The first-line pharmacological intervention in attention-deficit hyperactivity disorder (ADHD) is stimulant medication, particularly methylphenidate. It has shown short-term efficacy in reducing behavioural symptoms of ADHD Reference Banaschewski, Coghill, Santosh, Zuddas, Asherson and Buitelaar1,Reference Greenhill, Kollins, Abikoff, McCracken, Riddle and Swanson2 and improving cognitive tasks and academic performance. Reference Prasad, Brogan, Mulvaney, Grainge, Stanton and Sayal3,Reference Loe and Feldman4 However, although use of medication for ADHD is associated with a possible decrease in criminality, Reference Lichtenstein and Larsson5 there is little evidence of its impact on long-term impairments related to risky behaviours, psychiatric comorbidities and socio-occupational outcomes. Reference van de Loo-Neus, Rommelse and Buitelaar6,Reference Molina, Hinshaw, Eugene Arnold, Swanson, Pelham and Hechtman7 Moreover, ADHD medication is generally well tolerated but potentially exposes children to adverse effects on appetite, growth, sleep and the cardiovascular system. Reference Cortese, Holtmann, Banaschewski, Buitelaar, Coghill and Danckaerts8,9 In some cases ADHD medication is not adequately used since children without ADHD (false positives) or children with moderate forms can unduly be exposed to medication. Conversely, many children with severely impairing ADHD do not receive medication, even in countries with the highest rates of prescription. Reference Rey and Sawyer10 Accordingly, both expert assessment of diagnosis Reference Banaschewski, Coghill, Santosh, Zuddas, Asherson and Buitelaar1,11,Reference Wolraich, Brown, Brown, DuPaul and Earls12 and expert monitoring and management of prescription practices are required. Reference Cortese, Holtmann, Banaschewski, Buitelaar, Coghill and Danckaerts8 The international situation regarding ADHD medication prescription is variable. 13 In many high-income countries, pharmacoepidemiological surveys suggest an increase in the pattern of stimulant medication use over time. Reference Scheffler, Hinshaw, Modrek and Levine14 This is notable in the USA, where the overall prevalence of medicated children rose from 0.6% in 1987 to 2.7% in 1997 and to 3.5% in 2008, although the situation varies from state to state (over- and underuse). Reference Zuvekas and Vitiello15,Reference Olfson, Gameroff, Marcus and Jensen16 However, in other countries, there is insufficient access to pharmacotherapy.
The substantial impact of ADHD on health and quality of life calls for a better understanding of its healthcare determinants, particularly medication. This is pressing, as alternative non-pharmacological interventions for ADHD (dietary and psychological treatments) have shown limited benefit for reducing core ADHD symptoms. Reference Sonuga-Barke, Brandeis, Cortese, Daley, Ferrin and Holtmann17 Accordingly, medication still appears to be one of the most effective approaches to treating ADHD. However, there are risks of over-, under- and inadequate prescriptions. In addition, the concerns about the rising number of prescriptions, misuse and long-term safety have triggered worries among the stake-holders (the families of treated children, the media and the medical community), which may influence practices and acceptance of interventions. Reference Singh18,Reference Graham and Coghill19 Identifying factors that predict medication use beyond ADHD symptoms is of utmost importance as this knowledge could be used by clinicians in their treatment decisions.
