Patients with schizophrenia are more likely to smoke, and to smoke heavily, than other psychiatric or non-psychiatric populations (Reference de Leon, Dadvand and Canusode Leon et al, 1995). Smoking is 40-100% more prevalent in schizophrenia than in other diagnostic groups (Reference Goff, Henderson and AmicoGoff et al, 1992). The association with smoking remains even when allowing for other contributing factors such as age, gender, in-patient/out-patient status, education level, comorbid substance misuse, marital status, socio-economic status and neuroleptic medication (Reference de Leon, Dadvand and Canusode Leon et al, 1995). Patients with schizophrenia who smoke score higher on rating scales for psychotic symptoms (Reference Goff, Henderson and AmicoGoff et al, 1992; Reference Ziedonis, Kosten and GlazerZiedonis et al, 1994) than their non-smoking peers. Both reports suggest that smoking and nicotine may have some influence on development or amelioration of the symptoms of schizophrenia. We hypothesised an association between smoking and psychotic symptomatology in general, rather than specifically with schizophrenia. To test this hypothesis we examined smoking behaviour in a population with bipolar affective disorder. We anticipated an increase in smoking prevalence and heavier smoking in patients with a history of psychotic symptoms.
METHOD
Subjects
The sample consisted of patients ascertained from the Republic of Ireland for a UK—Irish Collaborative Study into the Genetics of Bipolar Disorder (Reference Craddock and GillCraddock & Gill, 1998). Patients were ascertained from community and hospital psychiatric services nationally, private psychiatric hospitals, general practitioners and directly through local and national media. Following a full explanation of the procedures involved, written informed consent was obtained. The first interviewed member of each family to meet ICD—10 diagnostic criteria for bipolar affective disorder (World Health Organization, 1993) was included in this study.
Interview schedule
Participants were interviewed on a single occasion by one of four trained clinicians (A. C., E. O'M., C. C., R. O'C.). The diagnosis was made by structured interview using the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) Version 2.1 (World Health Organization, 1992). Additional information was collected from available medical records and collateral data (from family and/or treating clinician). Subjects were asked for information about their current and previous smoking behaviour; where possible this information was verified with family and/or care workers. Each interviewer completed a detailed case summary and generated a diagnosis using the computer package OPCRIT (Reference McGuffin, Farmer and HarveyMcGuffin et al, 1991). Each case was reviewed independently by another clinician blind to the diagnosis (M. G., N. C.). Consensus diagnosis meetings were held, cases were discussed if consensus was not reached between SCAN, chart review, case summary and OPCRIT diagnosis. Only cases where a consensus diagnosis of bipolar affective disorder could be reached were included in the study.
Measures
Individuals were considered as current smokers if they had been smoking daily for at least 3 months prior to interview. Individuals who had never smoked or those who had stopped smoking at least a month prior to interview were considered non-smokers. Cigarette use was further classified as ‘moderate’ (defined as ≤20 cigarettes/day) or ‘heavy’ (> 20 cigarettes/day) smoking.
Data were converted into ordinal and nominal variables for statistical analysis. Patients were classified as having psychoses if they had a history of at least one psychotic symptom (delusion or hallucination) as defined by SCAN, and thus met criteria for a psychotic mood episode as defined by ICD—10 (World Health Organization, 1993). Patients with a history of psychosis were further divided into those with a history of ‘low-grade’ symptoms (not significantly contributing to overall illness) and those with ‘severe’ symptoms (defined as at least 25% of total illness duration). This approach was based on the dimensional classification for bipolar affective disorder as described by Craddock et al (Reference Craddock, Jones and McCandless1997). The other variables, namely: gender, age at onset, course of illness, number of manic episodes, number of depressive episodes, duration of illness, comorbid alcohol or non-alcohol substance misuse/dependency, education, socio-economic status and current neuroleptic usage were treated as nominal variables. These variables were selected by a review of smoking and schizophrenia literature as those most likely to confound any association between smoking and psychosis.
Individuals were divided into categories according to age at onset of illness: <20 years, 20-29 years and ≥ 30 years old. Duration of illness was categorised as <10 years, 10-19 years, 20-29 years and ≥ 30 years. The numbers of episodes of mania or depression were grouped as 0, 1-2, 3-5, 6-9 and 10+ total lifetime episodes. Course of illness (defined as in ICD—10) was simplified to ‘good’ if single/multiple episodes with good recovery between episodes occurred, and ‘poor’ if recovery between episodes was only partial or the illness was continuous. Alcohol-related pathology was defined categorically as a lifetime history of ICD—10 alcohol abuse or dependency. Non-alcohol substance misuse was defined as: ‘never’; ‘occasional’ if less than monthly; ‘regular’ if monthly or more frequently, but abstinent for the last month; and ‘current’ if used in the month prior to interview. Education was defined as completion of primary, secondary or tertiary level education. Socio-economic status was defined using standard social class measures: (I) professional, (II) managerial and technical, (III) non-manual, (IV) skilled manual, (V) semi-skilled, (VI) unskilled, (VII) all others. For analysis these were divided into three groups representing (I) and (II); (III) and (IV); (V)—(VII). Neuroleptic use was defined as a daily dosage for at least the month prior to interview (including current depot treatment).
