Epidemiological studies have consistently documented a marked rise in the prevalence of depression occurring between the ages of 13 years and 15 years (e.g. Reference Hankin, Abramson and MoffittHankin et al, 1998). As biological and social changes occur in this period, behavioural genetic theory provides a plausible account of these age-related increases. Larger genetic effects on depressive phenotypes in adolescence compared with childhood have been reported (Reference Rice, Harold and ThaparRice et al, 2002). ‘Developmental’ genes (Reference Scourfield, Rice and ThaparScourfield et al, 2003) and environmental factors (Reference O'Connor, Neiderhiser and ReissO'Connor et al, 1998; Reference Silberg, Pickles and RutterSilberg et al, 1999) also emerge in adolescence. As these studies have only assessed change between two time points in samples spanning a wide age range, it is unclear whether these ‘new’ influences emerge in the transition from childhood to adolescence or during adolescence. We aimed to clarify this by examining developmental changes in genetic and environmental factors across three time points from adolescence through early adulthood.
METHOD
Participants
The sample consisted of twins and siblings from the G1219 longitudinal study (Reference Lau, Rijsdijk and EleyLau et al, 2006). Recruitment for this study was through a random selection of twins born between 1985 and 1988 identified by the UK Office for National Statistics, and the offspring of adults from a large-scale population-based study. Initial response rates for these two samples were 47% and 40% respectively. Only twin and sibling pairs aged 12–19 years were retained, leaving data from 3640 individuals of 1820 families. Questionnaires were sent to participants at three time points, the first of which was the initial contact (wave 1). Wave 2 questionnaires were completed approximately 8 months (range 0–2 years) after first contact by 2651 individuals from 1372 families (73% of the original sample at wave 1). Wave 3 questionnaires were returned approximately 25 months (range 1–4 1–4 years) after wave 2 by 1597 adolescents from 824 families (44% of the original sample at wave 1). The mean age of participants at each time point was 14 years 5 months (s.d.=19 months, range 12–19 years), 15 years (s.d.=20 months, range 12–21 years) and 17 years and 17 years 8 months (s.d.=20 months, range 13–23 years) respectively.
Zygosity was established through a parent-report questionnaire assessing physical similarity between twins (Reference Cohen, Dibble and GraweCohen et al, 1975). Classifications gave 168 monozygotic male twin pairs, 199 monozygotic female twin pairs, 138 dizygotic male twin pairs, 190 dizygotic female pairs and 463 opposite-gender dizygotic pairs; 235 pairs were of unknown twin zygosity. There were also 109 male sibling pairs, 132 female sibling pairs and 186 opposite-gender sibling pairs.
The sample comprised roughly equal numbers of females and males with 51.7%, 56.1% and 58.7% of the sample being female at waves 1, 2 and 3 respectively. Levels of parental education were somewhat higher (39% educated to A-level or above) in these participants than in a large, nationally represented sample of parents (32% educated to A-level or above; Reference Meltzer, Gatward and GoodmanMeltzer et al, 2000). Parents in the G1219 study were also somewhat more likely to own their own houses (82%) than those in the nationally representative sample (68%). Gender of the child, parental education and housing tenure predicted attrition rates at waves 2 and 3 such that responses were more likely from females and from individuals whose parents reported higher educational qualifications and were owner-occupiers. Individuals with higher scores on a self-reported adolescent behaviour measure were also less likely to respond at wave 3. To reduce the impact of initial response biases associated with educational level and to account for attrition between waves, a weighting system was derived by assigning scores on predictor variables of initial and subsequent participation, and this was included in all statistical analyses. Full details of the recruitment process, sample characteristics and the weighting variable have been described elsewhere (Reference Lau, Rijsdijk and EleyLau et al, 2006).