Prior research found that a variety of individual characteristics, sociodemographic and environmental factors are associated with the prescription of medication for ADHD: youth psychopathology (ADHD, externalising disorders, internalising disorders), being male, ethnicity, non-intact families, parental psychopathology, low maternal education, family income, negative family influences, low academic functioning, and previous receipt of stimulant medication. Reference Olfson, Gameroff, Marcus and Jensen16,Reference Leslie and Wolraich20-Reference Winterstein, Gerhard, Shuster, Zito, Johnson and Liu26 For some predictors, particularly disruptive comorbidity, divergent findings have been reported (protective in some studies v. risk factor in others). In addition, these studies had limitations: (a) medication use was often measured only at one point in time; (b) some studies were cross-sectional, implying a concomitant evaluation of risk factors and outcomes; (c) potential confounders were not taken into account; (d) a categorical diagnosis of psychiatric problems was generally used; (e) early childhood factors were not studied prospectively in a population-based sample. These limitations impeded the correct longitudinal appraisal of the role of risk factors, exposed the data to confounding biases and did not allow a dimensional approach to behavioural problems. The aim of the present study was to go beyond these limits by using a population-based birth cohort to assess the baseline and longitudinal influences of environmental and behavioural predictors on ADHD medication between the ages of 3.5 and 10 years. We tested the hypotheses that: (a) hyperactivity-impulsivity symptoms and inattention symptoms would predict medication use in this population-based sample; (b) other risk factors could heighten/lessen the likelihood of receiving ADHD medication.
Method
Participants and procedure
Data were drawn from the Quebec Longitudinal Study of Child Development (QLSCD). The QLSCD protocol was approved by the Quebec Institute of Statistics and the St Justine Hospital Research Center ethics committees. Data were collected by trained interviewers through repeated (n = 10) home interviews with the person most knowledgeable about the child (the mother for 98% of children) in order to obtain information on child, parent and family characteristics and behaviours. Informed written consent was obtained from all participating families at each assessment. Assessments were conducted at the following ages: 5 months, 1.5, 2.5, 3.5, 4, 5, 6, 7, 8 and 10 years. The initial sample was selected from birth registries and comprised 2120 children evaluated at 5 months and representative of children born in the province of Quebec (Canada) in 1997/1998. The average response rate over the 10 years of data collection was 83% (range: 63-100%) with an average completeness of data equal to 79% (range: 61-91%). The sample with complete data at the first assessment comprised 1920 children. This sample (n = 1920) was largely similar to the initial sample (n = 2120) regarding sociodemographic characteristics. However, there was a tendency for underprivileged families, non-intact families and immigrant families (P<0.0001 for all) to have missing values. Table 1 describes the sociodemographic characteristics of the complete data sample at the first assessment (n = 1920).
Measures
Outcome: use of ADHD medication
Use of ADHD medication was reported by parents in a question referring to the preceding 12 months ‘Does [your child] take any of the following prescribed medication on a regular basis: Ritalin or any other medication for treating hyperactivity or inattention?’ at ages (years) 3.5 (0.2% of the sample), 4 (0.2% of the sample), 5 (0.2% of the sample), 6 (1.5% of the sample), 8 (5.6% of the sample), and 10 (8.6% of the sample).
Explanatory variables
Time-varying covariates: children’s mental health and parenting. Symptoms of hyperactivity-impulsivity, inattention, anxiety, opposition and emotional problems were reported through the Interviewer Computerized Questionnaire when the children were 2.5, 3.5 4, 5, 6, and 8 years of age. Ratings relied on the early childhood behaviour scale from the Canadian National Longitudinal Study of Children and Youth. 27 This tool incorporates items from the Child Behavior Checklist, Reference Achenbach28 the Ontario Child Health Study Scales Reference Boyle, Offord, Racine, Sanford, Szatmari and Fleming29 and the Preschool Behavior Questionnaire. Reference Tremblay, Desmarais-Gervais, Gagnon and Charlebois30 For each dimension the items used were as follows:
-
(a) hyperactivity-impulsivity: ‘could not sit still, was restless or hyperactive’, ‘could not stop fidgeting’, ‘was impulsive, acted without thinking’, ‘had difficulty waiting for his/her turn in games’, ‘couldn’t settle down to do anything for more than a few moments’;
-
(b) inattention: ‘was unable to concentrate, could not pay attention for long’, ‘was easily distracted, had trouble sticking to any activity’, ‘was inattentive’;
-
(c) anxiety: ‘was too fearful or anxious’, ‘was worried’, ‘was nervous, high strung or tense’, ‘cried a lot’;
-
(d) opposition: ‘was defiant or refused to comply with adults’ requests or rules’, ‘didn’t seem to feel guilty after misbehaving’, ‘punishment didn’t change his/her behavior’, ‘had temper tantrums or hot temper’;
-
(e) emotional problems: ‘seemed to be unhappy or sad’, ‘was not as happy as other children’, ‘had no energy, was feeling tired’, ‘had trouble enjoying him/herself’.
