The pathophysiology of the striatum is one of the key elements in our understanding of schizophrenia, particularly of psychotic states. Reference Howes, Egerton, Allan, McGuire, Stokes and Kapur1,Reference Howes and Kapur2 Increased striatal dopamine has been suggested as a ‘final common pathway to psychosis’. Reference Howes and Kapur2 This idea is supported by findings of increased striatal dopamine transmission during prodromal and psychotic states. Reference Howes, Montgomery, Asselin, Murray, Valli and Tabraham3,Reference Kegeles, Abi-Dargham, Frankle, Gil, Cooper and Slifstein4 Levels of hyperdopaminergia correlate with psychosis severity and antidopaminergic drugs reduce psychotic symptoms in most cases. Reference Howes and Kapur2,Reference Agid, Mamo, Ginovart, Vitcu, Wilson and Zipursky5 Recent in-vivo positron emission tomography studies indicate that particularly the presynaptic dopamine concentration is increased, mainly in the dorsal striatum. Reference Kegeles, Abi-Dargham, Frankle, Gil, Cooper and Slifstein4 This regional specificity is further supported by resting-state functional magnetic resonance imaging (rs-fMRI) results, demonstrating that functional connectivity of ongoing activity within the striatum is selectively increased in the putamen. Such intra-striatal functional connectivity changes have been shown to be only present during psychosis and associated with the severity of positive symptoms, whereas functional connectivity changes within the ventral striatum were only found during psychotic remission and linked with negative symptoms. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 Because of striatum's involvement in cortico-basal-ganglia-thalamocortical loops, the question arises whether, beyond specifically aberrant intra-striatal connectivity, extra-striatal functional connectivity with cortical regions is also specifically changed, in particular for the putamen.
First, patterns of cortical extra-striatal functional connectivity are distinct for dorsal and ventral striatum. Reference Di Martino, Scheres, Margulies, Kelly, Uddin and Shehzad7 While ventral striatum functional connectivity includes the ventromedial prefrontal cortex and orbitofrontal cortex, the putamen is mainly linked with the anterior insula, anterior cingulate cortex and medial/lateral prefrontal cortex. Reference Di Martino, Scheres, Margulies, Kelly, Uddin and Shehzad7,Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna8 Previous imaging studies demonstrated changes in extra-striatal connectivity in patients with schizophrenia. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 For example, during cognitive tasks such as attentional oddball, decreased frontostriatal functional connectivity was found in patients, with progressive decreases being associated with disorder severity and task duration. Reference Morey, Inan, Mitchell, Perkins, Lieberman and Belger9 Resting-state studies of ongoing brain activity revealed aberrant functional connectivity from various frontal regions including the anterior cingulate cortex, dorsolateral prefrontal cortex and orbitofrontal cortex with the striatum in prodromal state and patients with psychosis. Reference Salvador, Martinez, Pomarol-Clotet, Sarro, Suckling and Bullmore10–Reference Tu, Lee, Chen, Li and Su13 Most recently, altered striatal functional connectivity with cortical regions has been observed from increased to decreased connectivity along a ventral–dorsal axis within the striatum in patients with first-episode psychosis and their relatives; this result indicates complex and disease risk-related reorganisation of extra-striatal functional connectivity across striatal subregions in schizophrenia. Reference Fornito, Harrison, Goodby, Dean, Ooi and Nathan14 Particularly across different striatal subsystems, this complex pattern suggests less distinctiveness of functional connectivity for different subregions especially in psychosis. More specifically, accounting for putamen's prominent role in psychosis and its intra-striatal reorganisation, we hypothesised that – relative to the ventral striatum – putamen functional connectivity with the cortex might be increased for regions that are normally more strongly connected with the ventral striatum.
Second, from the perspective of intrinsic networks (i.e. consistent spatial patterns of coherent ongoing brain activity), the putamen is intimately associated with the salience network. Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna8 The salience network covers insula, anterior cingulate cortex and parts of the dorsomedial and dorsolateral prefrontal cortex. It processes emotionally salient stimuli from the body and external world and controls, particularly via its right anterior insula, interactions between other networks such as default mode or central executive network. Reference Menon and Uddin15 Recently, aberrant salience network connectivity and control function was observed in patients in a psychotic episode and associated with hallucinations with aberrations converging on the right anterior insula. Reference Manoliu, Riedl, Zherdin, Muhlau, Schwerthoffer and Scherr16,Reference Palaniyappan and Liddle17 These findings were specific for psychosis, since during psychotic remission left rather than right anterior insula connectivity was relevant for aberrant network interactions and patients' negative symptoms. Reference Manoliu, Riedl, Zherdin, Muhlau, Schwerthoffer and Scherr16 Together with intra-striatal changes, these data suggest that selective changes of the putamen and right anterior insula in psychosis might be related. Based on these findings, we hypothesised that putamen's extra-striatal functional connectivity with the right anterior insula is specifically altered in patients in a psychotic episode and less distinct relative to the ventral striatum.
