Families provide most of the care to people with dementia living at home. Family carers have worse physical health, more absences from work, lower quality of life and are more likely to be anxious or have depression than non-carers.Reference Mahoney, Regan, Katona and Livingston1–Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames4 Currently around 50 million people globally have dementia, projected to nearly triple by 2050, and the present annual global cost is US$818 billion.Reference Prince, Wimo, Guerchet, Ali, Wu and Prina5 Nearly 85% of costs are family and social rather than medical costs.Reference Winblad, Amouyel, Andrieu, Ballard, Brayne and Brodaty6
The START (STrAtegies for RelaTives) multicomponent intervention for family carers is individually delivered by supervised psychology graduates (with a first degree in psychology and no clinical training) and was tested by our research team in a randomised controlled trial (RCT). It was the first trial to show both clinical effectiveness (reduced anxiety and depressive symptoms, decreased depression ‘caseness’, improved quality of life) and cost-effectiveness for family carers of people with dementia.Reference Livingston, Barber, Rapaport, Knapp, Griffin and King7,Reference Knapp, King, Romeo, Schehl, Barber and Griffin8 Methods and results up to 2 years of follow-up have been reported in detail elsewhere.Reference Livingston, Barber, Rapaport, Knapp, Griffin and King7–Reference Sommerlad, Manela, Cooper, Rapaport and Livingston11 We found that START carers had a decrease in symptom score that was greater than the minimum clinically important difference and at 8 months were one-fifth as likely to have case-level depression. These benefits persisted for 2 years,Reference Livingston, Barber, Rapaport, Knapp, Griffin and King9 when the intervention was also cost-neutral when considering health and care services used by both family carers and patients.Reference Livingston, Barber, Rapaport, Knapp, Griffin and King9 To the best of our knowledge, there are no clinically and cost-effective interventions that have demonstrated effects beyond 5 years,Reference Sorensen, Duberstein, Gill and Pinquart12–Reference Mittelman, Roth, Clay and Haley14 and none are manualised; so the intervention can be delivered consistently to participants; by graduates without clinical training, with potential to implement at scale.
Objectives
Our aim was to determine the long-term (up to 6 years from baseline) clinical effectiveness of START for family carers' affective symptoms and costs compared with treatment as usual (TAU) in terms of:
(1) our primary outcome – Hospital Anxiety and Depression Scale – total score (HADS-T)Reference Zigmond and Snaith15,Reference Spinhoven, Ormel, Sloekers, Kempen, Speckens and VanHemert16 in carers of people with dementia;
(2) secondary outcomes were:
(a) anxiety and depression caseness and scores,
(b) time until care home admission and death of the person with dementia,
(c) time spent at home,
(d) cost of care for both people with dementia and carers.
Method
We registered a trial protocol before recruitment began at https://doi.org/10.1186/ISRCTN70017938. After recruitment, the research team (with approval from the funding body while the database was still locked) agreed that the primary outcome should be changed to the total score on HADS as this has been shown to have better sensitivity and positive predictive value than either of the individual anxiety and depression scores in identifying depression and registered this prior to analyses. A standard reporting protocol was used.
After registration we requested and received a 5-year no-cost extension to the trial.
Intervention and delivery
We recruited 260 participants to the study. We developed the eight-session START manual-based individual coping intervention for dementia family carers from the American ‘Coping with Caregiving’.Reference Gallagher-Thompson, Solano, McGee, Krisztal, Kaye and Coon17 We trained and supervised non-clinically trained psychology graduates to deliver the intervention (see supplementary Fig. 1, available at https://doi.org/10.1192/bjp.2019.160), and P.R. supervised them clinically as a group with additional time available for individual support. There was a strong practical focus in the training programme on how to deliver the therapy, potential clinical dilemmas, empathic listening, effective use of supervision, safe working practise and when to ask for help. They were trained to adhere to the manual and we used role-play with senior members of the team completing a competency checklist to ensure they could deliver each session competently. We monitored intervention fidelity using a checklist out of a possible five points, and it was satisfactory. Therapists worked with carers to identify individual difficulties and to find workable solutions rather than give answers or recommendations, and implement strategies including: behavioural management, communication strategies, identifying and changing unhelpful thoughts, positive reframing, accessing support, future planning and increasing pleasant events. Each session included a relaxation exercise and we asked carers to put into practise the individualised strategies and relaxation between sessions. The final session was used to agree a plan of what to do in the future based upon what that carer had felt worked. The carer kept their own manual and relaxation compact discs.