All items referred to the preceding 12 months and were coded on a frequency scale (never or not true: 0; sometimes or somewhat true: 1; often or very true: 2) and quantitative scores derived from scales were z-standardised. Owing to high correlations and to avoid multi-collinearity, hyperactivity-impulsivity and inattention were combined into a single variable hyperactivity-inattention.
The Parental Cognition and Conduct Toward the Infant scale Reference Boivin, Pérusse, Dionne, Saysset, Zoccolillo and Tarabulsy31 was used to assess coercive parenting when the children were 2.5, 3.5, 4, 5, 6, and 8 years of age, using the following items: ‘I have been angry with my child when he/she was particularly fussy’, ‘when my child cries, he/she gets on my nerves’, ‘I have raised my voice with, or shouted at, my child when he/she was particularly fussy’, ‘I have spanked my child when he/she was particularly fussy’, ‘I have lost my temper when my child was particularly fussy’, ‘I have left my child alone in his/her bedroom when he/she was particularly fussy’, ‘I have shaken my child when he/she was particularly fussy’. All items were rated on an 11-point scale (higher score, more coercive parenting).
Baseline covariates
The gender of the child was coded 1 for boys and 0 for girls. Family structure was coded 1 if the family was non-intact (i.e. single-parent families; or families composed of a couple, married or common-law, living with at least one child not born to them) and 0 if the family was intact (i.e. the child lives with his/her two biological parents regardless of the type of conjugal relationship). Maternal education was coded 1 if low (no high-school diploma) and 0 if medium (high-school or post-secondary diploma) or high (university degree). Maternal age at birth of the target child was coded 1 if 21 years or younger (10.5%) and 0 if older than 21 years. Household income was computed according to Statistics Canada’s guidelines accounting for the family zone of residence, the number of people in the household and the family income in the past year. Income was coded 1 if insufficient and 0 if sufficient. Parental immigration status corresponded to non-immigrant (mother and father born in Canada) v. immigrant (mother or father born outside Canada; 74% belonging to racial/ethnic minorities). Maternal and paternal depressive symptoms were assessed with the abbreviated version (12 items) of the Center for Epidemiological Studies Depression Scale (CES-D). Reference Radloff32 Parents reported the frequency of depressive symptoms in the previous week. Each item was coded on a four-point scale. Informant total ratings were z-standardised. All baseline variables were assessed when the target child was 5 months of age.
Statistical analyses
Longitudinal risk factors (i.e. multiple measurements) were treated as time-varying covariates in all analyses. We first described the sample characteristics and the patterns of unadjusted associations between the main risk factors and ADHD medication use by computing the instantaneous hazard (equivalent to the proportion of the population using ADHD medication per unit time) on the basis of Kaplan-Meier survival functions. Second, we conducted bivariate and multivariate analyses using survival analysis with time-dependent covariates Reference Therneau33 to determine associations between baseline/longitudinal risk factors and subsequent use of an ADHD medication. The method made it possible to estimate hazard ratios (HR) for the use of ADHD medication (endpoints at waves: 3.5, 4, 5, 6, 8, and 10 years of age) adjusting for the effects of other time-varying covariates (measured at waves: 2.5, 3.5, 4, 5, 6, and 8 years of age). To select predictors included in the multivariate models, we estimated bivariate associations between risk factors and the outcome (survival analysis). Variables with P<0.05 were entered into the multivariate models. Each participant was specified as a cluster of correlated observations, as medication status at any given time can depend on previous medication status. A robust variance was thus estimated to account for this pattern. In order to test the robustness of the findings, sensitivity analyses were performed using multiple imputation models (number of imputation, 100) under the missing-at-random (MAR) non-response mechanism. Finally, interactions between independent variables kept in the final model were tested. A P<0.05 was considered statistically significant.