Measures
Depressive symptoms at all three waves of the study were assessed using the self-report version of the Short Mood and Feelings Questionnaire (Reference Angold, Costello and MesserAngold et al, 1995). This consists of 13 items assessing core depressive symptoms occurring over the previous 2 weeks. As one of the initial aims of the G1219 study was molecular genetic analysis of extreme-scoring groups (Reference Eley, Sugden and GregoryEley et al, 2004), a four-point response format (never, sometimes, often, always) was used at the first two waves of data collection to allow better discrimination of the lower end of the spectrum. The standard three-point scale was used at wave 3. Total symptom scores were created by summing individual items. Internal consistency statistics indexed by Cronbach's alpha were comparable with previous studies, at 0.88, 0.90 and 0.88 for waves 1, 2 and 3 respectively. Reasonable sensitivity (0.60–0.75) and specificity (0.61–0.74) in discriminating between those with and without depression has been reported for this questionnaire (Reference Thapar and McGuffinThapar & McGuffin, 1998).
Statistical analyses
As the analyses were performed on data from twins and siblings, all statistical analyses controlled for the non-independence of data that this design incurs. A structural equation modelling package (Mx; Reference Neale, Boker and XieNeale et al, 1999) that incorporates sampling weights into analyses was used for all descriptive and model-fitting procedures.
Descriptive analyses
Saturated models, which estimate the variances, covariances and means of measured variables, were fitted to data at each time point. As these summary statistics are obtained for each gender-specific zygosity group, differences between males and females and between zygosity groups can be formally assessed through the comparison of various sub-models. For example, gender differences in mean symptom scores were ascertained by comparing a model that estimates separate means for males and females with one that constrains means to be the same across both genders. Similarly, zygosity differences in mean symptom scores can be tested, as well as the comparability of within-pair covariance between dizygotic twins and full siblings. The latter test is relevant as these zygosity groups are modelled similarly in subsequent genetic models. As each set of comparisons involves models that are nested within one another, any significant determination in fit between them, indexed by the change in χ2 values, reflects possible differences in means or covariance between males and females or zygosity groups.
Similar principles were applied to compare mean differences between depressive symptom scores across two time points. A model in which different means for symptom scores were allowed to differ at two time points was compared with a second model in which these means were equated. A significant deterioration in fit between them marks the presence of mean differences in symptom scores across time. Given that wave 3 data were collected using a different response format, this test was only applied to changes in scores between waves 1 and 2. Age trends and phenotypic correlations between variables were computed from covariance matrices specified in saturated models. As the distribution of scores at all three waves was positively skewed, a log transformation [ln(x + 1)] was applied to approximate normality. Descriptive analyses for depressive symptoms at waves 2 and 3 have been reported elsewhere (Reference Lau, Rijsdijk and EleyLau et al, 2006; further details available from authors on request).
Model-fitting analyses
Univariate analyses were first conducted to assess genetic and environmental effects on depressive symptoms at each time point. Estimates of genetic (a 2), shared environmental (c 2) and non-shared environmental (e 2) components can be derived through comparisons of within-pair similarity among monozygotic twins, who share 100% of their genetic make-up and dizygotic twins and/or full siblings, who share on average only 50% of genes. Greater monozygotic than dizygotic or full sibling resemblance is attributed to the increased genetic similarity among monozygotic twins, and is used to derive estimates of heritability (a 2). Within-pair similarity not resulting from genetic factors is assigned as shared environmental variance (c 2), which contributes towards resemblance among individuals growing up in the same family. Finally, non-shared environmental influences (e 2) create differences among individuals from the same family, and are estimated from within-pair differences between monozygotic twins. This term also includes any measurement error that might be present. This basic model can be extended to include a fourth source of variance, a twin similarity effect (t 2), which accounts for within-pair similarity among monozygotic and dizygotic twins over and above that between siblings. As such it was only included in models if the phenotypic similarity among dizygotic twins was not comparable to that between full sibling pairs, as shown in earlier descriptive analyses. Estimates of variance components can be specified to vary across males and females under different sub-models, to test for gender differences in the size and type of genetic and environmental parameters, and in the variance of each measure.