In summary START is a parallel-group, superiority, single-blind, RCT conducted in the UK (four sites). Participants were selected to represent varied clinical services so we could see if the intervention was generalisable – a mental health trust based in a large city; a trust in a semi-rural area, a tertiary neurological clinic for rare and young-onset dementia; and a mental health trust where patients were allocated to a specialist nurse (Admiral nurse). We recruited self-identified family carers providing at least weekly support to people with a clinical diagnosis of dementia, living in their own homes and referred to the service we recruited from during the previous year. We excluded those who were unable to give informed consent or who lived more than 1.5 h travelling time from the researchers' base. We recruited from 4 November 2009 to 8 June 2011 through three mental health trusts and a tertiary neurology clinic. Last follow-up was 28 April 2017. Standard treatment includes medical, psychological and social interventions, consisting of assessment, diagnosis and information-giving, risk assessment and management (for example fire, driving, adequate nutrition and self-care, vulnerability, managing money), drug treatment, cognitive stimulation therapy, practical support, treatment of neuropsychiatric and cognitive symptoms, assessment of capacity, help in making long-term decisions and carer support. Patients in both groups received TAU and the use of services in both groups has been described in detail.Reference Knapp, King, Romeo, Schehl, Barber and Griffin18
Randomisation and masking
Participants were randomised 2:1 to intervention:TAU in order to maintain study power given the potential for clustering of outcomes by therapist in the intervention arm. Randomisation was stratified by centre using random permuted blocks via an online computer-generated randomisation system from an independent clinical trials unit. Assessors were masked to randomisation status, but study participants knew their allocation.
Outcome measures
We collected carer and patient sociodemographic details at baseline and measured dementia severity using the clinical dementia rating.Reference Morris19 We also administered the Neuropsychiatric Inventory (NPI),Reference Cummings, Mega, Gray, Rosenberg-Thompson, Carusi and Gornbein20 as neuropsychiatric symptoms have been shown to be associated with carer psychological morbidity, and the Zarit Burden Interview.Reference Bjelland, Dahl, Haug and Neckelmann21 Each NPI item is scored as the product of severity and frequency giving a potential score of 0–12 and scores are summed giving a possible total from 0 to 144. Higher scores indicate more neuropsychiatric symptoms and more burden, respectively.
We also measured carers' anxiety and depressive symptoms, using the HADSReference Zigmond and Snaith15,Reference Spinhoven, Ormel, Sloekers, Kempen, Speckens and VanHemert16 at baseline, 4, 8, 12 and 24 months. In an agreed extension with our funders and ethics committees we continued to collect carer HADS scores and place of residence for patients 6 monthly from 24 until 72 months. We recorded the date that a patient was admitted to a care home or had died, and stopped measuring the HADS at that point. HADS is a scale, validated for all age groups and settings, in people who are physically well or ill, and in Asian and African ethnic groups;Reference Bjelland, Dahl, Haug and Neckelmann21 summarised as HADS-D (depression) HADS-A (anxiety) with scores from 0 to 21 and a total HADS score (HADS-T) from 0 to 42 (higher scores indicating more symptoms). The total score (HADS-T) is our chosen primary outcome as it has better sensitivity and positive predictive value than either of the individual scales in identifying depression, when compared with ICD depression diagnosis criteria.Reference Spinhoven, Ormel, Sloekers, Kempen, Speckens and VanHemert16 HADS-D and HADS-A are also validated as scores for ‘caseness’ and were dichotomised as ‘case’ and ‘non-case’, with a cut-point of ≥9.Reference Bjelland, Dahl, Haug and Neckelmann21
The Client Service Receipt Inventory (CSRI)Reference Beecham, Knapp, Thornicroft, Brewin and Wing22 measured health and social care service use retrospectively until 24 months, but not beyond that point. Each carer reported their own and the patient's service use over the previous 4 months, covering the full range of services.Reference Knapp, King, Romeo, Schehl, Barber and Griffin8 Service contacts were multiplied by their unit costs (2009–2010 prices) obtained from publicly available sources: National Health Service (NHS) reference costsReference Coon, Thompson, Steffen, Sorocco and Gallagher-Thompson23 for in-patient and out-patient attendances, and the Personal Social Services Research Unit volumeReference Curtis24 for other services. Costs were discounted to present values at an annual rate of 3.5%.25
Beyond the 24-month point, we estimated costs of services used by patients and carers up to the earliest of either withdrawal from the study, death of either patient or carer, or end of follow-up period (72 months). For patients who continued living in the community, we assumed that weekly costs remained the same as at 24 months. For patients moving to a care home, we attached a unit cost equal to the weekly charge for a private nursing home for older people,Reference Curtis24 the most likely type of admission for someone with dementia, for the duration of stay, and we assumed that carer service use costs continued. Costs were carried forward as long as the patient/carer remained alive.