To test these hypotheses, we measured blood oxygenation level-dependent (BOLD) activity in 42 healthy controls and 21 patients with schizophrenia in a psychotic episode by use of rs-fMRI. Imaging data of half of the healthy volunteers were used as independent regional priors for subsequent group comparisons between patients and the other half of the controls, to increase specificity of findings. Main outcome measures were individual β-maps of seed-based functional connectivity applied to putamen and ventral striatum. To estimate group differences, β-maps were compared across groups via voxelwise two-sample t-tests. To investigate the relationship between psychotic symptoms and aberrant extra-striatal functional connectivity in patients, correlation analysis was performed. To analyse distinctiveness of extra-striatal functional connectivity for putamen and ventral striatum, we defined distinct functional connectivity by averaged incongruent seed-target-functional connectivity values (i.e. averaged functional connectivity values of functional connectivity seed in the putamen and functional connectivity target as defined by the functional connectivity map of the ventral striatum and vice versa Reference Etkin, Prater, Schatzberg, Menon and Greicius18 ).
Method
Participants
In total, 42 healthy controls (control group) and 21 patients with schizophrenia in a psychotic episode (schizophrenia group) participated in the study. All patients and 21 healthy controls had been investigated in a previous study mentioned above, Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 which investigated intra-striatal functional connectivity changes. For the current study of extra-striatal functional connectivity, an additional 21 group-matched healthy controls were recruited as an independent control group to improve extra-striatal functional connectivity analysis sensitivity and specificity. Written informed consent in accordance with the Human Research Committee guidelines of the Klinikum Rechts der Isar, Technische Universität München was obtained from all participants. Patients were recruited from the Department of Psychiatry, Klinikum Rechts der Isar TU München and controls by word-of-mouth advertising. Participants' examination included medical history, psychiatric interview, psychometric assessment, urine drug screening and additionally blood tests for the schizophrenia group. The global level of social, occupational, and psychological functioning was measured with the Global Assessment of Functioning Scale (GAF). Reference Spitzer, Williams, Gibbon and First19 Psychiatric diagnoses relied on the DSM-IV. 20 To assess psychiatric diagnoses, the Structured Clinical Interview for DSM-IV (SCID-I) was used. Reference Spitzer, Williams, Gibbon and First19 For rating severity of clinical symptoms on the day of scanning, the Positive and Negative Syndrome Scale (PANSS) was applied. Reference Kay, Fiszbein and Opler21 Clinical psychometric assessment was completed by psychiatrists (D.S. and M.S.) who have been professionally trained for SCID and PANSS-based interviews with interrater reliability of more than 95%.
Inclusion criteria for the study were diagnosis of schizophrenia, acute psychosis, particularly during the fMRI session (at least three positive PANSS subscores ⩾3), and age between 18 and 60 years. Exclusion criteria were current or past neurological or internal systemic disorder, current depressive or manic episode, substance misuse (except for nicotine) and cerebral pathology on MRI.
All the schizophrenia group were diagnosed with paranoid schizophrenia during acute psychosis as indicated by clinical exacerbation and increased positive symptom scores on the PANSS (Table 1). In total, 7 out of 21 had significant hallucinations (PANSS P3 ⩾3), 15 delusions (PANSS P1 ⩾3). The mean duration of illness was 7.15 years (s.d. = 6.89), the mean number of hospital admissions was 2.98 (s.d. = 2.48). Concerning medication, three patients were free of any antipsychotic medication. All other patients received mono- or dual therapy with atypical antipsychotic medication (see Table 1 and online Table DS1). All patients have been treated previously with antipsychotic drugs (i.e. none of the patients was treatment naive). The control group were all free of any current or past psychiatric, neurological or systemic disorder or psychotropic medication.
Table 1 Demographic and clinical characteristics

| Measure | Independent control group (n = 21) |
Comparison control group (n = 21) |
Schizophrenia group (n = 21) |
Schizophrenia group (n = 21) v.
comparison control group (n = 21) a |
|
|---|---|---|---|---|---|
| Age, mean (s.d.) | 33.49 (12.9) | 33.57 (13.6) | 34.05 (12.27) | −0.121 | 0.904 |
| Gender (men/women), n | 10/11 | 10/11 | 10/11 | ||
| Positive and Negative Syndrome Scale, mean (s.d.) | |||||
| Total | – | 30.14 (0.65) | 80.76 (20.77) | 8.96 | <0.001* |
| Positive | – | 7.05 (0.22) | 19.4 (6.09) | 9.091 | <0.001* |
| Negative | – | 7.10 (0.44) | 21.14 (8.20) | 7.84 | <0.001* |
| General | – | 16.05 (0.22) | 39.81 (11.06) | 9.846 | <0.001* |
| Global Assessment of Functioning Scale, mean (s.d.) | – | 99.76 (1.09) | 39.62 (11.68) | −23.492 | <0.001* |
| Chlorpromazine-equivalent dose, mean (s.d.) | – | 388.61 (384.67) | |||
a. Two-sample t-test
* Significant at P<0.05 corrected for multiple comparisons.