Whereas univariate models quantify genetic and environmental influences on the variance of each measure, multivariate models decompose the covariance between variables to assess shared genetic and environmental components. A Cholesky decomposition of three variables partitions genetic, shared and non-shared environmental effects into three sets of factors (Fig. 1). A T1, C T1 and E T1 influence all three variables, A T2, C T2 and E T2 influence the second and third variables and A T3, C T3 and E T3 influence the third variable only. Although any possible ordering of the variables explains the variance–covariance matrix between variables equally well, the current variables were ordered according to the time sequence with which they were collected, allowing inferences of the direction of effects in the results. In particular, ‘stable’ genetic and environmental influences (continuity) contributing to the continuity of phenotypes across all three time points (A T1, C T1 and E T1) can be distinguished from ‘new’ aetiological influences (change) that become operational at time 2 (A T2, C T2 and E T2) and time 3 (A T3, C T3 and E T3). Thus the proportion by which a 3 accounts for the total genetic variance on depression at time 3 (a3 + a 5 + a 6) reflects the extent to which a ‘stable’ genetic factor is influential in later depression, whereas the corresponding proportions that a 5 and a 6 explain of the total genetic variance represent the effects of ‘new’ genetic influences at waves 2 and 3 respectively. Calculating these proportions at each time point allows inferences of when developmental factors become effectual.
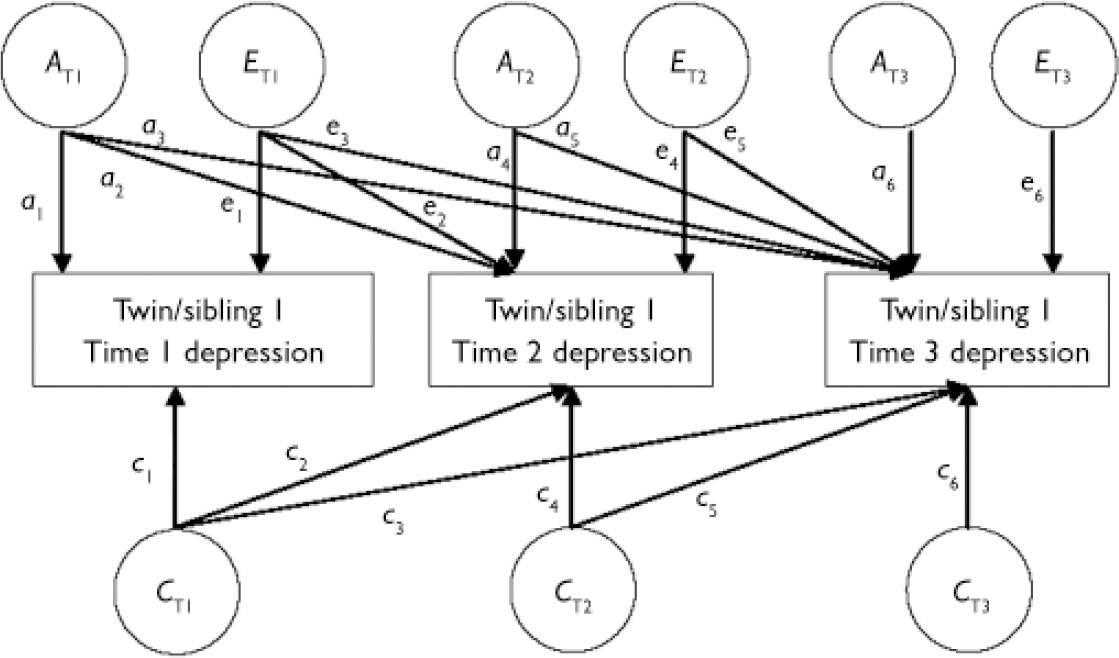
Fig. 1 Multivariate genetic analysis of longitudinal twin and sibling data for one member of a twin/sibling pair.
The program Mx uses maximum likelihood estimation procedures on raw data to generate parameter estimates of univariate and multivariate models that best explain the observed variance–covariance structure of the data. Statistics such as χ2 index the degree of fit. This is produced by subtraction of the –2LL (twice the negative log-likelihood) statistic generated in univariate and multivariate raw data models from the –2LL obtained in saturated models containing the same number of measured variables. A lower χ2 value relative to the degrees of freedom (i.e. a non-significant χ2) usually indicates less discrepancy between the expected and observed values and thus a better fit of the model to the data. Akaike's information criterion (AIC), which also takes into account parsimony (the number of estimated parameters relative to observed statistics) and is calculated as χ27d.f., is also often reported. A more negative AIC value indicates both good model fit and parsimony.