Statistical analysis
Analyses were conducted as intention-to-treat based on a predefined statistical analysis plan. Most analyses were carried out in Stata (version 14) for Windows, but some models (as detailed) were fitted using R.
HADS scores
HADS data included in the primary 72-month analysis are those collected while the carer was still actively looking after the patient (i.e. patient was still living at home). Data collected after the patient had died or was admitted to a care home were excluded.
To be included in the primary long-term analysis, the individual must have had at least one follow-up HADS-T score. Those excluded therefore have no follow-up measurements at any time point. Analyses compare the group as randomised, regardless of the number of therapy sessions attended in the intervention group
We used mixed-effects linear regression models to assess the effect of the START intervention on repeated measurements of HADS-T over 72 months. Initially we adjusted for treatment centre, HADS-T at baseline and time, but then extended this model to include adjustments for carer age, carer gender, baseline NPI score and Zarit score. We also investigated whether the treatment effect changed over time by including a treatment × time interaction. We chose not to allow for therapist clustering in these models as previous analyses of data up to 24 months had indicated that clustering effects were negligible. As a sensitivity analysis, however, models were refitted allowing for therapist clustering. For all cases estimates obtained were not substantially different.
We used scatter plots of residuals and fitted values to check model assumptions. The correlation structure assumed in the main analyses was compound symmetry; however, models were refitted in sensitivity analyses with alternative structures (autoregressive (order 1) and linear spatial correlation assumptions). For all models these investigations supported the models used for the main analyses.
The analyses described for the HADS-T were repeated for anxiety and depression subscales of the HADS. We investigated the effect of the START intervention on the occurrence of individuals with anxiety/depression, using mixed-effects logistic regression models, with a participant-level random effect.
If care home admission or death of the care recipient occurred prior to 72 months, the carer was not followed-up beyond the last visit prior to death or care home admission. Given the possibility of a relationship between HADS scores and death/care home admission, we conducted sensitivity analyses to consider the impact of such informative censoring. Joint mixed-effect models for the longitudinal HADS scores and time to admission to a home or death were fitted to account for the correlation between the longitudinal and survival outcomes.Reference Henderson, Diggle and Dobson26 The HADS component treatment effect estimates were compared with those obtained from the previously fitted mixed models. (Joint models were fitted using the JM package in RReference Rizopoulos27).
Time until care home admission
We employed a multistate model (depicted pictorially in supplementary Fig. 2)Reference Meira-Machado, de Una-Alvarez, Cadarso-Suarez and Andersen28 to analyse time until care home admission while accounting for the possibility of patient death. The model was set up to allow transition from living at home to one of two states, care home admission or death. Effect estimates from the model are ‘intensity ratios’ that are analogous to hazard ratio estimates in a Cox proportional hazards model but pertain to the specific transitions within the multistate model. As before, models were fitted adjusting for centre, carer age, carer gender, baseline NPI and Zarit score. (Multistate models were fitted using the msm package in RReference Meira-Machado, de Una-Alvarez, Cadarso-Suarez and Andersen28).
Time spent at home
In a further analysis of patient time spent at home (i.e. time prior to care home admission or death), we fitted models for time to admission or death using a standard survival analysis. We used a log rank test to make a comparison between the randomised groups and then fitted a Cox regression model to provide a treatment effect estimate adjusted for centre, carer age, carer gender, baseline HADS total, baseline NPI total and Zarit total score.
Costs
The difference in costs between treatment arms at 72 months was assessed using the non-parametric Wilcoxon Rank-Sum test.Reference Wilcoxon29
Patient involvement
This study was devised and conducted with patient and public involvement (PPI) in the questions of the study and PPI representatives were on the management and steering group. They helped shape the original questions, added qualitative questions about the experience and took part in interpreting the findings. They have also presented them.