Behavioural and imaging data from the schizophrenia group and 21 controls (the ‘comparison control group’) were used in a previous study, Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 which focused on intra-striatal functional connectivity. An additional 21 group-matched healthy controls were recruited as an independent control group to improve functional connectivity analysis sensitivity and specificity (see below).
MRI data acquisition
MRI was carried out using a 3T whole-body MR scanner (Achieva, Philips, The Netherlands) using an eight-channel phased-array head coil. T 1-weighted anatomical data were obtained by a magnetisation-prepared rapid acquisition gradient echo sequence (echo time (TE) = 4 ms, repetition time (TR) = 9 ms, inversion time (TI) = 100 ms, flip angle 5°, field of view (FoV) = 240 × 240 mm, matrix 240 × 240, 170 slices, voxel size 1 × 1 × 1 mm). Data from rs-fMRI were obtained by a gradient-echo echo-planar imaging (EPI) sequence (TE = 35 ms, TR = 2000 ms, flip angle 82°, FoV = 220 × 220 mm, matrix 80 × 80, 32 slices, slice thickness 4 mm, and 0 mm interslice gap). All participants underwent 10 min of rs-fMRI resulting in 300 volumes. As in most previous rs-fMRI studies (e.g. Di Martino et al, Reference Di Martino, Scheres, Margulies, Kelly, Uddin and Shehzad7 Seeley et al Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna8 ), we instructed participants to keep their eyes closed and not to fall asleep. We verified that participants stayed awake by interrogating via intercom immediately after the rs-fMRI scan.
MRI data analysis
Preprocessing
For each participant, the first three rs-fMRI scans were discarded because of magnetisation effects. SPM8 (Wellcome Department of Cognitive Neurology, London) was used for motion correction, spatial normalisation into the stereotactic space of the Montreal Neurological Institute (MNI) and spatial smoothing with an 8 × 8 × 8 mm Gaussian kernel. To ensure data quality, particularly concerning motion-induced artefacts, temporal signal-to-noise ratio (tSNR) and point-to-point head motion were estimated for each participant. Reference Murphy, Bodurka and Bandettini22,Reference Van Dijk, Sabuncu and Buckner23 Point-to-point motion was defined as the absolute displacement of each brain volume compared with its previous volume. Moreover, root mean square (RMS) of the translational head movement parameters was calculated for each participant. Reference Van Dijk, Sabuncu and Buckner23 Excessive head motion (cumulative motion translation >3 mm and mean point-to-point translation or rotation >0.15 mm or 0.1) was applied as exclusion criterion. None of the participants had to be excluded. Two-sample t-tests yielded no significant differences between groups regarding mean point-to-point translation or rotation of any direction (P>0.15), RMS (P>0.2), or tSNR (P>0.40). Further control for head motion effects was carried out in the individual-level functional connectivity analysis.
Individual-level functional connectivity analysis
Seeds of functional connectivity analysis were selected according to coordinates of group-different regions derived from our first study in patients during acute psychosis (putamen) and after remission (ventral striatum) relative to healthy controls. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 We created spherical regions of interest (ROIs, 6 mm radius) for the dorsal striatum, i.e. left and right putamen (+/−24, 12, 0) and the ventral striatum, i.e. nucleus accumbens (+/−12, 9, −9), respectively, by the use of MarsBaR (v0.42, http://marsbar.sourceforge.net/). Centres of ROIs were derived from the study of Martinez and colleagues and corresponded to centres in our previous study. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6,Reference Martinez, Slifstein, Broft, Mawlawi, Hwang and Huang24
After Butterworth bandpass-filtering of all voxel time courses for the frequency range from 0.009 to 0.08 Hz, we extracted voxel time courses of seed ROIs and reduced them to ROI-representative time courses by singular value decomposition. Each time course was entered into a first-level fixed-effects general linear model in SPM8, and four separate functional connectivity analyses (i.e. left/right ventral striatum, left/right putamen) were performed for each participant yielding four functional connectivity maps for subsequent second-level analyses. Regressors for global grey matter, white matter, cerebrospinal fluid (CSF) BOLD-signal, and six movement parameters for each participant were included as covariates of no interest in each model. Reference Di Martino, Scheres, Margulies, Kelly, Uddin and Shehzad7 As the global grey matter signal is thought to reflect a combination of physiological processes (such as cardiac and respiratory fluctuations) and scanner drift, it was included as a nuisance signal to minimise the influence of such factors. Reference Birn, Murphy and Bandettini25 To extract the nuisance covariate time series for grey matter, white matter and CSF, each individual's high-resolution T 1-weighted structural image was segmented. Mean images of study sample's T 1-segmentation were used to create ROIs for the extraction of grey matter, white matter and CSF nuisance signals.
