Bipolar disorder is a chronic psychiatric condition characterised by depressive and manic or hypomanic mood episodes that fluctuate over time. Despite the existence of a high genetic loading highlighted by familial and twin studies, genetic variants found to date explain only a small fraction of the risk for bipolar disorder. It thus appears important to integrate the role of environmental factors in the understanding of this disorder.Reference Etain, Henry, Bellivier, Mathieu and Leboyer1 Among the most robust environmental factors that increase the risk of psychiatric disorders, early-life adversity is particularly relevant. Several studies have shown that early-life adversities have a critical role in the development of bipolar disorder. Physical, sexual and emotional childhood maltreatments have indeed been more frequently observed in patients with bipolar disorder compared with control groups.Reference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2-Reference Etain, Mathieu, Henry, Raust, Roy and Germain6 Furthermore, about half of these patients had been exposed to a combination of different types of early-life traumatic events.Reference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2 These epidemiological findings strongly suggest that early-life stress could act as an important risk factor for the development of bipolar disorder. Childhood traumatic events may moreover influence the clinical expression of the disorder. People with bipolar disorder and a history of childhood maltreatment are found to have (among other severity indices) earlier onset of the disease,Reference Etain, Henry, Bellivier, Mathieu and Leboyer1,Reference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2,Reference Garno, Goldberg, Ramirez and Ritzler7 rapid cycling course,Reference Leverich and Post4,Reference Garno, Goldberg, Ramirez and Ritzler7 increased psychotic symptoms,Reference Romero, Birmaher, Axelson, Goldstein, Goldstein and Gill8 and suicidal behaviour.Reference Leverich and Post4,Reference Garno, Goldberg, Ramirez and Ritzler7 Thus, childhood trauma may not only increase the risk of bipolar disorder, but also modulate its clinical course and outcome. From this perspective it appears crucial to understand how, at the biological level, early-life adverse events may lead to psychopathological conditions such as bipolar disorder in adulthood.
Translational research indicates that early-life stress can modify different steps in the development of neuronal circuits and homeostatic systems.Reference Lupien, McEwen, Gunnar and Heim9 In rodents and humans, early-life stress is linked to a variety of long-term modifications including changes in the hypothalamic-pituitary-adrenal (HPA) axis and abnormal brain development.Reference Lupien, McEwen, Gunnar and Heim9-Reference Frodl and O'Keane11 Effects of early-life stress on brain circuit assembly and maturation during critical developmental periods could thus represent a key mechanism determining vulnerability to emotional and mood psychiatric disorders. Epigenetic mechanisms could play an important part in these programming events and are likely to involve the regulation of a large number of genes. Among target genes, increased methylation of the promoter region of the glucocorticoid receptor gene (NR3C1) has been the focus of much translational research. In rodents, increased NR3C1 methylation in the hippocampus of rats with a history of low maternal care has been correlated with decreased levels of hippocampal glucocorticoid receptor expression and increased glucocorticoid secretion.Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma and Seckl12,Reference Weaver, D'Alessio, Brown, Hellstrom, Dymov and Sharma13 In humans, early-life stress during pregnancy has been associated with increased NR3C1 methylation in blood cells of newborns and appears to correlate with increased salivary cortisol stress responses.Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin14,Reference Radtke, Ruf, Gunter, Dohrmann, Schauer and Meyer15 In adults, increased NR3C1 methylation has been observed post mortem in the hippocampal tissues of people who died by suicide and had a history of childhood maltreatment.Reference McGowan, Sasaki, D'Alessio, Dymov, Labonte and Szyf16 More recently we found that increased NR3C1 methylation in peripheral blood lymphocytes was associated with increased loading of childhood maltreatment in people with borderline personality disorder, suggesting that peripheral blood could represent a proxy of the epigenetic modifications of NR3C1 occurring in the central nervous system.Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 Taken together, these data suggest that increased NR3C1 methylation could represent a general epigenetic mark of early-life stress that could be observed in different psychiatric populations exposed to early-life adversity and experiencing emotional and/or mood deregulation. To test this hypothesis and try to extend our previous study of borderline personality disorder to patients with bipolar disorder, we collected peripheral blood DNA from participants with bipolar disorder and assessed their levels of childhood maltreatment by means of a self-report questionnaire. In this sample we sought to determine whether the severity of early-life stress (number, intensity and types of abuse and neglect) correlated with the methylation status of the exon 1F NR3C1 promoter.