Results
Table 1 summarises the characteristics of the sample at baseline and the number of person-years and events during follow-up. Figure 1 shows the instantaneous hazard rates of ADHD medication use: raw rates (Fig. 1(a)), by levels of hyperactivity-inattention symptoms (>+1 s.d., +1 s.d. to median, <median) (Fig. 1(b)), by levels of maternal education (low/medium/high) (Fig. 1(c)), and by parental immigration status (immigrant v. non-immigrant) (Fig.1(d)).
Table 2 provides the results of proportional hazards regressions with time-dependent covariates. It shows the hazard ratios and 95% confidence intervals for the use of ADHD medication. The fully-adjusted model (number of persons-years observed, 9165; number of events, 210) was significant (Wald χ2 = 88.1, d.f. = 8, P<0.0001). All variables respected the hazard proportional assumptions (the contribution of each predictor was constant over time) except family income, which was not significantly associated with the outcome in multivariate models. To deal with this issue, we stratified on this variable, which still controls for the variable but does not provide an estimate of its contribution. Hyperactivity-inattention, male gender, low maternal education and immigration status were significantly associated with the use of ADHD medication. There was no statistically significant interaction. Additional analyses using multiple imputed data showed the same patterns of associations: hyperactivity-inattention (P<0.0001), gender of the child (P<0.0001), low maternal education (P = 0.006) and immigration status (P = 0.036) were significantly associated with the use of ADHD medication. Complementary analyses dichotomising hyperactivity-inattention into two dimensions hyperactivity-impulsivity and inattention showed that both dimensions were significantly associated with ADHD medication exposure (online Table DS1).
Table 1 Characteristics of the sample at entry and number of person-years during follow-upFootnote a
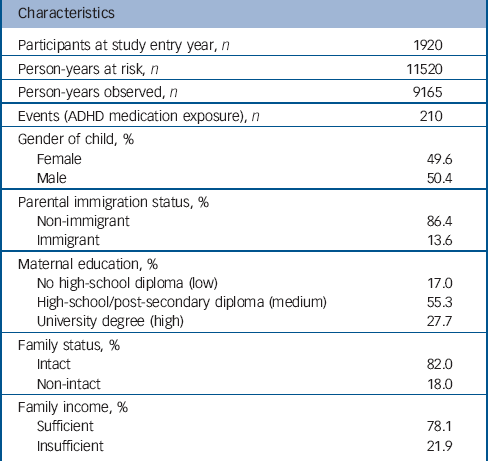
| Characteristics | |
|---|---|
| Participants at study entry year, n | 1920 |
| Person-years at risk, n | 11520 |
| Person-years observed, n | 9165 |
| Events (ADHD medication exposure), n | 210 |
| Gender of child, % | |
| Female | 49.6 |
| Male | 50.4 |
| Parental immigration status, % | |
| Non-immigrant | 86.4 |
| Immigrant | 13.6 |
| Maternal education, % | |
| No high-school diploma (low) | 17.0 |
| High-school/post-secondary diploma (medium) | 55.3 |
| University degree (high) | 27.7 |
| Family status, % | |
| Intact | 82.0 |
| Non-intact | 18.0 |
| Family income, % | |
| Sufficient | 78.1 |
| Insufficient | 21.9 |
ADHD, attention-deficit hyperactivity disorder.
a. Data are courtesy of the Quebec Institute of Statistics.
Discussion
Potential risks and possible misprescription have led some clinicians to highlight the need for caution in medicating ADHD. Reference Singh18 Our findings suggest that ADHD medication is significantly predicted by core ADHD symptoms but also over and above these symptoms by additional risk factors: being male, low maternal education and immigrant status.
