Depression is a common disorder (Reference Blazer, Kessler and McGonagleBlazer et al, 1994) and recurrent depression is a major cause of disability (Reference Hays, Wells and SherbourneHays et al, 1995). Whereas personal and psychosocial risk factors for the development of depression have been elucidated (Reference KendlerKendler, 1998), the neurobiological mechanisms involved in mediating vulnerability have not been well defined. Study of conditioned responses to aversive stimuli allows investigation of the neural circuits related to emotional experiences (Reference Buchel, Morris and DolanBuchel et al, 1998; Reference LaBar, Gatenby and GoreLaBar et al, 1998; Reference Morris, Ohman and DolanMorris et al, 1998) and we previously have adapted approaches to allow dynamic monitoring of responses to thermal pain and its anticipation using functional magnetic resonance imaging (fMRI) (Reference Ploghaus, Tracey and GaitiPloghaus et al, 1999). Given the recognised potential for conditioning responses to define traits relevant to depression (Reference Eysenck, White and EynsenckEysenck et al, 1976), we have employed our fMRI conditioning paradigm to contrast responses of healthy controls with those of subjects who had a history of recurrent depression and who had made a full recovery and were off antidepressant medication in an effort to identify a potential marker of vulnerability to depression.
METHOD
Subjects
Eighteen right-handed women underwent fMRI. Ten of these had a history of recurrent major depressive disorder and had suffered at least two episodes of DSM-IV depressive disorder (American Psychiatric Association, 2001) but had been recovered and off medication for at least 6 months. The remaining eight subjects (healthy controls) had no history of psychiatric disorder and were medication-free. Psychiatric history and current state were assessed at interview using the Structured Clinical Interview for DSM-IV (Reference Spitzer, Williams and GibbonSpitzer et al, 1987). Current depressive symptomatology was assessed with the Beck Depression Inventory (BDI; Reference Beck, Ward and MendelsonBeck et al, 1961).
Behavioural procedure
A Peltier thermode was used to apply thermal stimuli to the dorsum of the left hand, as described previously (Reference Ploghaus, Tracey and GaitiPloghaus et al, 1999). Stimulus intensities were chosen with the subject in the scanner, and for each subject individually; two stimuli that were consistently described by the subjects as ‘painfully hot’ and ‘clearly warm, but not hot’ were chosen. Three coloured light-emitting diodes (LEDs) (red, green, blue) were mounted at the subjects' feet and could be viewed through a mirror in the magnet bore. During the experiment, subjects received seven noxious and seven comfortable warm stimulations in pseudorandom order. Each type of stimulation was signalled consistently by a certain colour LED for each subject, randomised across subjects (Fig. 1). The coloured LED signals preceded the onset of thermal stimulation by a pseudo-randomly varied interval with a mean of 7.5 s (s.d.=5 s) and stayed on during thermal stimulation, which was applied for 11 s. Between conditioning trials subjects had a rest period that also was pseudo-randomly varied (mean duration=26.5 s, s.d.=9 s) and signalled by the third coloured LED. Subjects were instructed to work out the contingency between LED colour and thermal stimulation.