Sole reliance on the χ2 test has the problem that its power varies with sample size, such that for a large sample it is almost certainly significant even when the model provides a good fit to the data. Conversely, with small samples, fit statistics may be adequate even when fit is bad. The root mean squared error approximation (RMSEA) takes into account sample size and is often used in studies with large numbers of participants. Values falling below 0.10 indicate a model of good fit, with values below 0.05 suggesting excellent fit. An RMSEA was obtained for all univariate and multivariate models. Gender differences in genetic and environmental parameters or in the variance of each measure were determined by selection of the univariate sub-model with the lowest fit statistics.
Univariate and multivariate models were performed on age-regressed and log-transformed scores to minimise mean effects associated with age and to correct for positive skewness of the symptom data. Means of each measure were modelled separately for each gender-specific zygosity group in each model to minimise mean differences associated with gender or zygosity. Gender-specific effects found in univariate models were included in the multivariate model. Univariate models for depressive symptoms data at waves 2 and 3 have been reported elsewhere (Reference Lau, Rijsdijk and EleyLau et al, 2006), but the multivariate analyses of the longitudinal associations between time points are unique to the current study.
RESULTS
Descriptive analyses
Table 1 presents the depressive symptom scores for the sample at waves 1, 2 and 3 (further information on the comparison of sub-models is available from the authors upon request). Females reported more symptoms than males at all time points. Full siblings scored higher than twins in both gender groups at waves 1 and 2. Differences in within-pair covariance between dizygotic twins and full siblings at waves 1 and 3, with greater correlations in dizygotic twins, were also found. Among individuals with data at all three time points, wave 2 depressive symptom scores were significantly higher than those at wave 1, indicating an increase in rates of symptoms between these points. Given the different response format used to collect data at wave 3, corresponding comparisons between waves 2 and 3 could not be made. Correlations between age and depressive symptoms assessed at each time point were modest, at r=–0.03 (NS), r=–0.06 (P<0.01) and r=0.01 (NS) for waves 1, 2 and 3 respectively. Strong stability of depressive symptoms across time was found. Correlations were 0.58 (P<0.001), 0.45 (P<0.001) and 0.40 (P<0.001) between waves 1 and 2, 2 and 3, and 1 and 3 respectively.
Table 1 Depressive symptom scores measured at waves 1, 2 and 3, analysed according to gender and zygosity

| Wave 1 | Wave 2 | Wave 3 | |
|---|---|---|---|
| Whole sample | |||
| Age: mean | 14 years 5 months | 15 years 0 months | 17 years 8 months |
| Score: mean (s.d.) | 6.93 (5.74) | 8.07 (6.64) | 6.27 (5.32) |
| n | 3542 | 2526 | 1557 |
| Males | |||
| Score: mean (s.d.) | 6.20 (5.20) | 6.69 (5.47) | 5.03 (4.73) |
| n | 1687 | 1100 | 616 |
| Females | |||
| Score: mean (s.d.) | 7.59 (6.11) | 9.13 (7.24) | 7.08 (5.52) |
| n | 1855 | 1426 | 941 |
| MZ twins | |||
| Score: mean (s.d.) | 6.28 (5.40) | 7.06 (6.18) | 5.58 (5.07) |
| n | 875 | 705 | 442 |
| DZ twins | |||
| Score: mean (s.d.) | 6.86 (5.70) | 8.07 (6.55) | 6.56 (5.45) |
| n | 1830 | 1277 | 814 |
| Full siblings | |||
| Score: mean (s.d.) | 7.79 (6.08) | 9.34 (7.21) | 6.51 (5.25) |
| n | 823 | 530 | 287 |
Univariate genetic analyses
Twin and sibling correlations are presented in Table 2. Univariate sub-models incorporating qualitative and quantitative differences in genetic and environmental effects, and variance differences between males and females, were tested. Furthermore, as dizygotic twins had a greater within-pair covariance compared with full siblings at waves 1 and 3, a twin similarity effect was estimated first in univariate genetic models of these variables. Removing this latent factor from the model did not result in changes of fit at either time point: Δχ2(1)=0.00 (NS) for both waves 1 and 3. This suggests that twins do not share more similar depression-relevant environments than do siblings. This parameter was thus dropped from subsequent analyses.