Method
Ninety-nine adult participants receiving out-patient treatment for bipolar disorder type 1 or 2 were recruited from a centre specialising in the treatment of this disorder at the Department of Mental Health and Psychiatry of the University Hospitals of Geneva. All participants were interviewed by trained psychiatrists or psychologists using a French version of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I).Reference First, Spitzer and Williams18 The patients were interviewed when they no longer met the criteria for depressive or manic/hypomanic episode. The Childhood Trauma Questionnaire (CTQ), a self-report questionnaire that examines five types of trauma (sexual abuse, physical abuse, physical neglect, emotional abuse and emotional neglect), was used to assess childhood traumatic experience.Reference Bernstein and Fink19 To ensure the independence of our study, none of the 99 participants had taken part in our previous investigation.Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 The study was approved by the ethics committee of the University Hospitals of Geneva. Informed written consent was obtained from all participants.
Genetic analysis
DNA extraction
Samples of DNA were extracted from peripheral blood leucocytes using the Nucleon kit (Bioscience Amersham, GE Healthcare, Glatbrugg, Switzerland).
DNA methylation status
After extraction, 2 μg of genomic DNA was bisulfite-modified by using the EpiTect Bisulfite Kit (Qiagen, Germantown, Maryland, USA) according to the manufacturer’s instructions. Polymerase chain reactions were carried out with 80 ng of genomic bisulfite-modified DNA using the KAPA2G Robust HotStart Kit (Kappa Biosystem, Cape Town, South Africa) in a final volume of 20 μl containing 1×buffer A, 1×enhancer 1, 0.02 mmol/l deoxynucleoside triphosphates (dNTPs), 7.5 μmol/l of each primer, 0.01 mmol/l HotStart polymerase and 0.04 μmol/l EvaGreen fluorescent intercalating dye (Invitrogen, Eugene, Oregon, USA). Amplification conditions are available from the authors upon request. We analysed a portion of the exon 1F NR3C1 promoter similar to the one explored in our earlier study,Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 containing eight CpG sites. The degree of methylation at each CpG site was determined using Pyro Q-CpG Software (Biotage AB, Uppsala, Sweden). All samples were analysed in duplicate and the mean percentage was then calculated and used for the study analyses. Samples were processed and analysed masked to demographic variables, psychiatric diagnosis and childhood maltreatment history.
Statistical analysis
Linear or logistic regression with adjustment for age and gender and age at onset where applicable (number of mood episodes, for instance) was used to assess the effect of childhood maltreatment on continuous and categorical clinical variables. The sum score of abuse and neglect was calculated, as described in our earlier study, by summing the different maltreatments, each treated as a binary outcome (yes or no).Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 Linear regression with adjustment for age and gender was used to assess the effect of childhood maltreatment on NR3C1 methylation status. Mean methylation percentage of the eight NR3C1 CpG sites was used as the dependent variable. For the association with NR3C1 methylation status, severity of each type of trauma as described by Bernstein & Fink,Reference Bernstein and Fink19 based on four levels of maltreatment (none, low, moderate and severe), was also used. In a secondary analysis all CpGs were analysed separately. The results of regression models are presented as standardised regression coefficients (β) with 95% confidence intervals which can be interpreted as effect size. The threshold for significance was P = 0.05. All analyses were performed using Stata release 10 for Windows.
Results
Table 1 describes the demographic and clinical characteristics of the sample. A majority (56%) of the participants were women; over half of the sample had the diagnosis bipolar disorder type 1 and three-quarters reported at least one type of childhood maltreatment.