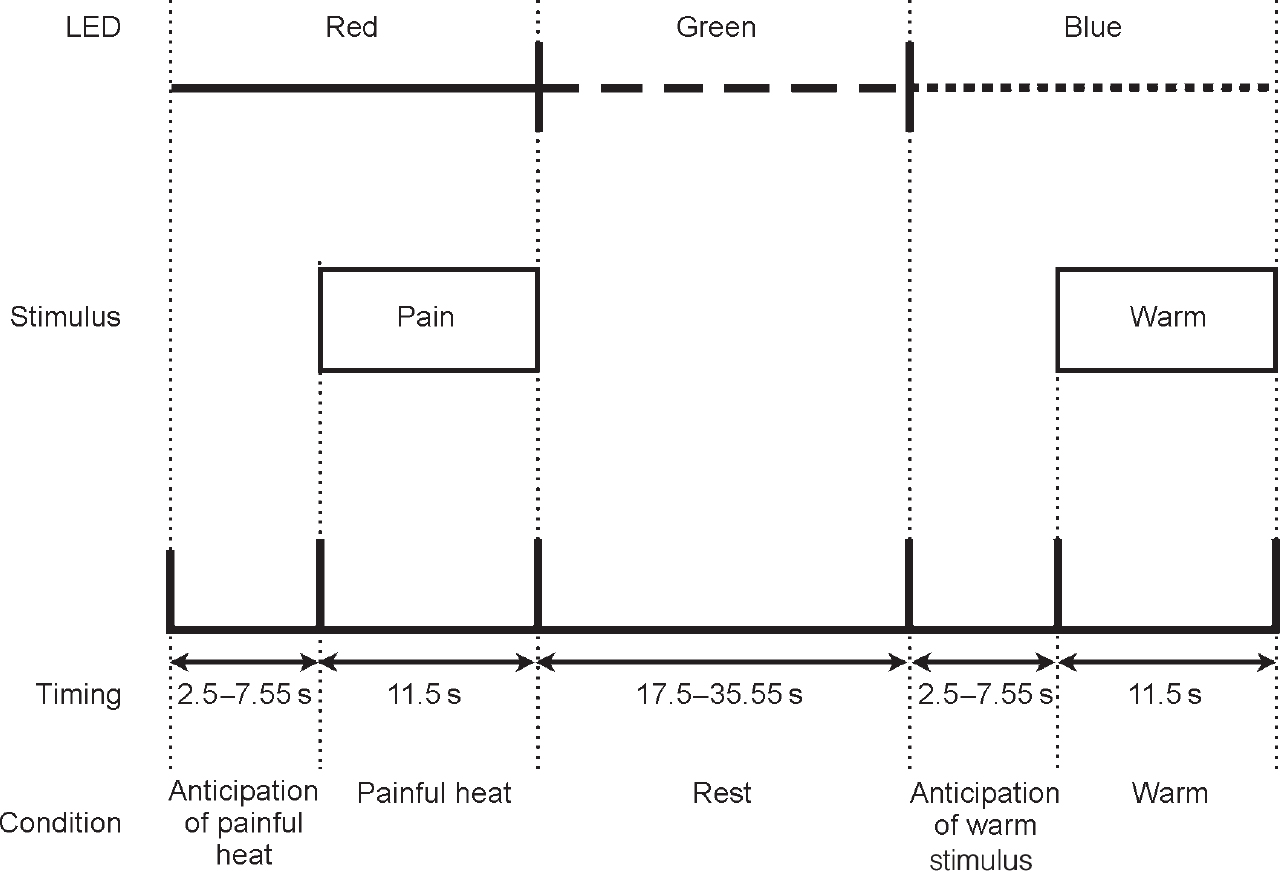
Fig. 1 Diagrammatic representation of study method (light-emitting diode (LED) colours were randomised across subjects).
Subjects rated their mood before the experiment, immediately after (in the scanner) and at the end of the scan using visual analogue rating scales. After the experiment, subjects rated the two thermal stimuli on intensity and unpleasantness using a 7-point modified form of the McGill pain scale (Reference MelzackMelzack, 1975). Neuroticism was assessed at baseline using the Eysenck Personality Questionnaire (Reference Eysenck, Eysenck and BarrettEysenck et al, 1985).
Data acquisition
We used a 3-tesla Varian INOVA magnetic resonance imaging system (Palo Alto, CA, USA) with a multislice gradient echo planar imaging (EPI) sequence (repetition time, TR=3000 ms, echo time, TE=30 ms, flip angle=90°, field of view, FOV=256 mm2, matrix=642, 21 6-mm axial slices). We also acquired a high-resolution T1-weighted anatomical scan for each subject.
Image processing and statistical analysis
Image analysis was performed within MEDx (Sensor Systems, Inc., Sterling, VA, USA). Each subject's data was first motion corrected using AIR (http://www.loni.ucla.edu/NCRR/Software/AIR.html), spatially filtered using a Gaussian kernel of full width at half maximum of 5 mm, global (volumetric) mean intensity normalised, and high-pass filtered (period=180.0 s). Statistical analysis was then carried out using FMRIB's Easy Analysis Tool (FEAT) (www.fmrib.ox.ac.uk/fsl) to localise regions of significant change. Differences in signal responses to noxious pain and comfortable warm stimulations were localised, as were differences in their preceding anticipation periods, during the conditioning experiment. The parameter estimate images for each subject were warped into the Montreal Neurological Institute 152 average brain space using FMRIB's Linear Image Registration Tool (FLIRT) and a random-effects statistic was used to generate group Z-statistic images. Cluster detection was applied to the group Z-statistic images (Z>2.3, P<0.1; Reference Poline, Worsley and EvansPoline et al, 1997).