Results
Participant flow and recruitment
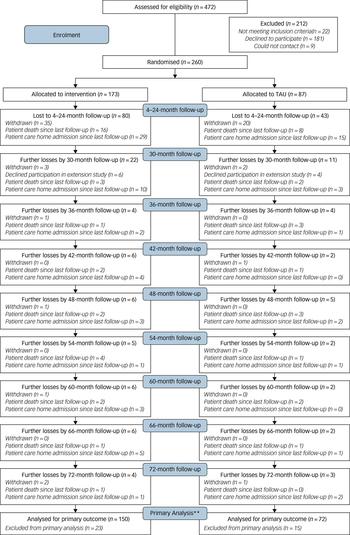
The Consort diagram (Fig. 1) shows participant flow through the study. We randomised 260/472 (55%) of the carers referred. Others refused (n = 181; 38%), did not meet inclusion criteria (n = 22; 5%) or were uncontactable (n = 9, 2%). We randomised 173 (67%) participants to intervention and 87 (33%) to TAU. The characteristics of the randomised groups generally achieved good balance in terms of sociodemographic and clinical characteristics (see supplementary Table 1). Carers were mostly spouses/partners (109; 42%) or children (113; 44%). The proportions of patients who died (before they were admitted to a care home), were admitted to care homes and withdrew by randomised group is shown in supplementary Table 2. There is no evidence of significant differences in the proportions of participants in each end-status category between the START and TAU groups.

Fig. 1 CONSORT diagram for long-term outcomes (up to 72 months).
Intervention adherence and fidelity
In total, 130 (75%) carers in the intervention group attended ≥5 therapy sessions, 8 (5%) withdrew before any therapy sessions. Ten therapists delivered the intervention, to between 11 and 32 carers each. The mean fidelity score was 4.7 (s.d. = 0.66).
Primary outcome
Table 1 summarises HADS-T scores at each follow-up point. Analysis of HADS-T, adjusting for centre, baseline score, time and factors related to outcome (carer age and gender, NPI, Zarit) over the 6-year period, showed an average improvement in HADS-T of 2.00 points compared with TAU (95% CI −3.38 to −0.63; P = 0.005) (Table 2). In the model adjusting only for centre, baseline score and time, average score decrease was smaller but still significant and in favour of the intervention group (Table 2). A model including an interaction with time showed no evidence of differential effects of the intervention over time (P = 0.98).
Table 1 Summaries of Hospital Anxiety and Depression Scale (HADS) total score at each follow-up time by treatment group
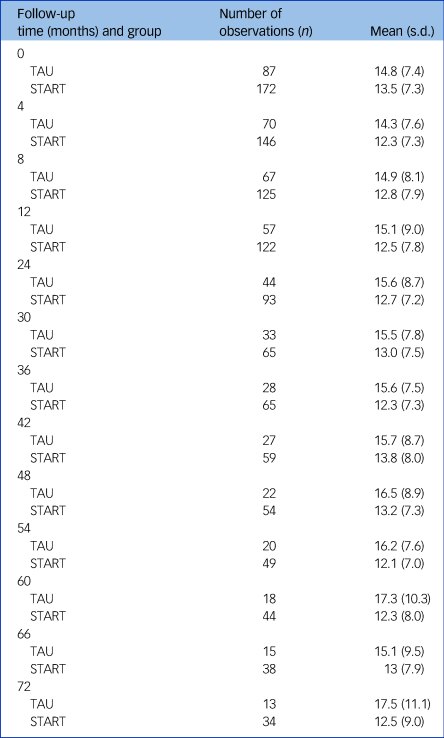
TAU, treatment as usual; START, STrAtegies for RelaTives.
Table 2 Estimates of the effect of the START (STrAtegies for RelaTives) intervention compared with treatment as usual (TAU) on Hospital Anxiety and Depression Scale (HADS) measures over 6 years

HADS-D, HADS – depression subscale; HADS-A, HADS – anxiety subscale; NPI, Neuropsychiatric Inventory; Zarit, Zarit Burden Interview.
Secondary outcomes
Depression and anxiety caseness and scores
In the fully adjusted analyses there was a reduced odds of HADS-depression caseness in the intervention group compared with TAU, (odds ratio (OR) = 0.20, 95% CI 0.08–0.52, P = 0.001). Reduction in HADS-anxiety caseness, however, was not significant (OR = 0.50, 95% CI: 0.24–1.07, P = 0.07) (Table 2).
