Heschl's gyrus is located on the dorsal surface of the superior temporal gyrus and forms part of the primary auditory cortex. In schizophrenia the volume of the superior temporal gyrus and its posterior section, the planum temporale, are reduced (Reference Barta, Pearlson and BrillBarta et al, 1997) and both regions show activation during auditory hallucinations (Reference Shergill, Brammer and WilliamsShergill et al, 2000). However, although Heschl's gyrus forms the anterior border of the planum temporale and is functionally connected with it, few neuroimaging studies have focused on this gyrus and consistent changes have not been observed (Reference Sumich, Chitnis and FannonSumich et al, 2002). There has been no quantitative neuropathological study of this region in schizophrenia (Reference HarrisonHarrison, 1999). Therefore, we proposed to characterise neuronal and glial cell density and cortical thickness within Heschl's gyrus in people with schizophrenia, major depressive disorder and bipolar disorder. We focused on layers 3 and 5 of this region because previous investigations have indicated that the pyramidal neurons of these layers (Reference Pierri, Volk and AuhPierri et al, 2001) might be particularly vulnerable in schizophrenia.
METHOD
Human brain specimens were obtained from the Stanley Foundation Brain Consortium. The specimens were derived from 60 individuals: 15 were normal controls, 15 had had a diagnosis of schizophrenia, 15 of bipolar disorder and 15 of major depressive disorder. Demographic, clinical and histological information relating to this sample is given by Torrey et al (Reference Torrey, Webster and Knable2000).
Tissue was available from one hemisphere of each brain. Hemispheres were fixed in 10% phosphate buffered formalin, and the temporal lobes and superior temporal gyrus were dissected into a series of 1 cm-thick blocks. Each block was embedded in paraffin wax and tissue sections of 25 μm thickness were cut; one of these was randomly selected and stained with cresyl violet. This series of tissue sections, spanning the rostrocaudal extent of the superior temporal gyrus, was examined and sections containing Heschl's gyrus (Brodmann area 41) were identified. Where more than one gyrus was present, only the most anterior was categorised as the Heschl's gyrus. Four tissue sections that included Heschl's gyrus were selected for examination from the specimens derived from each individual.
Neurons and glial cells within cortical layers 3 and 5 were identified by standard criteria and stereologically based quantitation was undertaken as described for a previous study of this brain series (Reference Cotter, Mackay and LandauCotter et al, 2001). We used an optical disector of depth 10 μm with guard depths above and below of 3 μm. Cell density estimations were made with the aid of image analysis software (Kinetic Imaging version 5.0 for Windows, Histometrix, Liverpool, UK) according to the stereological optical disector method. The dimensions of the disector used were 50.5 μm × 37.5 μm in the x and y axes. We defined the cortical layers of interest by tracing the layer borders at ×20 magnification, and a systematic random sampling strategy was employed within these. An average of 75 fields were counted per layer per case for neuronal and glial estimations, and an average of 169 neurons and 215 glia were counted in each layer of each case. Values for the coefficient of error of the density estimates were all less than 5%. Neuronal and glial cell densities are expressed as cells × 103 per mm3. The width of each cortical layer was assessed using an image analysis system (Image-Pro Plus 4.0 for Windows, Media Cybernetics, Silver Spring, Maryland, USA) and methods described by Chana et al (Reference Chana, Landau and Beasley2003).
Statistical analysis
Neuronal and glial cell density in layers 3 and 5, and the thickness of each of the six cortical layers, were analysed using the same statistical methods applied in our previous study of this brain series (Reference Cotter, Mackay and LandauCotter et al, 2001). A forward selection procedure with 10% inclusion threshold was used to identify demographic and histological variables predicting outcome. Group comparisons of cell densities and layer thickness were adjusted for potential confounders and empirical predictors. For cortical thickness the Bonferroni-adjusted significance level was 0.05/6=0.0083, and for cell density the adjusted significance level was 0.05/2=0.025. For the analysis of cell density we used Poisson modelling to compare densities between groups. The random-effects Poisson models were fitted using the procedure GLMM in the statistical package Genstat 5 (for Windows; VSN International Ltd), which employs Schall's method to fit a generalised linear mixed model. Accumulated analysis of deviance using the experimental method was used to test for differences between the patient groups and the control group. Standard multiple regression models were used to assess group differences in layer thickness. All layer thickness analyses were carried out in the Statistical Package for the Social Sciences, version 11 for Windows. Cortical thickness data are summarised by their means, and densities by their medians.
RESULTS
All modelling of layer thickness and cell density was adjusted for gender, postmortem interval and fixation time.