RESULTS
Subject characteristics
There were no significant differences in age between the two groups. As expected, the subjects recovered from depression had a higher overall rating of neuroticism. BDI scores were also higher in this group, although all BDI scores were within the normal range (Table 1).
Table 1 Baseline characteristics of subjects
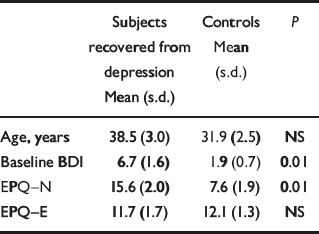
| Subjects recovered from depression Mean (s.d.) | Controls Mean (s.d.) | P | |
|---|---|---|---|
| Age, years | 38.5 (3.0) | 31.9 (2.5) | NS |
| Baseline BDI | 6.7 (1.6) | 1.9 (0.7) | 0.01 |
| EPQ—N | 15.6 (2.0) | 7.6 (1.9) | 0.01 |
| EPQ—E | 11.7 (1.7) | 12.1 (1.3) | NS |
Ratings of mood and experience of pain
There were no significant differences between groups in ratings of mood or of the intensity or affective quality of the painful and warm stimuli. There were also no differences between groups for visual analogue ratings of happy, sad and anxious (data not shown). Interviews after the experiment confirmed that all subjects learned the correct relation between the coloured light cues and the temperature of the subsequent thermal stimulation (noxious heat or warm).
Neuroimaging results
A contrast of responses during noxious heat with non-noxious warm stimulation in both groups showed activations in brain regions previously reported in neuro-imaging studies of pain (Table 2 & Fig. 2). No significant differences in activation extent, magnitude or localisation, or site of activation were found between the control group and the recovered depression subjects.

Fig. 2 Fixed-effects analysis of the response to thermal pain (painful heat v. non-noxious warm stimulus) in the healthy control subjects. The group response has been overlaid onto one subject's structural image. Activation responses are seen in areas previously identified to respond to pain: 1, secondary somatosensory cortex; 2, primary sensorimotor cortex; 3, insular cortex; 4, anterior cingulate cortex; 5, thalamus; 6, basal ganglia; 7, premotor cortex; 8, prefrontal cortex; 9, precuneus. Cerebellar activation also was seen but is not shown here.
Table 2 Brain volumes activated by noxious pain v. non-noxious warm stimulation in controls. Coordinates of centre of mass and maximum Z-score for each of the neuroanatomically defined regions are given
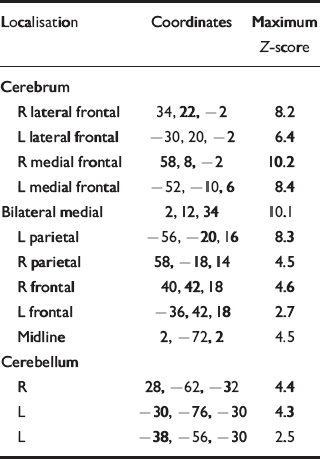
| Localisation | Coordinates | Maximum Z-score |
|---|---|---|
| Cerebrum | ||
| R lateral frontal | 34, 22, -2 | 8.2 |
| L lateral frontal | -30, 20, -2 | 6.4 |
| R medial frontal | 58, 8, -2 | 10.2 |
| L medial frontal | -52, -10, 6 | 8.4 |
| Bilateral medial | 2, 12, 34 | 10.1 |
| L parietal | -56, -20, 16 | 8.3 |
| R parietal | 58, -18, 14 | 4.5 |
| R frontal | 40, 42, 18 | 4.6 |
| L frontal | -36, 42, 18 | 2.7 |
| Midline | 2, -72, 2 | 4.5 |
| Cerebellum | ||
| R | 28, -62, -32 | 4.4 |
| L | -30, -76, -30 | 4.3 |
| L | -38, -56, -30 | 2.5 |
To investigate differences between the two groups in activation to noxious heat and non-noxious warm stimulation during the conditioning period, an analysis was performed comparing activation during anticipation of painful stimulation with activation during anticipation of non-noxious warm stimulation for the two groups. Direct contrast identified significant differences between controls and subjects recovered from depression in the lateral cerebellum and the cerebellar vermis (Fig. 3). Direct measurement of the absolute signal intensities revealed a higher cerebellar response with anticipation of pain relative to warmth (mean signal intensity increase in group images, 0.4%) in the controls relative to those recovered from depression (mean signal intensity increase, 0%).