Fully adjusted models for HADS-A and HADS-D continuous scores indicated significant beneficial intervention effects over 6 years, with average decreases of −0.97 (95% CI −1.78 to −0.15) and −1.06 (95% CI −1.78 to −0.35), respectively. Models showed no evidence of differential intervention effects with time for HADS-A or HADS-D (P = 0.98 and P = 0.94, respectively).
Adjusted joint models were used as sensitivity analyses to allow for the possibility of a relationship between HADS scores and time to care home admission or death and these gave similar results to previous models for HADS-T, HADS-D and HADS-A (HADS-T: 2.01 (95% CI −3.38 to −0.63), HADS-D: −1.07 (−1.78 to −0.37), HADS-A: −0.97 (−1.78 to −0.16)). This suggests that censoring by death/care home admission is not problematic.
Analysis of time until patient care home admission and death
Figure 2 shows the cumulative incidence of care home admission and death over time by randomised group and indicates little difference between the groups for either outcome. The multistate model adjusted for centre, carer age, carer gender, baseline HADS-T, baseline NPI and baseline Zarit gave intensity ratios for the START intervention versus TAU of 0.88 (95% CI 0.58–1.35) for the home-to-care-home transition and 0.81 (95% CI 0.50–1.30) for the home-to-death transition, both showing no evidence of a between-group difference in transition rates to care homes or in death rates.
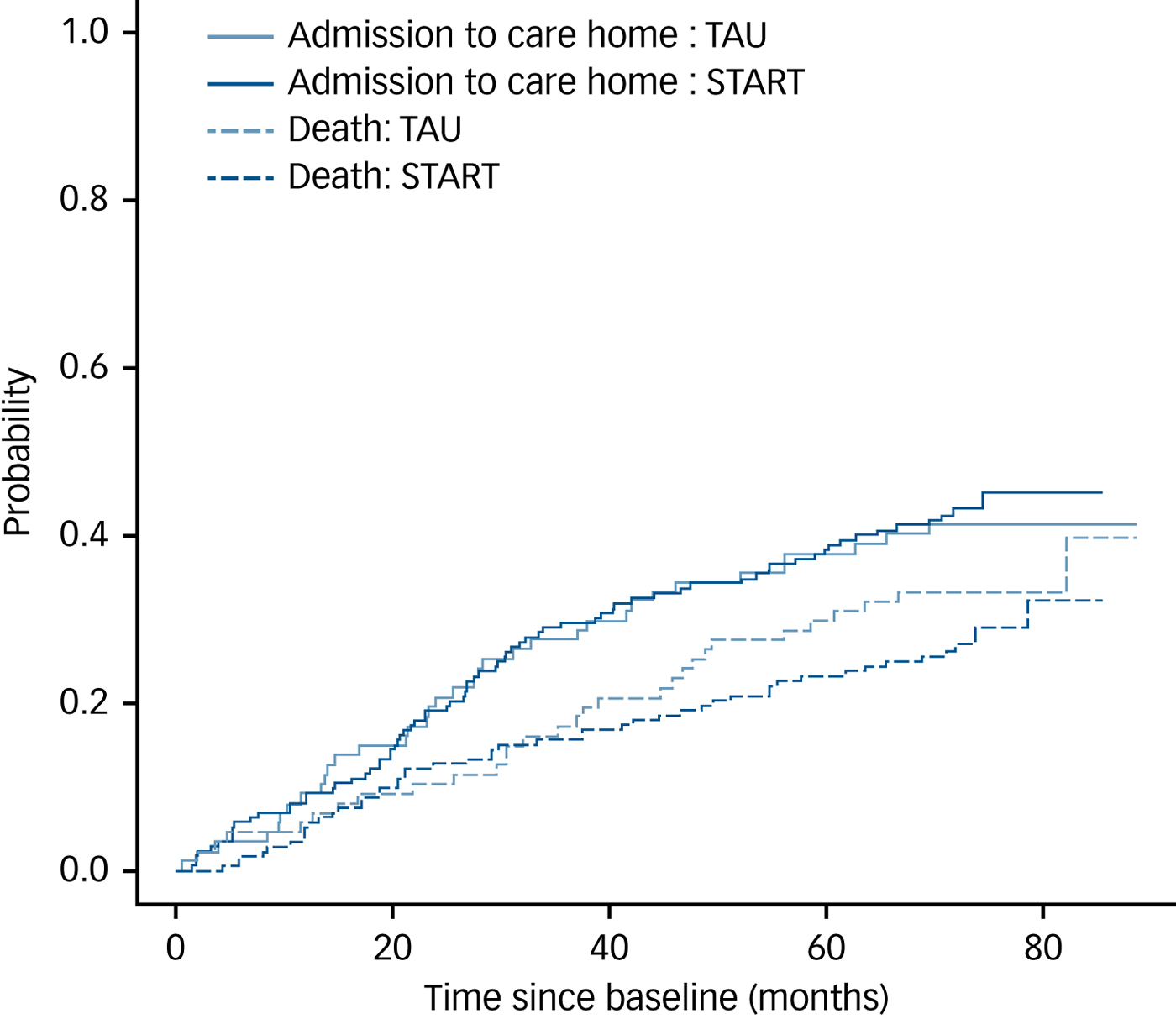
Fig. 2 Plot of estimated cumulative incidence functions for the events ‘care home admission’ and ‘death’ over time, stratified by treatment group.