Statistical methods
Basic frequency data were compiled, followed by a χ2 analysis of the relationship between smoking, heavy smoking and presence/absence of a history of psychotic symptoms. Individual χ 2 testing of all variables was avoided to limit multiple testing. To further explore the association we chose to use an ordinal logistic regression model using the computer package JMP (SAS Institute Inc, 1995). This model analysed the relationship between the dependent variable ‘psychotic symptoms status/severity’ and the variable ‘smoking status/severity’ controlling for the effects of all other variables. Within the model we examined the correlation between variables and multi-co-linearity was excluded.
RESULTS
Psychosis and smoking data
Our total sample was of 92 patients with an ICD—10 diagnosis of bipolar affective disorder, of whom 49 (53.3%) were female. The mean age of participants was 44 years (s.d.=11.8) (range 22-72). Sixty-four participants (69.6% of the total population) had a history of at least one psychotic symptom; of these 23 (25%) had symptoms in the defined low-grade category and 41 (41.6%) had a history of severe psychotic symptoms. In this sample, 57.6% of participants (n=53) were smokers; data on smoking behaviour were confirmed by collateral history in 88% of cases (n=81). Only four of the non-smokers had ever been regular smokers (10.2%). Smoking was particularly prevalent among patients with psychosis (68.7%, n=44); by contrast in the group with no history of psychotic symptoms the smoking prevalence was 32.1% (n=9). The latter figure is remarkably consistent with the current smoking prevalence in Ireland of 32% (Health Research Board, 1998). A significant relationship was detected between smoking/heavy smoking and a history of psychotic symptoms (χ2=11.68, d.f.=2, P=0.003 (two-tailed)) (Fig. 1).
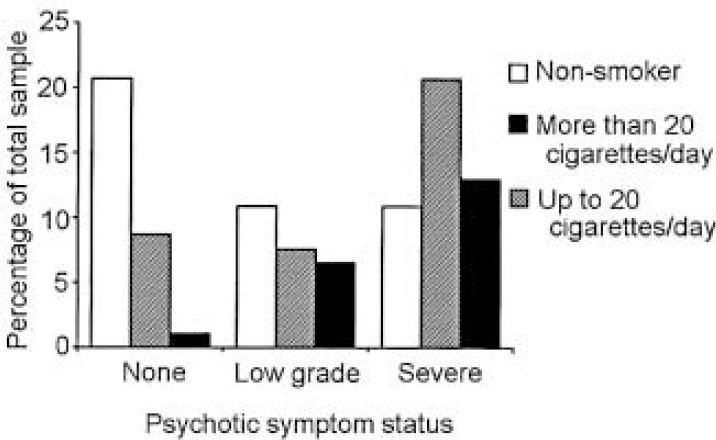
Fig. 1 Psychotic symptom status grouped by smoking status.
Other clinical and epidemiological variables
Twenty of the participants (21.7%) fulfilled the ICD—10 criteria for alcohol dependence/abuse. Forty-five patients (48.9%) were taking neuroleptic medication, 38 (41.3%) were not, and these data were unavailable on the remaining nine patients (9.8%). Details of social class and education were available for 88% of the total sample. Nineteen participants (20.7%) reported a history of non-alcohol substance use, and nine of these (9.8%) admitted to ever having used substances monthly. All patients denied current substance misuse, and only one described regular use of a substance other than cannabis (Ecstasy).
Ordinal logistic regression
We performed an ordinal regression analysis to examine the relationship between each individual test variable and history and severity of psychotic symptoms, controlling for all other test variables (Table 1). Analysis confirmed that smoking/heavy smoking predicted psychotic symptom status and a history of severe psychotic symptoms (χ2=10.73, P=0.027). Examination of the parameter estimates showed this to be explained by a significant difference in psychotic symptom severity between non-smokers and moderate smokers (χ2=10.14, P=0.0015). No significant difference for psychotic symptom severity was detected between the moderate and heavy smoking groups. Of the other tested variables, only current use of neuroleptic medication (as we would expect) predicted a history of psychotic symptoms (χ2=6.25, P<0.001); this was independent of all other variables, including smoking.