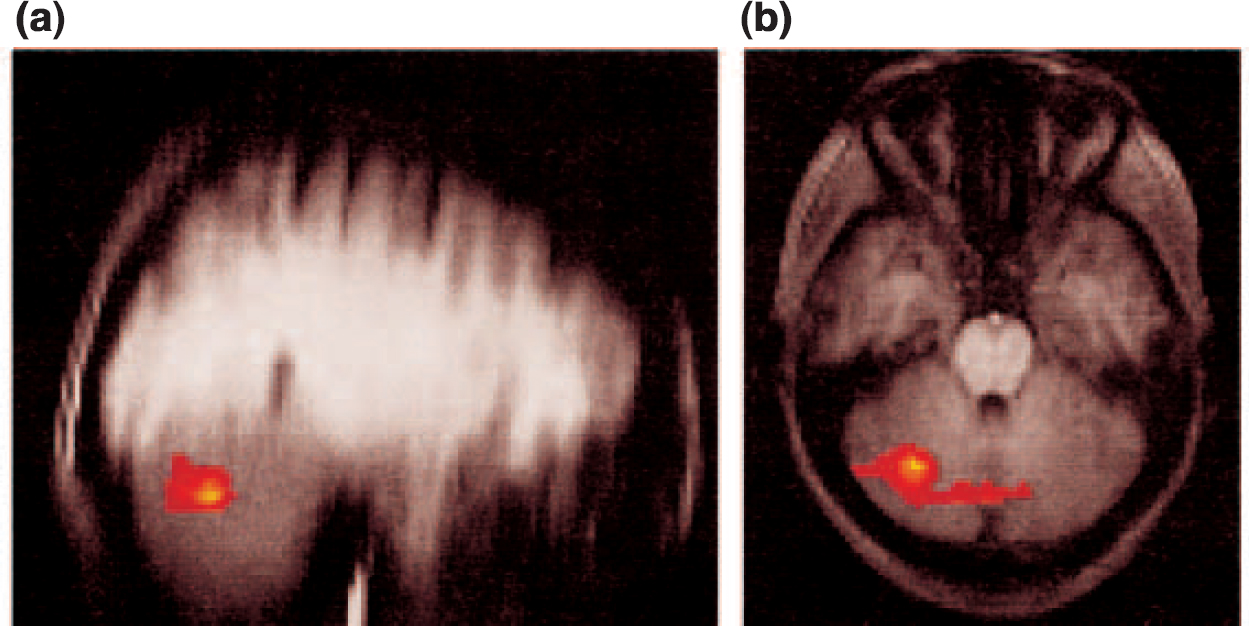
Fig. 3 Fixed-effects analysis of the differences in group responses between healthy controls and subjects recovered from depression to the noxious (painful) and non-noxious (warm) stimuli during the conditioning phase. The activation differences are shown on (a) sagittal and (b) axial slices. These differences demonstrate a reduced conditioning phase response in the subjects that had recovered from depression.
A possible confound could have been greater movement during scanning by the subjects recovered from depression, which could decrease the magnitude of response. Indices of movement derived from the motion correction were therefore tested for differences in subject motion between the two groups. The index used for each subject was the mean (over time points) of the relative motion from each time point to the next, where this motion is summarised as the mean displacement (in mm) over all brain voxels. Mean displacements during scanning for the individual control subjects (0.08, 0.07, 0.18, 0.11, 0.14, 0.06, 0.07 and 0.17 mm) were not significantly different (2-tailed t-test, P=0.29; Wilcoxon rank sum test, P=0.36) from those for subjects recovered from depression (0.16, 0.16, 0.15, 0.20, 0.05, 0.13, 0.09, 0.11, 0.16 and 0.12 mm).
DISCUSSION
Neuroticism, depression and classical conditioning
Not unexpectedly, the group that had recovered from depression showed greater neuroticism than the healthy controls. Neuroticism is a known risk factor for depression and subjects with a history of major depression have an increased risk of experiencing further episodes (Reference KendlerKendler, 1998). This conclusion is supported by theories of classical conditioning (Reference Eysenck, White and EynsenckEysenck et al, 1976; Reference Graeff, Guimaraes and De AndradeGraeff et al, 1996).