Table 1 Clinical and demographic characteristics of the sample (n = 99)

| Characteristic | |
|---|---|
| Age, years | |
| Age at baseline: mean (s.d.) | 44.6 (10.2) |
| Age at onset: mean (s.d.) | 19.7 (8.6) |
| Number of mood episodes: mean (s.d.) | 17.3 (10.7) |
| Duration of illness, years: mean (s.d.) | 24.9 (10.9) |
| Gender (female), n (%) | 55 (56) |
| Bipolar disorder type 2, n (%) | 45 (45) |
| History of suicide attempt, n (%) | 36 (36) |
| Substance use disorder, n (%) | 23 (23) |
| Alcohol use disorder, n (%) | 24 (24) |
| Psychotic mood episodes, n (%) | 68 (69) |
| Reported trauma in childhood, n (%) | |
| Emotional abuse | 48 (48) |
| Emotional neglect | 55 (56) |
| Physical abuse | 33 (33) |
| Physical neglect | 29 (29) |
| Sexual abuse | 30 (30) |
Childhood maltreatment and adult clinical characteristics
After adjustment for age and gender there was an association between the type of bipolar disorder and childhood maltreatment. People with bipolar disorder type 2 reported more childhood maltreatments than those with type 1 disorder (OR = 1.31, 95% CI 1.02-1.69, P = 0.033). This was mainly due to more reports of emotional abuse (60% v. 39%; OR = 2.42, 95% CI 1.05-5.58, P = 0.037), physical abuse (44% v. 24%; OR = 2.36, 95% CI 1.01-5.53, P = 0.047) and physical neglect (40% v. 20%; OR = 2.41, 95% CI 1.00-5.85, P = 0.05) in the bipolar disorder type 2 group compared with type 1. Participants with a history of substance or alcohol use disorder also reported more childhood maltreatment compared with the group as a whole (substance misuse, OR = 1.57, 95% CI 1.14-2.16, P = 0.006; alcohol misuse, OR = 1.43, 95% CI 1.06-1.92, P = 0.017) which was mainly accounted for by more physical abuse (substance misuse, 56% v. 26%; OR = 3.55, 95% CI 1.31-9.57, P = 0.012; alcohol misuse, 50% v. 28%; OR = 3.08, 95% CI 1.17-8.10, P = 0.022). No other variable was associated with measures of childhood maltreatment.
Childhood maltreatment and NR3C1 methylation
After adjustment for age and gender, higher numbers of childhood maltreatments were associated with higher percentages of NR3C1 methylation (β = 0.52, 95% CI 0.46-0.59) (Fig. 1). With the exception of CpG1, the association between percentage of methylation and the number of types of abuse and neglect was observed for each CpG in the same direction independently (Fig. 2). Percentage of NR3C1 methylation was significantly associated with the severity of each type of maltreatment independently, emotional abuse being the most significantly associated (β = 0.64, 95% CI 0.52-0.76) (Fig. 3). The association with the severity of other type of maltreatments was as follows: sexual abuse β = 0.53 (95% CI 0.36-0.69), physical abuse β = 0.56 (95% CI 0.38-0.74), emotional neglect β = 0.68 (95% CI 0.54-0.81) and physical neglect β = 0.66 (95% CI 0.44-0.87). All associations were significant at P<<0.0001.

Fig. 1 Percentage of NR3C1 methylation according to the number of types of childhood abuse and neglect reported by the participants with bipolar disorder. Means are indicated by horizontal rules; each circle represents one participant.

Fig. 2 Methylation of the exon 1F NR3C1 promoter region, showing the percentage of methylation observed at each CpG site according to the number of types of childhood abuse and neglect reported. Asterisks indicate significance at P<0.0001.
Methylation status and clinical characteristics
Percentage of NR3C1 methylation was significantly associated with a history of alcohol use disorder (β = 0.54, 95% CI 0.079-1.00, P = 0.022). No other clinical or demographic variable was associated with percentage of methylation.
Discussion
In a university hospital out-patient clinic sample of 99 people with bipolar disorder, a high percentage reported a history of different types of childhood maltreatment events including sexual abuse (30%), physical abuse (33%), physical neglect (29%), emotional abuse (48%) and emotional neglect (56%). At one end of the severity spectrum a quarter of patients did not report any early-life traumatic events, whereas 7% at the other end had experienced a combination of five different types of childhood maltreatment events. These rates are comparable to those reported in other studies on bipolar disorder and exceed those reported in a general population assessed using the CTQ or other measures of childhood maltreatment.Reference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2,Reference Goldberg and Garno3,Reference Conus, Cotton, Schimmelmann, Berk, Daglas and McGorry5,Reference Etain, Mathieu, Henry, Raust, Roy and Germain6,Reference Finkelhor, Hotaling, Lewis and Smith20,Reference MacMillan, Fleming, Trocme, Boyle, Wong and Racine21 These findings strengthen the idea that childhood trauma represents a risk factor for bipolar disorder and that clinicians should pay attention to a history of childhood maltreatment when evaluating a patient. Replicating our previous findings,Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 we observed that the number of childhood traumatic events was strongly associated with the overall percentage of NR3C1 methylation in blood lymphocytes. Furthermore, NR3C1 methylation was associated with the severity of each type of childhood maltreatment, with emotional abuse showing the most significant association. These findings in a sample of people with bipolar disorder strengthen our previous results showing higher methylation status of the exon 1F NR3C1 promoter in people maltreated in childhood.Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 Together, these results strongly suggest that early-life adversity has a long-lasting effect on NR3C1 methylation, an effect that can be measured in the peripheral blood. The functional consequences of these specific epigenetic modifications are difficult to assess in humans. In rodents, hippocampal methylation of the exon 17 NR3C1 promoter region reduces the binding of the nerve growth factor-inducible protein A, leading to decreased transcription of the NR3C1 gene and decreased protein expression of hippocampal glucocorticoid receptors.Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma and Seckl12,Reference Weaver, D'Alessio, Brown, Hellstrom, Dymov and Sharma13 Decreased levels of glucocorticoid receptor expression alter the negative hippocampal feedback loop on hypothalamic corticotropin-releasing hormone neurons and lead to increased HPA axis reactivity.Reference Weaver, Cervoni, Champagne, D'Alessio, Sharma and Seckl12 Disturbances in HPA axis reactivity have been reported in patients with bipolar disorder,Reference Watson, Gallagher, Ritchie, Ferrier and Young22-Reference Salvadore, Quiroz, Machado-Vieira, Henter, Manji and Zarate24 and epigenetic modifications of NR3C1 in relation to early-life adversity could thus contribute to this process in a subset of patients with a history of childhood trauma. Among the different types of childhood maltreatments, the severity of emotional abuse showed the highest association with NR3C1 methylation. This highlights the importance of the caregiving function at early age and the fact that early-life alterations in the attachment process may produce long-lasting biological consequences.Reference Yates25,Reference De Bellis, Keshavan, Clark, Casey, Giedd and Boring26 Since retrospective evaluation in adult patients of early-life stress is influenced by memory biases (one limitation of this study), a prospective sample assessing the impact of early-life stressors would be valuable.
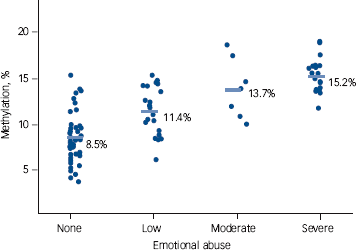
Fig. 3 Percentage of NR3C1 methylation according to the severity of emotional abuse. Means are indicated by horizontal rules; each circle represents one participant.
A similar association between the methylation status of the exon 1F NR3C1 promoter and severity of early-life adversity has been reported by our group in a sample of patients with border-line personality disorder and histories of childhood trauma.Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 Our new data from the bipolar disorder sample add weight to our initial findings, further strengthening the robustness of the impact of early-life adversity on NR3C1 epigenetic processes. It must be noted, however, that a limitation of this study resides in the fact that we did not assess the borderline personality disorder status of our bipolar disorder group. There is high degree of comorbidity between bipolar disorder and borderline personality disorder and there is much debate over whether borderline personality disorder belongs to the bipolar spectrum.Reference Coulston, Tanious, Mulder, Porter and Malhi27 Childhood maltreatment predisposes to both disorders and it has been reported that patients with bipolar disorder and a history of trauma have increased comorbidity with borderline personality disorder.Reference Carballo, Harkavy-Friedman, Burke, Sher, Baca-Garcia and Sullivan28 The association between borderline personality disorder and bipolar disorder is more frequently observed in patients with bipolar disorder who experience milder forms of mania such as bipolar disorder type 2 or bipolar spectrum disorder, or in those with rapid-cycling bipolar disorder.Reference Coulston, Tanious, Mulder, Porter and Malhi29,Reference Bassett30 Interestingly, in our bipolar disorder sample the number of childhood maltreatment events correlated with bipolar disorder type 2 and a history of substance and alcohol use disorder, which is more frequently observed in patients with bipolar disorder and a history of childhood maltreatment.Reference Romero, Birmaher, Axelson, Goldstein, Goldstein and Gill8,Reference Goldstein, Strober, Birmaher, Axelson, Esposito-Smythers and Goldstein31 Although one has to keep in mind that our sample may be too small to state firm conclusions concerning bipolar disorder subtypes, our results suggest that childhood trauma may change the course of bipolar disorder towards a type 2 disorder with comorbid substance use disorder, possibly through altered HPA axis functioning. Childhood trauma indeed modulates the clinical expression and course of bipolar disorder. It has been reported that bipolar disorder with history of childhood maltreatment is associated with an earlier onset of bipolar disorder,Reference Etain, Henry, Bellivier, Mathieu and Leboyer1,Reference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2 decreased premorbid functioning,Reference Conus, Cotton, Schimmelmann, Berk, Daglas and McGorry5 increased psychotic symptoms,Reference Romero, Birmaher, Axelson, Goldstein, Goldstein and Gill8 increased mood episodes,Reference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2 fast cyclingReference Leverich, McElroy, Suppes, Keck, Denicoff and Nolen2 and increased chronicity.Reference Angst, Gamma, Rossler, Ajdacic and Klein32 In contrast to these studies, the severity and number of childhood maltreatment events in our bipolar disorder sample was not associated with severity criteria of bipolar disorder such as the presence of psychotic symptoms, the age at onset and the total number of mood episodes. In our sample of severely affected people with bipolar disorder, we found an association between childhood trauma and clinical characteristics closely linked to borderline personality disorder (bipolar disorder type 2, alcohol and substance use disorder). Our data suggest that these characteristics (not reported here) should be assessed systematically in such a sample in order to improve our understanding of the impact of early-life adversities on the clinical course of bipolar disorder. Future studies should thus test whether increased levels of NR3C1 methylation are significantly associated with a subset of bipolar disorder patients presenting a type 2 pattern, a more severe history of childhood trauma and comorbidity with borderline personality disorder and substance misuse.