Table 2 Twin and sibling correlations and model-fitting statistics from univariate genetic models of depressive symptoms at waves 1, 2 and 3

| Twin and sibling correlations | Proportions of variance (95% CI) owing to1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ twins | DZ twins | Full siblings | |||||||||
| M | F | M | F | Opp | M | F | Opp | a 2 | c 2 | e2 | |
| Wave 1 | |||||||||||
| Depressive symptoms: | 0.52 | 0.57 | 0.33 | 0.49 | 0.34 | 0.22 | 0.14 | 0.27 | 45 (32-58) | 19 (9-29) | 36 (31-41) |
| -2LL=9529.34, d.f.=3507, χ2(20)=34.64, P=0.02, AIC=-5.36, RMSEA=0.02 | |||||||||||
| Wave 2 | |||||||||||
| Depressive symptoms: | 0.30 | 0.50 | 0.13 | 0.39 | 0.24 | 0.21 | 0.21 | 0.32 | 40 (21-55) | 9 (0-23) | 51 (44-59) |
| -2LL=6621.57, d.f.=2367, χ2(20)=34.69, P=0.02, AIC=-5.31, RMSEA=0.07 | |||||||||||
| Wave 3 | |||||||||||
| Depressive symptoms: | 0.43 | 0.33 | 0.27 | 0.32 | 0.25 | 0.19 | 0.01 | 0.15 | 45 (22-31) | 0 (0-16) | 55 (47-65) |
| -2LL=3239.77, d.f.=1523, χ2(21)=17.36, P=0.69, AIC=-24.64 | |||||||||||
Model-fitting statistics and parameter estimates of the univariate models with best fit are also displayed in Table 2. There was no qualitative or quantitative gender difference in genetic and environmental influences on symptoms of depression, but a model including variance differences between the genders fits best at waves 1 and 2. A single set of parameters was thus reported for the whole sample across all waves (details of the comparisons of submodels are available from the authors upon request). Of note, RMSEA was incalculable for the wave 3 univariate model of depression given that the degrees of freedom exceeds the value of χ2 leading to a negative result that requires the square root to be taken. In this instance the low χ2 and AIC values are sufficient to demonstrate good fit. A rather uniform pattern of moderate genetic effects with the remaining variance attributable to non-shared environmental variance emerges at each time point. Significant shared environmental effects were apparent at wave 1, but declined across time.
Multivariate genetic analyses
A Cholesky decomposition model was applied to assess the effects of stable and new genetic and environmental factors across three time points in adolescence and early adulthood. Summary modelfitting statistics and parameter estimates of these models are presented in Table 3. As there was no gender difference in the size of genetic and environmental parameters in univariate genetic models, a single set of parameters for the whole sample was presented for this model too. Very good fit statistics were obtained. The total estimated genetic and environmental effects on each depression measure can be obtained by summing the contributions of common and specific components. As such, the estimated heritability of symptoms at wave 1 is A T1, at wave 2 it is A T1 + A T2 and at wave 3 it is A T1 +A T2+ A T3. In general the total genetic and environmental effects estimated for each measure in these multivariate models are consistent with those derived in univariate genetic models. The most notable difference is the shared environmental component of wave 1, which is non-significant in the multivariate model. Such slight variations in parameter estimates are a result of additional information available in cross-twin/sibling, cross-measure covariance.