Comparison with previous findings and interpretation
As expected, the strongest clinical predictors of ADHD medication use were symptoms of hyperactivity-inattention. This finding is reassuring since hyperactivity-inattention symptoms are the core behavioural symptoms of ADHD explicitly targeted by this medication. Interestingly, both dimensions (i.e. hyperactivity-impulsivity and inattention) were independently related to medication. Comorbid psychiatric symptoms assessed longitudinally (i.e. anxiety, emotional problems and opposition) were not independently associated with ADHD medication use when other risk factors were accounted for. In other words, these other symptoms (particularly symptoms of opposition that are often correlated with ADHD symptoms) did not induce medication. This means that clinicians did not medicate children who showed oppositional/anxious problems beyond what their ADHD status required. These results are particularly relevant in the context of public discussions regarding the ethics of medicating youths with psychotropic medications.

Fig. 1 Instantaneous hazards of attention-deficit hyperactivity disorder (ADHD) medication use.
(a) ADHD medication use; contribution of (b) hyperactivity-inattention; (c) maternal education; and (d) parental immigration status.
Table 2 Survival models predicting exposure to attention-deficit hyperactivity disorder (ADHD) medicationa
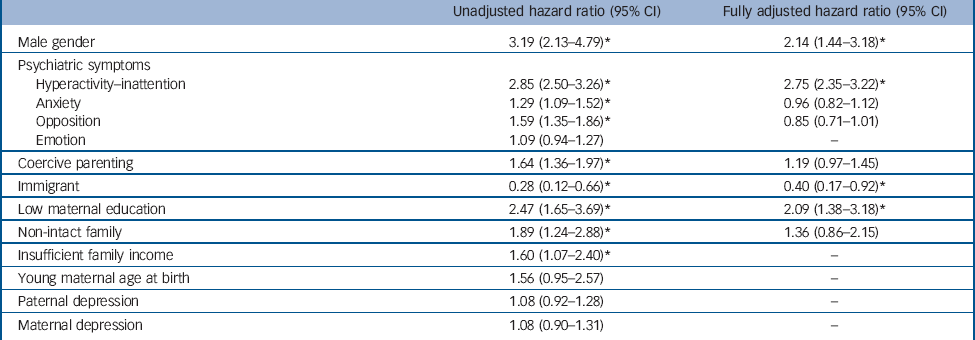
| Unadjusted hazard ratio (95% CI) | Fully adjusted hazard ratio (95% CI) | |
|---|---|---|
| Male gender | 3.19 (2.13-4.79)Footnote * | 2.14 (1.44-3.18)Footnote * |
| Psychiatric symptoms | ||
| Hyperactivity-inattention | 2.85 (2.50-3.26)Footnote * | 2.75 (2.35-3.22)Footnote * |
| Anxiety | 1.29 (1.09-1.52)Footnote * | 0.96 (0.82-1.12) |
| Opposition | 1.59 (1.35-1.86)Footnote * | 0.85 (0.71-1.01) |
| Emotion | 1.09 (0.94-1.27) | - |
| Coercive parenting | 1.64 (1.36-1.97)Footnote * | 1.19 (0.97-1.45) |
| Immigrant | 0.28 (0.12-0.66)Footnote * | 0.40 (0.17-0.92)Footnote * |
| Low maternal education | 2.47 (1.65-3.69)Footnote * | 2.09 (1.38-3.18)Footnote * |
| Non-intact family | 1.89 (1.24-2.88)Footnote * | 1.36 (0.86-2.15) |
| Insufficient family income | 1.60 (1.07-2.40)Footnote * | - |
| Young maternal age at birth | 1.56 (0.95-2.57) | - |
| Paternal depression | 1.08 (0.92-1.28) | - |
| Maternal depression | 1.08 (0.90-1.31) | - |
Data are courtesy of the Quebec Institute of Statistics.