Analysis of time spent at home
Based on Kaplan Meier estimates, the estimated median time spent at home (i.e. time until death or admission to a care home) for the TAU group was 39.0 months (95% CI 31.1–49.4) and for START was 42.2 months (95% CI 33.3–54.7). Cox regression with adjustments for centre, carer age, baseline HADS total, NPI score and Zarit score, showed no evidence of a difference between the randomised groups (hazard ratio estimate: 0.81 (95% CI 0.59–1.11)).
Costs
Costs for carer and patient service use are shown in Table 3. Costs of services used by patients were much higher than costs for services used by carers across the full study period. In the final year of follow-up (61–72 months) median patient service use costs were £16 964 for TAU and £5 759 for START (P = 0.072). Median carer service use costs were £377 for TAU and £274 for START.
Table 3 Annual costs of services used by carers and patients, by year, from 25 to 72 monthsa

a. Differences between groups were tested using the non-parametric Wilcoxon rank-sum test. None were statistically significant, although the difference for 61–72 months approached statistical significance (P = 0.0717).
Discussion
Main findings
This is the first RCT to demonstrate that family carers of people with dementia referred to specialist care experience benefits from an intervention delivered by supervised psychology graduates in terms of depression and anxiety symptoms and depression caseness, not only in the short term but for up to 6 years. The difference is small but is statistically significant, greater than the minimally clinically important difference (that which is clinically significant to patients) and is sustained.Reference Puhan, Frey, Buchi and Schunemann30 The difference in costs appears to be economically large (cost per patient in the intervention group is around a third of the cost in the TAU group) although there was no significant difference in time to care home admission or death. The reduced sample size, however, means that the test for differences in cost is underpowered (particularly given highly skewed cost data), but the estimated costs of health and care services used by patients appear to be lower for the intervention group compared with TAU in the final year of follow-up. It is encouraging that this intervention does not therefore increase costs, and might actually be cost-saving. Carers in the control group were five times more likely to have clinically significant depression on a rating scale validated against caseness using ICD criteria. Predictably, health and social care costs increase over time for both groups, as a result of the worsening condition. There is a bigger increase in TAU group.
Strengths and limitations
The trial is randomised, with masked follow-ups and we recruited the numbers of participants needed according to the power analysis based on the primary clinical outcome.Reference Livingston, Barber, Rapaport, Knapp, Griffin and King7 The intervention was manual-based, standardised and supervised. High fidelity ratings and very low intercluster correlations show the results do not differ according to therapists, suggesting that the intervention can be delivered consistently.
We planned a pragmatic trial to include all family carers who presented to services so they had varied sociodemographic and clinical characteristics and came from a variety of services; consequently, our study has some external generalisability, that is, it suggests the intervention can be used in a variety of NHS settings. We did not have the power to analyse whether this intervention was more effective in subgroups; for example, those with more education or without a mental health history or with more family support. At the time of the START intervention most patients had only recently presented to services and thus the intervention can be offered at the beginning of the patient pathway but may not be applicable to those who have had the diagnosis for many years. It was preventative as well as improving depression and so can be offered to those with and without depression.Reference Livingston, Barber, Rapaport, Knapp, Griffin and King7 Previously we have found that carers used different components of the intervention and some continued to use these consciously over 2 years but we did not ask about this at 6-year follow-up.Reference Sommerlad, Manela, Cooper, Rapaport and Livingston11 Only patient care home admission and death and carer HADS were directly collected after 2 years and therefore the economic analysis involved modelling. Although the differences in costs were striking, the nature of dementia, which inevitably meant attrition by death of some of those with it over 6 years, meant the numbers were smaller. In addition, the data were skewed and they only approached the usual level taken as significant.