Table 3 Summary model-fitting statistics and parameter estimates of multivariate longitudinal genetic models of depression between waves 1, 2 and 3

| Time 1 factors1 | Time 2 factors1 | Time 3 factors1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A T1 | C T1 | E T1 | A T2 | C T2 | E T2 | A T3 | C T3 | E T3 | |
| Wave 1 | 58 (40-71) | 9 (0-23) | 33 (28-40) | ||||||
| Wave 2 | 26 (12-43) | 5 (0-24) | 4 (2-8) | 10 (1-21) | 7 (0-14) | 48 (43-55) | |||
| Wave 3 | 16 (5-30) | 1 (0-14) | 3 (1-7) | 27 (5-35) | 1 (0-10) | 1 (0-4) | 0 (0-22) | 0 (0-11) | 51 (44-59) |
| -2LL=11559.63, d.f.=4982, χ2(149)=183.92, P=0.03, AIC=-114.08, RMSEA=0.02 | |||||||||
Results show that a stable genetic factor (A T1) influences symptoms at all three time points, accounting for 72% (26/26 + 10) and 37% (16/16 + 27) of the total genetic variance at waves 2 and 3 respectively. A new genetic factor emerges at wave 2, which also contributes to 63% of the total genetic variance at wave 3. No more new significant genetic influences are apparent by wave 3. There is a common shared environmental factor between waves 1 and 2 and waves 2 and 3, although the contribution of this factor to depressive symptoms at all time points is nonsignificant. Non-shared environmental effects, although significant, are generally specific to each time point. Given the wide age range of our sample, we also estimated separate parameters of the multivariate model for younger and older participants, classified according to a median split of age at wave 1 (12–14 and 15–19 years); this model did not show a significant improvement in fit (Δχ2(18)=17.85, NS), suggesting that genetic and environmental contributions to symptoms across time may be reasonably consistent across the age range of our sample.
DISCUSSION
Genetic and environmental effects at different time points
In the first set of analyses we assessed the relative effects of genetic and environmental factors on depressive symptoms at three time points, at which the mean ages of the sample were 14 years 5 months, 15 years and 17 years 8 months respectively. Results showed moderate genetic influence at each time point, accounting for just under half of the phenotypic variance. Shared environmental effects were significant at the first time point but declined across development. Finally, there were substantial nonshared environmental effects apparent at all three time points. A similar set of results characterised younger and older participants, indicating that overall the sizes of genetic and environmental effects are fairly consistent across adolescence and young adulthood. The size of estimates also falls roughly within the range of heritability and environmental effects reported in studies that have used a variety of phenotypic measures, including questionnaires (Reference Scourfield, Rice and ThaparScourfield et al, 2003), symptom counts (Reference Eaves, Silberg and MeyerEaves et al, 1997) and diagnostic categories (Reference Glowinski, Madden and BucholzGlowinski et al, 2003). Furthermore, these results are in keeping with age-related trends noted in earlier research, where genetic factors become increasingly important and shared environmental effects decline in adolescence compared with middle childhood (e.g. Reference Scourfield, Rice and ThaparScourfield et al, 2003). No gender difference was demonstrated in our estimates, although given the lack of consensus concerning the role of gender in moderating genetic and environmental effects in previous studies, there is no benchmark with which to compare this finding.
Stable and new genetic and environmental factors
Multivariate models addressed the effect of continuity and change of genetic and environmental factors on depressive symptoms across development. These showed that ‘stable’ genetic influences operational at the first time point (mean participant age 14 years 5 months) accounted partly for continuity of symptoms at the second (mean age 15 years) and third (mean age 17 years 8 months) time points. However, ‘new’ genetic effects also emerged at the second time point, which mainly contributed towards later depressive symptoms at the third point. ‘New’ non-shared environmental effects were also evident at each time point, and overall non-shared environment contributed to change rather than stability of symptoms across time. Consistent with univariate analyses, shared environmental influences were small and nonsignificant at each time point. Overall, results were similar across younger and older participants, suggesting that the findings of ‘stable’ and ‘new’ genetic and environmental factors may apply equally to transitions within adolescence as experienced by the younger subsample, and the transitions between adolescence and young adulthood of the older subsample.
Of the previous studies examining changes in genetic and environmental influences on depressive symptoms in young people, two identified ‘stable’ genetic influences contributing to continuity, with ‘new’ environmental variance effecting change (Reference O'Connor, Neiderhiser and ReissO'Connor et al, 1998; Reference Silberg, Pickles and RutterSilberg et al, 1999). In contrast, another study reported ‘new’ genetic as well as ‘new’ environmental effects (Reference Scourfield, Rice and ThaparScourfield et al, 2003). These studies all examined two time points, and with one exception (Reference Silberg, Pickles and RutterSilberg et al, 1999) used samples that included child participants. The current results are in agreement with both sets of findings, demonstrating that across three time points from adolescence through to young adulthood, genetic factors contribute primarily towards stability of symptoms but also to change.