Group-level functional connectivity analysis
Group analyses were performed using β-maps from individual-level functional connectivity analysis in separate flexible factorial models of analysis of variance (ANOVA). More specifically, all ANOVA models included covariates of no interest (gender, age, seed regional volumes of ventral striatum and putamen, respectively; see voxel-based morphometry (VBM) analysis below) and were restricted to explicit masks of ventral striatum and putamen functional connectivity, respectively. Masks were created by use of a flexible factorial ANOVA model of putamen/ventral striatum functional connectivity images of the independent control group (factors: hemisphere with levels left/right and seed-ROI with levels ventral striatum/putamen); appropriate post-hoc t-tests revealed positively correlated functional connectivity maps for the ventral striatum and putamen, respectively (P<0.05 uncorrected for masks; P<0.05 family-wise error (FWE)-corrected on voxel level for online Fig. DS1). Then, to analyse group differences of putamen functional connectivity, a flexible factorial ANOVA (P<0.05 FWE-corrected on voxel level and restricted to a mask of the independent control group) was applied to putamen functional connectivity images of currently symptomatic patients and the comparison control group with factors group (levels: patient/control) and seed-ROI (levels: left/right putamen). Analogous ANOVA was applied for ventral striatum functional connectivity. For both ANOVA models, the main effect of group (and corresponding post-hoc t-tests to reveal direction of change) was the effect of interest. Reported voxel coordinates correspond to standardised MNI space. To visualise results, we used MRIcroN (http://www.nitrc.org/projects/mricron).
Brain–behaviour relationship
To investigate the relationship between striatal functional connectivity differences and psychotic symptoms, β-values of regional group differences in putamen and ventral striatum functional connectivity for each cluster and patient were averaged across voxels and entered into partial correlation analyses. According to previous results, we chose subscores instead of the summed positive symptom score. Since only hallucinations and delusions correlated with striatal functional connectivity decreases, we limited analyses to these subscores. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 Covariates of no interest were age and gender as well as medication levels reflected by chlorpromazine-equivalent units (CPZ). Reference Woods26 The significance threshold was set to P<0.007, Bonferroni-corrected for seven group-different clusters of functional connectivity decreases (the seven clusters are listed in Table 2).
Table 2 Decreased functional connectivity in the schizophrenia group: schizophrenia group < control group

| Seed | Anatomical regions | Side | Cluster size in voxels, k |
MNI, peak voxel coordinates, x, y, z |
z-score | P a |
|---|---|---|---|---|---|---|
| Putamen | Inferior frontal gyrus (opercular, orbital) Anterior insula |
Right | 236 | 45, 15, −6 | 5.21 | <0.0001 |
| Superior frontal gyrus | Right | 87 | 51, 36, 6 | 4.57 | 0.027 | |
| Inferior frontal gyrus (triangular) Middle frontal gyrus |
Right | 61 | 12, 12, 48 | 5.50 | <0.0001 | |
| Middle frontal gyrus | Right | 48 | 36, 48, 30 | 4.16 | 0.044 | |
| Middle frontal gyrus | Left | 36 | −36, 48, 27 | 4.27 | 0.029 | |
| Middle cingulate cortex | Right | 20 | 9, 15, 39 | 4.33 | 0.023 | |
| Ventral striatum |
Anterior insula Inferior frontal gyrus (orbital) |
Left | 26 | −36, 21, −9 | 4.37 | 0.011 |
MNI, Montreal Neurological Institute.
a. Two-sample t-test, P<0.05 familywise error -corrected for multiple comparisons on voxel level.
Distinct functional connectivity
We expected that in the schizophrenia group, putamen's functional connectivity is changed both with the anterior insula and with areas that are typically connected more strongly with the ventral striatum. To examine such altered distinctiveness of putamen and ventral striatum connectivity, we defined distinct functional connectivity in terms of averaged incongruent seed-target functional connectivity values of striatal seeds. For example, the connectivity of the putamen with regions that were normally coupled more strongly with the ventral striatum than the putamen is referred to as incongruent seed-target connectivity of the putamen. By the use of appropriate post-hoc t-tests for the above-mentioned ANOVA model (factors seed (putamen/ventral striatum) and side (left/right) of the independent control group) (P<0.05 FWE-corrected), we first obtained distinct functional connectivity maps of the putamen (main effect seed putamen> ventral striatum) and ventral striatum (main-effect seed ventral striatum>putamen). Reference Etkin, Prater, Schatzberg, Menon and Greicius18 We used these distinct functional connectivity maps as masks to calculate averaged incongruent seed-target functional connectivity values (for example, for a given participant and the putamen as seed, β-values of putamen functional connectivity map were averaged across all voxels of the ventral striatum-distinct functional connectivity mask). Incongruent seed-target-functional connectivity values reflect distinctiveness of striatal functional connectivity and were compared across the schizophrenia and comparison control group by use of two-sample t-tests (P<0.05). Accordingly, increased incongruent seed-target connectivity for a striatal seed would be interpreted as reduced distinctiveness of striatal functional connectivity because of an enlarged functional connectivity for this seed.