Cortical layer thickness
The forward selection procedure identified age as a predictor of total cortical layer thickness (F (1, 55)=4.3, P=0.044), with thickness estimated to decrease by 71.3 μm with every additional 10 years of age at death. Hence, layer-wise group comparisons were also adjusted for age. No group difference in layer-wise cortical thickness was observed, other than an increase in layer 4 in major depressive disorder (P=0.047), but this effect could not be maintained after adjusting for multiple layer-wise comparisons (data presented as a data supplement to the online version of this paper and available from the authors on request).
Cell density
Summaries of the neuronal and glial densities and the results of the statistical comparisons are shown in Table 1. Tissue pH was found to be an empirical predictor of neuronal density (Wald test χ2(1)=12.1, P=0.0005) and glial density (Wald test χ2(1)=18.4, P<0.0001). Hence, the layer-wise group comparisons were also adjusted for tissue pH. There was no significant group difference in neuronal densities. We detected decreased glial density in major depressive disorder relative to controls in layer 3 (P=0.038), but this finding did not remain significant after adjusting for two layer-wise comparisons.
Table 1 Comparison of neuronal and glial cell densities between the control group and patient groups adjusted for gender, fixation time, post-mortem interval and tissue pH
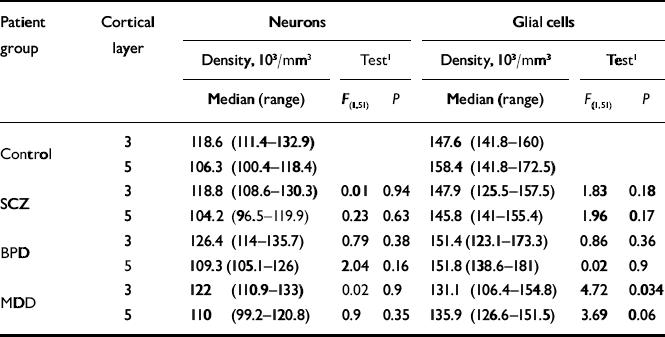
| Patient group | Cortical layer | Neurons | Glial cells | ||||
|---|---|---|---|---|---|---|---|
| Density, 103/mm3 | Test1 | Density, 103/mm3 | Test1 | ||||
| Median (range) | F (1, 51) | P | Median (range) | F (1, 51) | P | ||
| Control | 3 | 118.6 (111.4-132.9) | 147.6 (141.8-160) | ||||
| 5 | 106.3 (100.4-118.4) | 158.4 (141.8-172.5) | |||||
| SCZ | 3 | 118.8 (108.6-130.3) | 0.01 | 0.94 | 147.9 (125.5-157.5) | 1.83 | 0.18 |
| 5 | 104.2 (96.5-119.9) | 0.23 | 0.63 | 145.8 (141-155.4) | 1.96 | 0.17 | |
| BPD | 3 | 126.4 (114-135.7) | 0.79 | 0.38 | 151.4 (123.1-173.3) | 0.86 | 0.36 |
| 5 | 109.3 (105.1-126) | 2.04 | 0.16 | 151.8 (138.6-181) | 0.02 | 0.9 | |
| MDD | 3 | 122 (110.9-133) | 0.02 | 0.9 | 131.1 (106.4-154.8) | 4.72 | 0.034 |
| 5 | 110 (99.2-120.8) | 0.9 | 0.35 | 135.9 (126.6-151.5) | 3.69 | 0.06 | |
DISCUSSION
Our main findings are that cortical thickness and neuronal and glial cell densities in cortical layers 3 and 5 of Heschl's gyrus are unaltered in schizophrenia and affective disorders. These are important negative findings for schizophrenia, as this region has been implicated in these disorders by neuroimaging investigations but has not yet been assessed using cytoarchitectural morphometric methods. We now demonstrate that there is no overt cellular abnormality within these cortical layers in this region in schizophrenia. Previous morphometric studies of bipolar disorder and major depression within prefrontal cortical regions have demonstrated deficits in glial cell densities and counts (Reference Rajkowska, Miguel-Hidalgo and WeiRajkowska et al, 1999; Reference Cotter, Mackay and LandauCotter et al, 2001). Our negative cell counting data on Heschl's gyrus in these disorders suggest that glial cell deficit in affective disorders might be localised to prefrontal cortical association regions, sparing primary sensory cortical regions. Taken together, these findings indicate that the cellular neuropathology of major psychiatric disorders demonstrates regional vulnerability. A current challenge is to determine the basis of such regional vulnerabilities.
Acknowledgements
The study was funded by the Wellcome Trust, the Medical Research Council (UK) and the Theodore and Vada Stanley Foundation. Post-mortem brains were donated by the Stanley Foundation Brain Bank Consortium, courtesy of Drs. Llewellyn B. Bigelow, Juraj Cervenak, Mary M. Herman, Thomas M. Hyde, Joel Kleinman, Jose D. Paltan, Robert M. Post, E. Fuller Torrey, Maree J. Webster and Robert Yolken.




eLetters
No eLetters have been published for this article.