Changes in genetic and environmental effects may explain age-related increases in depressive symptoms
The principal implication of these results is that the emergence of ‘new’ increased genetic effects and ‘new’ individual-specific environmental experiences may be instrumental in precipitating the observed rise in depressive conditions. It is interesting to note that these new genetic factors appear at a time (mean age 15 years) that coincides with increases in depressive symptoms in this as well as other studies (e.g. Reference Hankin, Abramson and MoffittHankin et al, 1998). As adolescence is characterised by a host of biological and pubertal changes and by maturation of various areas of cognition, it is plausible that developmentally sensitive genetic factors are ‘switched on’ at critical periods to enact these changes (Reference Pickles, Pickering and SimonoffPickles et al, 1998), which in turn have strong effects on the rates of depressive symptoms (Reference Angold, Costello and WorthmanAngold et al, 1998). In addition, adolescents are also confronted with novel socialisation practices both in the family and in their peer group, and have been reported to experience higher levels of stressful life events in this developmental period. Although these genetic and environmental influences may alone have adverse effects on the prevalence of depression, there are also suggestions that these factors could correlate and interact with one another during adolescence to account for symptoms (Reference Silberg, Rutter and NealeSilberg et al, 2001; Reference Rice, Harold and ThaparRice et al, 2003). That is, genetic factors may simultaneously influence exposure towards high-risk environments such as negative events or negative parental relationships (gene–environment correlation) and yet the occurrence of these stressors could in turn elicit other genetically driven vulnerabilities to influence the onset of depression. In summary, our results are in keeping with the many biological and social changes occurring in this period that have been linked to the sudden onset of depressive conditions.
Limitations
Although this study shows developmental changes both in genetic and environmental influences on adolescent depressive symptoms, which might account for the rise in prevalence, these implications need to be considered in the context of several limitations. First and foremost, a wide age range characterised our sample. Although comparable results were found among younger and older participants, indicating similar changes in genetic and environmental factors across adolescence and in the transition to young adulthood, the variability in age at each time point makes it difficult to attribute the emergence of developmental influences to specific ages or stages of development. Thus, conclusions about when genetic and environmental influences become effectual during development are based tentatively on the mean age of the sample at a particular wave of data collection. A related issue was that we defined ‘development’ purely by age rather than pubertal status. Given that biological, cognitive and social changes are likely to correspond with stage of puberty rather than chronological age, it would be interesting for future studies to examine the emergence of new factors across transitions associated with puberty rather than age.
A second issue concerns the generalisability of the results. These are entirely based upon questionnaire scores of depressive symptoms and it is unlikely that a single dimension of mood-related symptoms is representative of the complexities of diagnostic criteria. Moreover, participants were volunteers from the general community, and are likely to underrepresent individuals from economically disadvantaged backgrounds; this could potentially underestimate environmental effects despite the weighting system used. Finally, this sample consisted primarily of twins, invoking the issue of differences between these individuals and singletons. The unexpected finding of a difference in mean symptom scores between twins and siblings in our study reinforces this potential design-related caveat. Together these study characteristics emphasise the usual maxim of the importance of replication of results with alternative study designs.
A third limitation concerns the accuracy of the estimates of heritability and environmental effects derived from the genetic modelling procedures. Most notably the various genetic models tested in the current study did not incorporate effects associated with gene–environment correlation and interaction, which have been demonstrated to be important to depression symptoms in other studies and in the current sample (details available from author on request). Excluding these effects may inflate genetic and non-shared environmental parameters at the expense of shared environmental effects. A further drawback associated with the use of questionnaire measures to assign zygosity was that any incorrect classification might affect the size of monozygotic relative to dizygotic twin correlations, upon which estimates of heritability are based.
Notwithstanding these shortcomings, this twin and sibling study suggests changes in both genetic and environmental factors across development. Whether these explain the marked rise in the rates of depressive symptoms during adolescence, mediated through biological and social challenges, remains to be fully clarified.
Acknowledgements
This study was supported by the W.T. Grant Foundation, the University of London Central Research Fund and Medical Research Council Training Fellowship and Career Development Award to T.C.E.







eLetters
No eLetters have been published for this article.