Comparison with other studies, meaning and implications
The practical nature of the intervention, in which carers were encouraged to develop and continue to use successful strategies, might also account for the longevity of the positive effects on carer mental health that we found – the most successful strategies were likely to be used repeatedly and therefore remembered and integrated into caring routines. The intervention included a final session on planning for the future. It is likely that the nature of caring difficulties will have evolved over 6 years. Intervention group participants were given a manual in which strategies they had found helpful for managing caring challenges, as well as pleasant events, were recorded and it also included recordings of the relaxation exercises so that they had something to refer to during future caring.
Our findings would suggest that carers were able to continue using the skills and strategies they had practised in the longer term; a focus on planning for the future, accessing support and explicit consideration of how difficulties may change and emotion-focused and acceptance-based strategies, might have helped support this. It is also possible that carers revisited previously less personally relevant aspects of the manualised intervention as certain issues or challenges became more salient to their caring.
Many interventions for family carers of people with dementia have not worked in improving mood.Reference Farran, Paun, Cothran, Etkin, Rajan and Eisenstein31–Reference Waldorff, Buss, Eckermann, Rasmussen, Keiding and Rishoj33 Others have been effective but the effects have not been sustained.Reference Moore, Chattillion, Ceglowski, Ho, von Känel and Mills34 Most have not considered prevention. In general, those that have been effective are multicomponent and delivered to individuals rather than groups for at least six sessionsReference Selwood, Johnston, Katona, Lyketsos and Livingston35,Reference Dickinson, Dow, Gibson, Hayes, Robalino and Robinson36 and our study was designed to follow this model. Some earlier interventions for family carers have been effective and had sustained effects that have continued for between 1 and 5 years.Reference Sorensen, Duberstein, Gill and Pinquart12–Reference Mittelman, Roth, Clay and Haley14 Our study is in line with this but because it is manual-based and delivered by non-clinically trained psychology graduates it is designed to be scalable and practical and has economic findings to support this. We have more fully considered cost than most other studies although there is some evidence that interventions can generate saving.Reference Knapp, Lemmi and Romeo37,Reference Clarkson, Davies, Jasper, Loynes and Challis38 There is little evidence that carer stress predicts care home admission in community-dwelling older people in generalReference Donnelly, Hickey, Burns, Murphy and Doyle39 but psychological interventions for family carers may reduce care home admission for people living with dementia, with a meta-analysis of the best-quality studies finding a significant reduction in the odds of care home admission, although the time to admission difference did not reach significance.Reference Spijker, Vernooij-Dassen, Vasse, Adang, Wollersheim and Grol40 Family carers become more anxious and depressed over time without intervention; thus we included carers who were not depressed at presentation.Reference Goren, Montgomery, Kahle-Wrobleski, Nakamura and Ueda3,Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames4
Future research
The START intervention is clinically effective, improving carer mood over 6 years. It does not increase patient or carer service-related costs and thus should be made available. The numbers of people with dementia and the diversity of culture, geographic location and available NHS resources mean that further research is necessary to widen access and optimise implementation. For example, to consider whether the intervention can be delivered remotely (through a skype or similar application), through the existing voluntary sector carer support infrastructure (as some carers do not see themselves as patients) and be adapted for ethnic groups with different cultures.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.160.
Funding
This project was funded by the National Institute for Health Research Health Technology Assessment (NIHR HTA) Programme (project number 08/14/06). The authors analysed results and prepared this manuscript independently of the funding body.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the HTA programme, NIHR, NHS or the Department of Health. The study was sponsored by UCL. Neither funders nor sponsors had a role in the study design and the collection, analysis, and interpretation of data and the writing of the article and the decision to submit it for publication. The researchers were independent from funders and sponsors. All researchers could access all the data. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.







eLetters
No eLetters have been published for this article.