Responses to pain and its anticipation
The conditioning model employed here has been used previously with normal volunteers to distinguish brain regions involved in the processing of pain and its anticipation (Reference Ploghaus, Tracey and GaitiPloghaus et al, 1999). Patients recovered from depression were similar to controls in their responses to thermal pain; this is not unexpected, as the groups did not report differences in the conditioning response or pain perception. However, there was a significant difference in processing the anticipation of the noxious heat relative to the non-noxious warm stimulus; subjects recovered from depression had a reduced response in the cerebellum. This was noted both in the cerebellar vermis and right hemisphere.
The cerebellum and emotional processing
Lateral cerebellar activation in healthy controls during the thermal pain conditioning task was noted in our previous study (Reference Ploghaus, Tracey and GaitiPloghaus et al, 1999) but it was unclear whether this represented part of a motor-related response (e.g. suppression of hand withdrawal) or was an intrinsic element of the primary conditioned response to pain. The cerebellum clearly plays a cognitive role in forms of conditioning, potentially consistent with the latter hypothesis (Reference SchmahmannSchmahmann, 1997).
Previous studies have suggested that the cerebellum could show functional abnormalities in patients with depression. A single photon emission computed tomography (SPECT) study of patients with depression showed increased cerebellar blood flow after antidepressant treatment (Reference Halloran, Prentice and MurrayHalloran et al, 1999). Such changes could be related to specific elements within the broader spectrum of symptoms of depression. For example, as anxiety levels increase in patients with depression, cerebellar glucose metabolism decreases (Reference Osuch, Ketter and KimbrellOsuch et al, 2000). In addition, subjects with depression show attenuated activation of the cerebellum during the Tower of London planning task (Reference Elliott, Baker and RogersElliott et al, 1997). Both of the latter observations would be consistent specifically with our observation of a decreased response in those recovered from depression.
Modulation of cerebellar circuits by depression is also given credence by studies suggesting a role for the cerebellum in emotional processing (Reference Rapoport, van Reekum and MaybergRapoport et al, 2000). There are extensive anatomical and functional connections between the cerebellum and limbic structures, including the hippocampus and amygdala (Reference Heath, Dempesy and FontanaHeath et al, 1978). Stimulation of the cerebellum, particularly the vermis, can reduce aggression and produce pleasure reactions in animals. In humans, acute stimulation of the cerebellum can induce emotional states, including fear and anxiety, whereas chronic stimulation appears to reduce anxiety and depression (Reference Cooper, Amin, Cullinan and CooperCooper et al, 1978). Induction of sadness in healthy volunteers is associated with increased blood flow in the cerebellar vermis (Reference Mayberg, Liotti and BrannanMayberg et al, 1999).
Implications for the pathophysiology of depression
Subjects recovered from depression have a substantial risk for recurrence (Reference KendlerKendler, 1998). From our results and those of others, it appears that patients recovered from depression have a number of differences in brain function relative to healthy controls (Reference GoodwinGoodwin, 1996). We have shown a specific difference in brain activity during a conditioning response to a noxious v. a non-noxious stimulus. This difference could be more than simply a marker or ‘scar’ from the previous illness. A persistent processing abnormality of this kind could contribute to making subjects that have recovered from depression more vulnerable to abnormal mood regulation in the presence of aversive stimuli, as might occur, for example, during stressful life circumstances. If confirmed in a larger group, such a difference could help to identify those at risk of recurrent depression. Our observations suggest further that treatments modifying brain networks involved in conditioning responses to noxious stimuli could provide useful strategies for the treatment of depression.
CLINICAL IMPLICATIONS
-
▪ Subjects with a history of recurrent depressive disorder show a reduced relative cerebellar activation response during anticipation of noxious v. a non-noxious stimulation.
-
▪ In conjunction with previous work, these observations suggest that abnormal cerebellar function could be a vulnerability marker of liability to recurrent depression.
-
▪ Treatments modifying brain networks involved in conditioning responses to noxious stimuli therefore could provide a novel strategy for the treatment of depression.
LIMITATIONS
-
▪ The present study included only women: the findings cannot necessarily be extrapolated to men.
-
▪ Although a random effects analysis was employed to allow generalisation of conclusions beyond the specific population studied, the results are based on a relatively small number of subjects.
-
▪ It is uncertain whether the abnormality in cerebellar activity identified is itself specifically relevant to the pathophysiology of depression or is merely a potential biological marker.








eLetters
No eLetters have been published for this article.