* P<0.05.
Boys were more likely than girls to receive medication, even when controlling for the frequency of hyperactivity-inattention symptoms. This association could be related to a diagnostic bias towards boys. This could be attributable to a noisier clinical expression of the disorder in boys Reference Rucklidge34 and/or to cultural trends (mothering ideology and masculinity stereotypes). Reference Singh18 Consequently, boys could be more likely to benefit from a more precise ADHD diagnosis and treatment than girls.
Recent research on ADHD suggests that the disorder is a complex and aetiologically heterogeneous condition caused by a combination of genetic, environmental and epigenetic contributions. Reference Galéra, Côté, Bouvard, Pingault, Melchior and Michel35,Reference Sonuga-Barke and Halperin36 It has been argued that social and cultural characteristics may influence both diagnosis and prescription. Reference Singh18 The current study found associations consistent with this view. Most importantly and consistent with prior research, Reference Hjern, Weitoft and Lindblad22,Reference Miller, Kohen and Johnston24 low maternal education was associated with ADHD prescription. There are several possible explanations for this finding. First, clinicians may conclude that psychoeducational interventions alone are less efficient than medication because of parental difficulties and limited resources. This would lead them to opt more easily for medication that has a quick and direct effect on ADHD symptoms and may make the parents more available for psychoeducative interventions. Second, mothers with more education may more readily seek and access information on medication, which makes them sensitive to drug issues and consequently less prone to accept use of medication. Third, low maternal education could confer risk for more severe symptomatology through a combination of environmental and biological factors. Fourth, phenomena such as parent-blame (more particularly mother-blame linked to the mothering ideology) and parental feelings of inadequacy in response to children’s disruptive behaviour are thought to possibly bias ADHD diagnosis/treatment. Reference Singh18,Reference Singh37 All these potential social mechanisms need to be tested further by using qualitative and quantitative methods (for example randomised controlled trials) to examine the reasons for clinicians to suggest, and/or parents to accept, medication in families with different education levels, and examine the short- and long-term effectiveness of the different choices (for example to confirm or refute the benefit of more frequent prescriptions in lower-educated families). It is useful to note that other social variables were related to ADHD medication in univariate analyses (coercive parenting, non-intact family, insufficient family income) but were not associated with the study outcome in multivariate analyses, suggesting more distal influences or a less important role in medication use.
Parental immigrant status (which was strongly associated with racial/ethnic minorities) was related to lower ADHD medication use. This result is coherent with findings showing lower rates of ADHD diagnosis and less use of ADHD medication in children with a family immigrant background. Reference Singh, Yu and Kogan38,Reference Knopf, Holling, Huss and Schlack39 The negative association could have several causes. First, immigrant status may be a barrier to proper access to healthcare. It has been suggested that minorities could be underdiagnosed owing to lesser healthcare utilisation because of poorer resources. Reference Visser, Lesesne and Perou40 Second, parents from ethnic minority groups have been shown to report fewer ADHD symptoms than White parents, independently of their health or socioeconomic characteristics. Reference Pastor and Reuben41 This could be related to culturally different beliefs regarding children’s problematic behaviours and resistance to drug treatment. Third, USA children benefiting from Medicaid programmes, who come more often from racial/ethnic minorities, are less likely to benefit from interventions meeting quality of care standards and display higher unmet mental needs. Reference Zima, Bussing, Tang, Zhang, Ettner and Belin42 Our results suggest that these hypotheses require further investigation in countries with different cultures and different healthcare systems.
Strengths and limitations
This study has several strengths, including the nature of the sample, the time period encompassed and the analytic approach. First, the large community-based sample made it possible to extend inferences beyond clinical populations. Second, the repeated measurement of ADHD and other behavioural symptoms prior to medication exposure increases reliability and strengthens causal inferences in comparison with prior cross-sectional studies. Third, since methylphenidate initiation most commonly begins between ages 5 to 9 years, Reference Winterstein, Gerhard, Shuster, Zito, Johnson and Liu26 this study provides an opportunity to study the predictors of prescription during this crucial period.