Voxel-based morphometry
To control for effects of striatal structure on functional connectivity results, we included ventral striatum and putamen volumes as covariates of no interest into statistical models of group comparisons. We used previous results of VBM analysis in the schizophrenia group and the comparison control group as described in the online supplement DS1 and elsewhere. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 Briefly, VBM volumes of patients' left and right putamen and ventral striatum were not different from those of the comparison control group.
Results
In the independent control group, significant functional connectivity was found for the putamen with bilateral inferior, middle, and superior frontal gyrus, anterior insula, anterior and middle cingulate cortex, pallidum and caudate nucleus, for the ventral striatum with orbital parts of the inferior frontal gyrus, the medial superior frontal gyrus, anterior insula, anterior cingulate cortex and pallidum (P<0.05 FWE-corrected, online Fig. DS1, Table DS2). Putamen and ventral striatum functional connectivity maps of the schizophrenia group and the comparison control group were largely consistent with these patterns, indicating that the basic pattern of putamen and ventral striatum functional connectivity is preserved in patients (online Fig. DS1).
Group comparisons, which were masked by functional connectivity patterns of the independent control group, revealed that in the schizophrenia group putamen functional connectivity was decreased for bilateral middle frontal gyrus and superior frontal gyrus, the right opercular and triangular part of the inferior frontal gyrus, right anterior insula and right middle cingulate cortex (P<0.05 FWE-corrected, online Fig. DS2, Table 2). The schizophrenia group's ventral striatum functional connectivity was decreased with the left anterior insula, extending to the orbital part of the left inferior frontal gyrus (P<0.05 FWE-corrected, online Fig. DS2, Table 2).
The partial correlation analyses for the patients' decreased regional functional connectivity values and psychotic symptoms (P<0.007, Bonferroni-corrected for multiple testing because of seven group-different clusters) showed the decreased functional connectivity between putamen and right anterior insula to be negatively correlated with hallucinations (P<0.001, Fig. 1). Neither delusions nor other regions' group-different functional connectivity showed significant results.
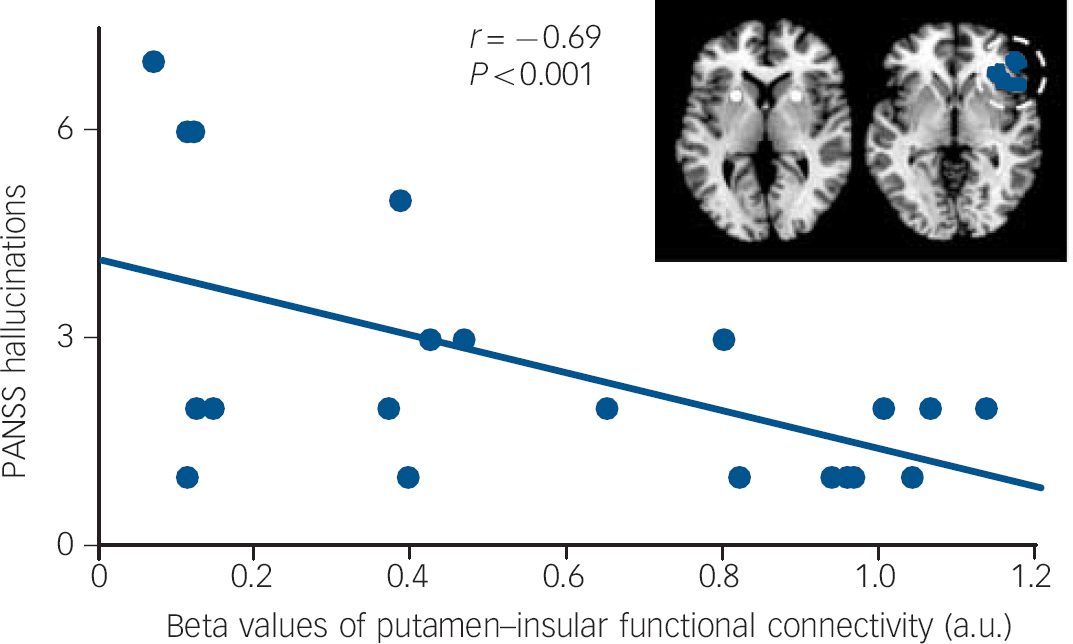
Fig. 1 Negative correlation between hallucinations and functional connectivity between putamen and right anterior insula.
Partial correlation analyses on averaged beta values of functional connectivity group-different clusters in online Fig. DS2 with hallucinations and delusion score on the Positive and Negative Syndrome Scale (PANSS) revealed only for the functional connectivity between putamen and right anterior insula a significant relationship with hallucinations (partial correlation coefficient r = −0,69, P<0.001, Bonferroni-corrected for multiple testing (i.e. number of group-different clusters). To control for confounding effects, medication levels (chlorpromazine-equivalent dose units), age and gender were included in partial correlation models. a.u., arbitrary units.