Table 1 Relationship between clinical and epidemiological variables and history and severity of psychosis (χ2 values are for the ordinal linear regression model)
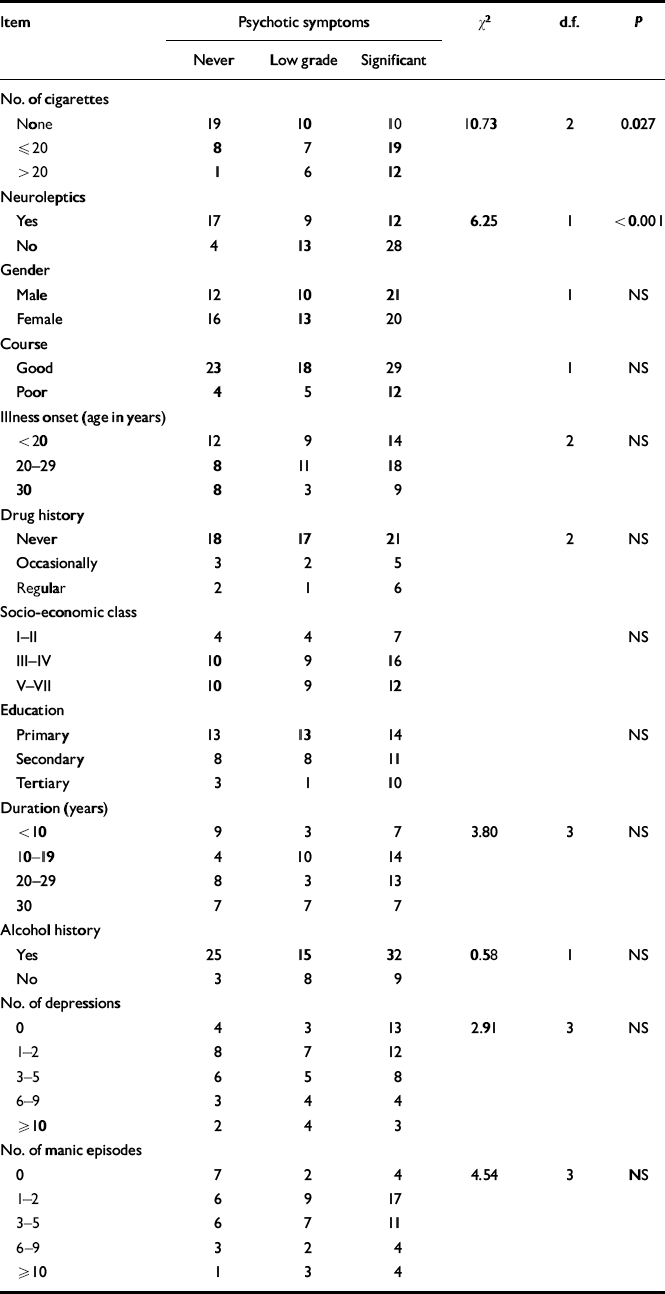
| Item | Psychotic symptoms | χ 2 | d.f. | P | ||
|---|---|---|---|---|---|---|
| Never | Low grade | Significant | ||||
| No. of cigarettes | ||||||
| None | 19 | 10 | 10 | 10.73 | 2 | 0.027 |
| ≤ 20 | 8 | 7 | 19 | |||
| > 20 | 1 | 6 | 12 | |||
| Neuroleptics | ||||||
| Yes | 17 | 9 | 12 | 6.25 | 1 | <0.001 |
| No | 4 | 13 | 28 | |||
| Gender | ||||||
| Male | 12 | 10 | 21 | 1 | NS | |
| Female | 16 | 13 | 20 | |||
| Course | ||||||
| Good | 23 | 18 | 29 | 1 | NS | |
| Poor | 4 | 5 | 12 | |||
| Illness onset (age in years) | ||||||
| <20 | 12 | 9 | 14 | 2 | NS | |
| 20-29 | 8 | 11 | 18 | |||
| 30 | 8 | 3 | 9 | |||
| Drug history | ||||||
| Never | 18 | 17 | 21 | 2 | NS | |
| Occasionally | 3 | 2 | 5 | |||
| Regular | 2 | 1 | 6 | |||
| Socio-economic class | ||||||
| I-II | 4 | 4 | 7 | NS | ||
| III-IV | 10 | 9 | 16 | |||
| V-VII | 10 | 9 | 12 | |||
| Education | ||||||
| Primary | 13 | 13 | 14 | NS | ||
| Secondary | 8 | 8 | 11 | |||
| Tertiary | 3 | 1 | 10 | |||
| Duration (years) | ||||||
| <10 | 9 | 3 | 7 | 3.80 | 3 | NS |
| 10-19 | 4 | 10 | 14 | |||
| 20-29 | 8 | 3 | 13 | |||
| 30 | 7 | 7 | 7 | |||
| Alcohol history | ||||||
| Yes | 25 | 15 | 32 | 0.58 | 1 | NS |
| No | 3 | 8 | 9 | |||
| No. of depressions | ||||||
| 0 | 4 | 3 | 13 | 2.91 | 3 | NS |
| 1-2 | 8 | 7 | 12 | |||
| 3-5 | 6 | 5 | 8 | |||
| 6-9 | 3 | 4 | 4 | |||
| 10 | 2 | 4 | 3 | |||
| No. of manic episodes | ||||||
| 0 | 7 | 2 | 4 | 4.54 | 3 | NS |
| 1-2 | 6 | 9 | 17 | |||
| 3-5 | 6 | 7 | 11 | |||
| 6-9 | 3 | 2 | 4 | |||
| ≥ 10 | 1 | 3 | 4 | |||
DISCUSSION
Main findings
Our results support the hypothesis that smoking is associated with a history of psychosis in bipolar affective disorder. We also found that smoking is associated with increased severity of psychotic symptoms in this population. While similar findings have been reported in patients with schizophrenia, this association has not previously been reported in bipolar affective disorder. We suggest that an association exists between smoking and psychotic symptomatology, and not with the categorical diagnosis of either schizophrenia or bipolar affective disorder.
