clear ‘gold standard’ against which to evaluate a new instrument, the strong correlation between the scale completed by the non-clinician and the semi-standardised expert clinician's rating, as well as the significant correlation between both scales and the neuropsychological and electrophysiological markers suggest that these scales are capturing the essence of fluctuating confusion.
Two clinical assessment instruments
The two scales are brief and easy to administer, and complement each other well. The Clinician Assessment of Fluctuation evaluates fluctuating confusion over the month prior to the interview, on the basis of an interview with an informant, and needs to be completed by an experienced clinician. It provides a method for standardising the clinical decision-making process. The One Day Fluctuation Assessment Scale evaluates fluctuating confusion over the 24 hours prior to the assessment, again on the basis of an interview with an informant. It can be completed by a variety of mental health professionals or research staff, and hence is well suited to research studies. The scoring system gives it good face validity for measuring change during intervention trials.
Future studies
Interrater reliability has not yet been determined for either scale, although the structured nature of the instrument should have advantages over expert clinical judgement alone. Within the current study, extensive discussion took place between the three raters, working through previously assessed cases in order to achieve consistent scores. The same procedure is recommended for groups of clinicians intending to use the Clinician Assessment of Fluctuation, as an aid to clinical evaluation.
Potential value for differential diagnosis
A preliminary evaluation was also undertaken to assess the potential value of the two scales in distinguishing between the different dementia groups. Just on the basis of fluctuation assessments, both scales were able to achieve a sensitivity and specificity in excess of 80%. Although there is a potential tautology for the Clinician Assessment of Fluctuation, as this information was used as part of the diagnostic procedure, the One Day Fluctuation Assessment Scale was completed independently of, and blind to, the diagnostic process, by a separate rater. It is therefore suggested that using this method to standardise the evaluation of fluctuating confusion, within the framework of the consensus clinical criteria for DLB, could have a significant impact upon diagnostic accuracy. This is potentially important, as poor sensitivity for the diagnosis of DLB has been a problem in some studies (Reference Mega, Masterman and BensonMega et al, 1996; Reference Litvan, Macintyre and GoetzLitvan et al, 1998), with the accurate identification of fluctuating confusion highlighted as a major difficulty. This is also highly relevant to everyday clinical practice, as good case identification is essential to optimise clinical management and to avoid neuroleptic sensitivity reactions.
APPENDIX I. CLINICIAN ASSESSMENT OF FLUCTUATION
‘Fluctuating confusion’ or ‘impaired consciousness’ are identified by a positive answer to either one or both of the following questions.
-
(a) Does the patient ever have spontaneous impaired alertness and concentration, i.e. appear drowsy but awake, look dazed, not be aware of what is going on around? (Clear examples demonstrating impaired consciousness with variations in performance/congnition are required to receive a positive rating.) Have these episodes occurred within the last month?
(0=no; 1=yes; 9=not known)
-
(b) Has the level of confusion experienced by the patient tended to vary a lot recently from day to day or week to week? Has it become worse then improved for a while, i.e. been up and down? (Significant fluctuation is regarded as present if distinct examples of differences in performance/cognition can be given on at least two occasions over the past month.)
(0=no; 1=yes; 9=not known)
If a positive rating of fluctuating confusion is present, a severity rating should be made.
Frequency of fluctuating confusion: Total score index
-
Frequency of fluctuating confusion
1=1 per month
2=monthly-weekly
3=weekly-daily
4=≥ daily
-
Duration of fluctuating confusion
0=seconds
1=≤5 minutes
2=5 minutes-1 hour
3=≥ 1 hour
4=≥ 1 day
-
Summary scores
Severity from Scale 1 —
Severity from Scale 2 —
APPENDIX 2. ONE DAY FLUCTUATION ASSESSMENT SCALE
-
1. Falls
Has the patient fallen today?
Yes — (1) No — (0)
If yes, how many times? —
Has the patient had any ‘near falls’ today? (a near fall is when the patient almost fell but was saved by somebody else or objects such as furniture or a walking aid)
Yes — (1) No — (0)
If yes, how many times? —
-
2. Fluctuation
-
(a) Has the patient had a period or periods today when he/she seemed to be confused and muddled and then a period or periods when he/she seemed to be improved and functioning better?
Yes — (1) No — (0)
If yes, how much of the day was he/she confused?
-
(i) 25% (¼) of the day or less — (1)
-
(ii) 25-75% (¼-¾) of the day — (2)
-
(iii) 75% (¾) or more of the day — (3)
-
-
(b) How great was the difference today between the worst period of function and the best period of function?
-
(i) a slight degree of variation — (0)
-
(ii) a moderate degree of variation which had a clear effect on his/her ability to function at the same level throughout the day — (1)
-
(iii) a marked degree of variation which had a large effect on his/her ability to function at the same level throughout the day — (2)
-
Give examples of worst period and best period of functioning:
Worst:
Best:
-
-
(3.) Drowsiness
Has the patient been excessively drowsy today?
Yes — (1) No — (0)
If yes, for how much of the day was he/she sleeping?
-
(i) 25% (¼) of today or less — (1)
-
(ii) 25-75% (¼-¾) to today — (2)
-
(iii) 75% (¾) or more to today — (3)
Were there any periods when he/she was unarousable (blackouts) today?
Yes — (1) No — (0)
-
-
(4.) Attention
Did the patient have difficulty focusing attention (for example, was he/she easily distractible, or did he/she have difficulty keeping track of what was being said) throughout the day?
Yes — (1) No — (0)
-
(5.) Disorganised thinking
Was the patient's thinking disorganised or incoherent (for example, with rambling or irrelevant conversation, unclear or illogical flow of ideas, or unpredictable switching from subject to subject) throughout the day?
Yes — (1) No — (0)
-
(6.) Altered level of consciousness
Overall, how would you rate this patient's level of consciousness today?
-
(i) Alert (normal) — (0)
-
(ii) Lethargic (drowsy, easily aroused) — (1)
-
(iii) Stuporous (difficult to arouse) — (2)
-
-
(7.) Communication
-
(a) How well does the patient understand what you communicate to him/her (you may use speaking, writing or gesturing)?
-
(i) Understands almost everything you communicate — (0)
-
(ii) Understands some of what you communicate — (1)
-
(iii) Understands almost nothing of what you communicate — (2)
-
-
(b) How well does the patient communicate (by speaking, writing or gesturing)?
-
(i) Well enough to make him/herself easily understood at all times throughout the day — (0)
-
(ii) Can be understood sometimes or with some difficulty — (1)
-
(iii) Can rarely or never be understood for whatever reason — (2)
-
-
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Brief standardised clinical scales are useful for identifying fluctuating confusion in patients with dementia.
-
▪ Standardised fluctuation scales may improve the accuracy of differential diagnosis between dementia with Lewy bodies and Alzheimer's disease.
-
▪ An electroencephalogram examination also contributes important information to the assessment of fluctuating confusion.
LIMITATIONS
-
▪ Not all diagnoses have been neuropathologically confirmed.
-
▪ No interrater reliability data were collected.
-
▪ Data from the One Day Fluctuation Assessment Scale were only collected for the subgroup of 40 patients who had a more detailed evaluation.






eLetters
No eLetters have been published for this article.