To investigate distinctiveness of striatal functional connectivity for putamen and ventral striatum, averaged incongruent seed-target functional connectivity was calculated for putamen and ventral striatum for each participant. In the schizophrenia group only, the putamen showed significantly increased incongruent seed-target functional connectivity values relative to ventral striatum and different to those of the control group (one- and two-sample t-test, P<0.05, Fig. 2).

Fig. 2 Distinctiveness of functional connectivity (FC) of putamen (PU) and ventral striatum (VS).
(a) White and blue maps represent binary spatial connectivity maps of the independent control group based on post-hoc t-tests (putamen<ventral striatum, putamen>ventral striatum) of ANOVA with factors seed (putamen, ventral striatum) and seed side (left, right), PFWE<0.05 corrected for family-wise error (FWE)). The idea of distinct functional connectivity is based on averaged incongruent seed-target functional connectivity for example averaged functional connectivity of the putamen in white with the regular target of the ventral striatum in blue. (b) Mean beta values for distinct functional connectivity for the schizophrenia and the control group are presented. **Indicates significant group differences in distinct functional connectivity (two-sample t-tests P<0.05). a.u., arbitrary units.
Discussion
Main findings
Extra-striatal cortical functional connectivity of the putamen and ventral striatum was studied in patients with schizophrenia during psychosis and in healthy controls using rs-fMRI and seed-based functional connectivity analysis. In patients, putamen's functional connectivity was reduced with the right anterior insula and the dorsomedial and dorsolateral prefrontal cortex, whereas ventral striatum's functional connectivity was decreased with the left anterior insula. Only putamen's aberrant functional connectivity with the right anterior insula was significantly associated with patients' hallucinations. Putamen's functional connectivity in patients was increased with areas regularly connected with the ventral striatum, indicating specifically less distinctive functional connectivity of the putamen relative to the ventral striatum. Data provide evidence that aberrant extra-striatal cortical functional connectivity during psychosis is centred on the putamen, with both less distinct connectivity focused on the putamen and decreased connectivity between putamen and right anterior insula specifically related to psychotic symptoms. Particularly the last finding suggests aberrant functional connectivity to link striatal and insular pathophysiology in psychosis.
Comparison with findings from other studies
In the schizophrenia group, we found reduced functional connectivity for both putamen and ventral striatum with cortical regions (online Fig. DS2, Table 2). Putamen functional connectivity was reduced in right anterior insula and dorsomedial and dorsolateral prefrontal cortex, whereas ventral striatum functional connectivity was decreased with the left anterior insula. Group differences were independent of age, gender and striatal volume effects, for which we controlled statistically. Spatial putamen and ventral striatum functional connectivity maps of those in the schizophrenia group were largely comparable with those of the control group, indicating that basic extra-striatal functional connectivity is preserved in schizophrenia (online Fig. DS1). Reduced functional connectivity between putamen and dorsal prefrontal cortex is in line with previous findings. Reference Zhou, Liang, Tian, Wang, Hao and Liu11–Reference Fornito, Harrison, Goodby, Dean, Ooi and Nathan14 For example, Zhou and colleagues Reference Zhou, Liang, Tian, Wang, Hao and Liu11,Reference Zhou, Liang, Jiang, Tian, Liu and Liu12 found reduced middle frontal gyrus functional connectivity with the dorsal striatum and Fornito and colleagues Reference Fornito, Harrison, Goodby, Dean, Ooi and Nathan14 recently observed reduced functional connectivity between the dorsocaudal putamen and dorsal prefrontal cortex in patients with first-episode psychosis, with striatal seed coordinates very near to those of our study. Furthermore, for the same prefrontal cortex areas, authors reported that putamen functional connectivity was also reduced in unaffected first-degree relatives of patients with schizophrenia, suggesting that these connectivity changes may express a specific disease risk more associated with disease trait than state-dependent symptoms. Our finding that reduced functional connectivity between putamen and dorsal prefrontal cortex is not related to psychotic symptoms, supports this suggestion.