Relationship to existing literature
Data on smoking behaviour in bipolar affective disorder are conflicting — two studies report increased smoking (Reference Hughes, Hatsukami and MitchellHughes et al, 1986; Reference Gonzalez-Pinto, Gutierrez and EzcurraGonzalez-Pinto et al, 1998) but a third found no change (Reference Diwan, Castine and PomerleauDiwan et al, 1998). From our finding we would predict that broadly defined mood disorder samples (Reference Diwan, Castine and PomerleauDiwan et al, 1998) would have a lower smoking prevalence, having more patients with unipolar depression, in which psychotic symptoms would be less frequent.
Implications for the pathophysiology of psychotic disorders
A regulatory effect for nicotine on the dopaminergic, serotonergic and glutaminergic systems implicated in schizophrenia has been suggested (for review see Reference Dalack and Meador-WoodruffDalack et al, 1998). Researchers studying abnormal auditory filtering (as measured by the P50 auditory evoked potential) in patients with schizophrenia have observed temporary reversal of the deficit with nicotine (Reference Adler, Hoffer and GriffithAdler et al, 1993). This group has also studied the role of nicotine in modulating gating abnormalities in a rat model (Reference Bickford and WearBickford & Wear, 1995). More recently they have reported genetic linkage between the human gating defect and a polymorphism of the α-7 nicotinic receptor sub-unit in families multiply affected with schizophrenia (Reference Freedman, Coon and Myles-WorsleyFreedman et al, 1997; Reference Leonard, Gault and MooreLeonard et al, 1998). Similar P50 gating abnormalities reported in bipolar disorder (Reference Baker, Staunton and AdlerBaker et al, 1990) had previously been interpreted as due to excess catecholamine release in the manic phase of illness. It is unclear whether excess smoking in psychotic bipolar affective disorder represents a different mechanism, or a shared mechanism in keeping with continuum (Reference CrowCrow, 1995) or alternative categorical models of psychosis (Reference Kendler, Karkowski and WalshKendler et al, 1998).
Methodological issues and future directions
The implications of our findings outside this sample require cautious interpretation, but we feel that the association warrants further investigation. In a follow-up study, we will include validated measures of symptom dimensions for psychosis. We were unable to measure nicotine or carbon monoxide levels in this study, but collateral information was available in most cases. There is also good evidence to support the accuracy of self-report of substance use in psychiatric patient populations (Reference McLellan, Luborsky and WoodyMcLellan et al, 1983). In future studies data derived from longitudinal rather than cross-sectional data may give the most accurate measure of true smoking behaviour, and in this regard hair analysis may provide a more objective measure (Reference Pichini, Altieri and PellegriniPichini et al, 1997). Our study failed to allow for variations in nicotine content of different brands of cigarette, although current thinking suggests that differential extraction of nicotine compensates for different amounts in cigarette brands. We also failed to find a difference in the severity of psychosis between moderate and heavy smoking groups. It is likely that we had insufficient sample size to distinguish such a difference should it exist, because of the relatively small groups involved.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Excessive smoking behaviour is associated with psychosis/severity of psychosis rather than specifically with bipolar affective disorder or schizophrenia.
-
• Research on the link between nicotine and bipolar affective disorder or schizophrenia may be interpreted as a link with presence and severity of psychotic symptomatology.
-
• Patients with psychotic bipolar illnesses should be targeted as a population at particular risk of smoking-related illnesses.
LIMITATIONS
-
• The results of this study require replication.
-
• Information on smoking behaviour was subjective: nicotine/metabolite levels were not objectively measured.
-
• Collateral information on comorbid substance misuse was unavailable for this sample.





eLetters
No eLetters have been published for this article.