The percentage of methylation was significantly associated with a history of alcohol use disorder. Recent data have suggested that exposure to alcohol or to drugs of misuse promotes changes in levels of DNA methylation and that this may possibly contribute to maintenance of addiction.Reference Bilinski, Wojtyla, Kapka-Skrzypczak, Chwedorowicz, Cyranka and Studzinski33 However, the main findings of our study were not changed when substance or alcohol use disorders were added as covariates in the analyses. The association between higher NR3C1 methylation levels and alcohol use disorder may thus be better accounted for by shared dimensions such as impulsivity, which is often found in those with borderline personality disorder or bipolar disorder and in those with substance use disorder; higher impulsivity here believed to result from childhood maltreatment.
Recent data using methylation arrays on hippocampal rodent and human post-mortem tissue indicate that variations in rodent maternal care or early-life adversity can lead to large-scale methylation changes in numerous genomic regions that include not only candidate gene promoters but also transcriptional and intragenic sequences.Reference Labonte, Suderman, Maussion, Navaro, Yerko and Mahar34,Reference McGowan, Suderman, Sasaki, Huang, Hallett and Meaney35 These data suggest that the specific NR3C1 methylation changes observed in this study will have to be integrated into the broad genomic methylation modifications induced by early-life adversity. The consequences of these early-life stress-induced epigenetic changes on gene expression and neural circuit development are only starting to be assessed in rodent models. Future work will be devoted to mapping these large-scale epigenetic changes in patients exposed to childhood maltreatment and to assessing how these epigenetic marks correlate with changes in neural circuit structure and function. Ultimately, it will be necessary to understand how these early-life stress-induced epigenetic changes interact with genetic variants to increase vulnerability to a variety of psychiatric disorders, including bipolar disorder.
Besides those described above, our study has other limitations. One is the possibility that our findings indirectly reflect an unmeasured variable that might have been influenced by both the report of childhood maltreatment and the measurement of methylation. However, several recent studies have consistently found an association between higher NR3C1 methylation status and early-life adverse events.Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin14,Reference Radtke, Ruf, Gunter, Dohrmann, Schauer and Meyer15,Reference McGowan, Suderman, Sasaki, Huang, Hallett and Meaney35 It is thus unlikely that our findings might be better accounted for by other unmeasured variables. In addition McGowan et al, investigating in rats a 7 million base pair region of chromosome 18 containing the NR3C1 gene, convincingly showed that some but not all regions displayed epigenetic modifications in relation to early environment, with many sequences showing little or no epigenetic modification.Reference McGowan, Suderman, Sasaki, Huang, Hallett and Meaney35 The NR3C1 promoter region was one of the modified regions, strengthening the hypothesis that early-life adverse events truly affect this region. A second caveat of our study is that the percentage of NR3C1 methylation may appear relatively low. However, it is in the range of previous studies, including our own, investigating NR3C1 methylation status in peripheral blood.Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin14,Reference Radtke, Ruf, Gunter, Dohrmann, Schauer and Meyer15,Reference Perroud, Paoloni-Giacobino, Prada, Olie, Salzmann and Nicastro17 We are thus confident in our results.
Implications
Our study shows that child maltreatment has a sustained effect on the methylation status of the exon 1F NR3C1 promoter, an effect that may be measured in the peripheral blood. This suggests that epigenetic processes, through enduring alteration of the HPA axis, may mediate the impact of early-life adversities on adult psychopathological disorder. Further studies should aim to improve the definition of clinical and environmental characteristics of patients in order to finely match epigenetic modifications with individual outcomes.







eLetters
No eLetters have been published for this article.