On the other hand, we found reduced functional connectivity with right and left anterior insula in patients for both putamen and ventral striatum (online Fig. DS2). This finding is in line with previous findings of reduced striatoinsular functional connectivity in patients in prodromal psychotic states and psychosis. Reference Fornito, Harrison, Goodby, Dean, Ooi and Nathan14,Reference Dandash, Fornito, Lee, Keefe, Chee and Adcock27 More specifically, Orliac and colleagues recently reported reduced striatal functional connectivity within the salience network centred on anterior insula in patients with schizophrenia. Reference Orliac, Naveau, Joliot, Delcroix, Razafimandimby and Brazo28 It should be noted, however, that in the study by Orliac et al, the salience network included the bilateral anterior insula whereas we identified reduced connectivity between the putamen and the right anterior insula in association with reduced connectivity between the ventral striatum and the left anterior insula. The salience network and especially the anterior insula is crucially involved in processing salient internal/external stimuli and controlling interactions between two distinct core cognitive networks, namely the default mode network – involved in self-referential cognition – and the central executive network – involved in goal-driven tasks. Reference Seeley, Menon, Schatzberg, Keller, Glover and Kenna8 In patients with schizophrenia, the anterior insula is characterised by various structural and functional alterations such as atrophy, impaired white matter structure and aberrant functional connectivity. In particular aberrant functional connectivity of the anterior insula within the salience network is associated with aberrant default-mode network/central executive network interactions and the severity of patients' symptoms. Reference Manoliu, Riedl, Zherdin, Muhlau, Schwerthoffer and Scherr16,Reference Manoliu, Meng, Brandl, Doll, Tahmasian and Scherr29 Our finding of reduced functional connectivity between striatum and anterior insula indicates that a disrupted connectivity between striatum and central components of the salience network may constitute a core mechanism underlying the pathophysiology of schizophrenia.
Concerning laterality of aberrant striatal functional connectivity, patients' functional connectivity was reduced for the putamen with the right anterior insula and for the ventral striatum with left anterior insula. Previously we found that only right anterior insula functional connectivity within the salience network was associated with positive symptoms during psychosis, Reference Palaniyappan and Liddle17 whereas during remission, left anterior insula connectivity was specifically linked with negative symptoms. Reference Manoliu, Meng, Brandl, Doll, Tahmasian and Scherr29 Correspondingly, for the striatum we found that during psychosis, only putamen intra-striatal functional connectivity was increased and linked with positive symptoms, whereas during remission, only ventral striatum functional connectivity was increased and linked with negative symptoms. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 Together, it seems that putamen and right anterior insula connectivity is related more with psychosis and positive symptoms, whereas ventral striatum and left anterior insula is related more with remission and negative symptoms. Since the control of vegetative nervous system activity is asymmetrically represented in the anterior insula with sympathetic parts more related to the right anterior insula whereas parasympathetic parts relate more with the left anterior insula, Reference Craig30 one might speculate whether lateralised striatal functional connectivity changes in putamen and ventral striatum in psychosis reflect this rather basic asymmetry. Future studies are necessary to investigate such a potential link.
Psychosis and putamen functional connectivity
With respect to psychosis, two additional findings specify decreased extra-striatal functional connectivity in schizophrenia: (a) aberrant functional connectivity seems to be centred on the putamen relative to the ventral striatum and (b) putamen's decreased functional connectivity with right anterior insula is specifically relevant for psychotic symptoms.
Abnormal distinctiveness of dorsal striatum functional connectivity
We found less distinct functional connectivity of the striatum in the form of increased putamen functional connectivity with regions regularly connected with the ventral striatum (Fig. 2). This finding suggests that in patients, areas which are preferentially linked with the ventral striatum, show on average increased functional connectivity with the putamen. This finding is focused on the putamen, as distinct functional connectivity of the ventral striatum relative to the putamen was normal in patients (Fig. 2). This spatial focus on the dorsal striatum in psychosis corresponds well with previous findings concerning other aspects of striatal pathophysiology. For example, intra-striatal functional connectivity is selectively increased in the putamen during psychosis, and presynaptic dopamine activity is increased during prodromal and psychotic states, especially in the dorsal striatum. Reference Howes, Egerton, Allan, McGuire, Stokes and Kapur1,Reference Howes, Montgomery, Asselin, Murray, Valli and Tabraham3,Reference Kegeles, Abi-Dargham, Frankle, Gil, Cooper and Slifstein4,Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 Since such relative dominance of the dorsal striatum is characteristic for habit-like behaviour, some theoretical accounts suggested that patients' pronounced changes in the dorsal striatum might point at a habit-like nature of psychotic symptoms. Reference Fletcher and Frith31,Reference Corlett, Taylor, Wang, Fletcher and Krystal32
Aberrant putamen functional connectivity and severity of hallucinations
In line with this relative dominance of dorsal striatum changes in psychosis, we found explicit evidence that only patients' decreased putamen functional connectivity with the right anterior insula is significantly linked with the degree of hallucinations (Fig. 1). Fornito and colleagues Reference Fornito, Harrison, Goodby, Dean, Ooi and Nathan14 argue that the previously reported relationship between reduced dorsal striatum functional connectivity and symptom severity may constitute a state-independent risk marker. However, they mainly discussed functional connectivity with dorsolateral and medial prefrontal cortex and did not consider PANSS subscores. Our finding fit with previous results very well: first, aberrant right anterior insula functional connectivity within the salience network is specifically associated with psychosis, particularly with hallucinations; second, putamen's intra-striatal functional connectivity is selectively associated with psychosis and the degree of psychotic symptoms. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6 The current result provides a link between these previous findings by extending isolated findings centred on either anterior insula or the dorsal striatum and showing that disrupted functional connectivity between putamen and right anterior insula is specifically related to psychotic key symptoms.