The study also has limitations. First, the study relies on parental reports, which makes the data subject to informant bias. Such reports might partly reflect higher or lower parental tolerance to ADHD behaviours. Of note, we adjusted for factors potentially associated with tolerance such as oppositional behaviours or non-intact family. Second, a full-blown categorical ADHD diagnosis was not a requirement. However, this limitation was offset by the use of a dimensional approach, thus providing a better assessment of the phenotype heterogeneity that leads to medication use. Reference Sonuga-Barke and Halperin36 Third, there were no data on functional impairment related to ADHD symptoms. Future investigations should take impairment severity into account, in order to explore factors associated with prescriptions in moderate and severe forms of ADHD, which could help in understanding the issue of potential overdiagnosis and overtreatment. Reference Thomas, Mitchell and Batstra43 Fourth, information relating to non-pharmacological ADHD interventions was not available. Fifth, we could not consider potential mechanisms explaining the observed associations, for example cultural influences such as school pressure, parental expectations, community representations, mothering ideology, masculinity stereotypes and healthcare system representations. These should be examined in future work with randomised controlled trials in order to enhance our understanding of medication determinants. Finally, it is to be acknowledged that the influences of social variables on prescription depend on the environmental, cultural and legal context. The findings cannot be directly generalised to other country settings, especially newly industrialised nations and countries with lower prescription rates. Further research could benefit from cross-cultural designs and comparisons between different countries.
Implications
The study’s findings have theoretical and practical implications. Physicians should bear in mind the possible contribution of social characteristics on their practices. Individual features of children are not the only variables influencing drug exposure. The role of social context (parental educational level, immigration status) and parenting style needs to be fully considered in order to choose effective therapeutic strategies. Caution needs to be taken since clinical decisions grounded solely on risk might stigmatise families and children on a long-term basis. However, within the set of possible explanations, both legitimate (i.e. accessibility to pharmacological and non-pharmacological interventions) and more questionable (i.e. social pressure) may link social variables to medication. An effort to design specific parenting and psychoeducative interventions for families with low educational levels may encourage clinicians to consider alternatives to drug prescription for this target population. Reference Boisjoli, Vitaro, Lacourse, Barker and Tremblay44 Clinicians may want to take parenting styles into account before prescribing and also develop specific psychoeducational interventions dedicated to this population. Furthermore, the present study suggests that some subgroups with less access to their healthcare system and medication could benefit from selective interventions to enhance their access to psychosocial services and consequently be offered the same opportunities as the rest of the population (i.e. have equal access to good mental healthcare, defined as appropriate and unbiased use of ADHD medications).
Major clinical practice guidelines Reference Banaschewski, Coghill, Santosh, Zuddas, Asherson and Buitelaar1,11,Reference Wolraich, Brown, Brown, DuPaul and Earls12 recommend that the management of the ADHD clinical situation should rely on the joint use of psychoeducation, psychotherapy and pharmacotherapy. The current findings support the recommendations regarding the necessity of enhancing child and family therapeutic education and physicians’ knowledge of prescription determinants. Future research will need to provide a better understanding of parental and practitioners’ views to determine what drives decision-making on treatment choice.
Acknowledgements
R.E.T. is the founding scientific director of the Quebec Longitudinal Study of Children. We thank the children and families whose ongoing participation made this study possible. We also acknowledge the considerable contribution of the staff and the coordinators of the Quebec Longitudinal Study of Child Development at the Quebec Institute of Statistics and the tireless work of all the interviewers who assessed the mothers and children during this study. We thank the staff of the Research Unit on Children’s Psychosocial Maladjustment for their help in data access, analyses and management.






eLetters
No eLetters have been published for this article.