Link between proximal salience, motivational salience and dopamine
More generally, these data suggest a link between two relevant models of psychosis in schizophrenia, namely the proximal salience model of Palaniyappan Reference Palaniyappan and Liddle17,Reference Palaniyappan, White and Liddle33 and the motivational salience model of Kapur. Reference Kapur34,Reference Kapur, Mizrahi and Li35 Although these two concepts integrate a lot of data that are critical for explaining psychotic symptoms in schizophrenia, they emphasise distinct neurocognitive mechanisms centred on distinct key regions, namely the anterior insula and the striatum. More specifically, the concept of proximal salience describes a momentary interoceptive state resulting from the appraisal of external and internal stimuli, which modulates both succeeding learning processes and the selection of actions/cognitions to improve future evaluation. Reference Palaniyappan and Liddle17 In contrast, the concept of motivational salience describes the assignment of a specific motivational value to an internal or external stimulus following its appraisal based on reward prediction error processes. Reference Kapur34,Reference Kapur, Mizrahi and Li35 In a neurobiological context, proximal salience has been proposed to be mediated by the anterior insula within the salience network, particularly via modulation of the interaction between other intrinsic connectivity networks, such as the default mode network and central executive network, whereas motivational salience depends highly on striatal activity, which, in turn, is strongly controlled by striatal dopaminergic activity. Reference Fletcher and Frith31,Reference Murray, Corlett, Clark, Pessiglione, Blackwell and Honey36 Latest studies provide strong evidence that psychosis in schizophrenia is characterised by altered functional connectivity within the dorsal striatum as well as in the right anterior insula within the salience network and by aberrant functional connectivity between intrinsic brain networks. Reference Manoliu, Riedl, Zherdin, Muhlau, Schwerthoffer and Scherr16,Reference Palaniyappan, Simmonite, White, Liddle and Liddle37 However, a direct link between these findings is still missing. Recently, Cole and colleagues Reference Cole, Oei, Soeter, Both, van Gerven and Rombouts38 demonstrated that striatoinsular functional connectivity is influenced by pharmacological modulation of striatal dopamine levels. Moreover, Lui et al Reference Lui, Li, Deng, Jiang, Wu and Tang39 reported increased functional connectivity between the dorsal striatum and the bilateral prefrontal cortex, the parietal cortex and the left superior temporal cortex after short-term treatment with second-generation antipsychotic medication in patients with first-episode schizophrenia. Of note, the increase in functional connectivity was associated with a reduction of clinical symptoms implicating that a pharmacologically induced alteration in functional connectivity leads to significant clinical improvement.
Future studies are required that focus explicitly on the linking potential of striatoinsular intrinsic connectivity between dopaminergic reward prediction error activity in the dorsal striatum, right anterior insula control function on interacting intrinsic networks and psychotic symptoms.
Methodological issues and limitations
The fact that almost all of the schizophrenia group in our study were medicated may limit the explanatory power of our findings. An influence of antipsychotic medication on functional brain connectivity has been shown. Reference Lui, Li, Deng, Jiang, Wu and Tang39,Reference Sambataro, Blasi, Fazio, Caforio, Taurisano and Romano40 However, a previous comparison between patient subsamples receiving antipsychotics and those who were medication-free did not yield a significant difference in striatal functional connectivity. Reference Fornito, Harrison, Goodby, Dean, Ooi and Nathan14 To control for the potential confound of medication, we included – with respect to the analysis of the relationship between aberrant functional connectivity and psychotic symptoms – CPZ units in the statistical model of patients' functional connectivity, which did not yield a significant explanatory effect of functional connectivity in the present study. Reference Sorg, Manoliu, Neufang, Myers, Peters and Schwerthoffer6,Reference Manoliu, Riedl, Zherdin, Muhlau, Schwerthoffer and Scherr16 Nevertheless, antipsychotic medication has to be considered when evaluating the current study's results. Future studies in patients who are drug-free might be helpful; however studies in patients who are drug-free but in a psychotic episode might have strong practical and ethical problems.
In conclusion, our results provide evidence that in patients with schizophrenia in a psychotic episode, striatal functional connectivity with the frontoinsular cortex is decreased. Changes were pronounced for the putamen relative to the ventral striatum and decreased connectivity between putamen and right anterior insula was specifically related to psychotic symptoms. Data suggest a link between striatal and anterior insular pathophysiology of psychosis via functional connectivity.
Funding
This work was supported by the German Federal Ministry of Education and Research ( to C.S.), the Alzheimer Forschung Initiative ( to V.R.) and the Kommission für Klinische Forschung of the Klinikum Rechts der Isar der Technischen Universität München ( to C.S). We are grateful to the participants of the study and the staff of the Department of Psychiatry and Neuroradiology for their help in recruitment and data collection.







eLetters
No eLetters have been published for this article.