Little is known about the clinical implications and prognostic significance of cannabis-induced psychotic symptoms, despite the fact that cannabis has been closely linked with the development of schizophrenia (Reference Arseneault, Cannon and WittonArseneault et al, 2004). It is well established that psychotic symptoms may follow cannabis intake, and a number of reviews have concluded that such symptoms are generally short-lived and that total remission can be expected (Reference TunvingTunving, 1985; Reference ThomasThomas, 1993; Reference JohnsJohns, 2001; Reference Hall, Degenhardt, Castle and MurrayHall & Degenhardt, 2004). However, the transient nature of the symptoms and the favourable prognosis have only been evaluated in case studies, and none of these has reported long-term follow-up data.
In this study we use an epidemiological approach to describe a substantial number of patients treated for cannabis-induced psychotic disorder with no prior history of psychotic symptoms. Patients were followed for at least 3 years, with the aim of determining the proportion and types of subsequent schizophrenia-spectrum disorders, the timing of onset, and whether the patients developed these conditions at an earlier age than other patients with schizophrenia but with no history of cannabis-induced psychotic symptoms.
METHOD
Sample
The first analyses describe follow-up data for a cohort of patients treated for cannabis-induced symptoms. These patients were included if they had received treatment at a Danish psychiatric hospital with a diagnosis of cannabis-induced psychotic disorder (code F12.5 in ICD-10; World Health Organization, 1992) between 1 January 1994 and 1 July 1999. Patients previously treated for any type of psychotic symptoms or bipolar disorder were excluded. The included patients were followed for at least 3 years, and the end-point for the whole sample was 1 July 2002. Data for those who died between 1 January 1994 and 1 July 2002 were censored.
The second analysis was concerned with the average age at the time schizophrenia-spectrum disorders were first diagnosed. The cannabis group was compared with a group consisting of all first-time referrals with schizophrenia-spectrum disorders between 2002 and 2003, with no history of cannabis-induced psychotic symptoms. In the comparison group, those with a history of psychotic episodes according to ICD-8 (assigned before 1994) were also excluded. The latter exclusion criterion was necessary because the diagnostic categories from ICD-8 cannot be translated into ICD-10 diagnoses in a meaningful way.
The registers
Data were extracted from the Danish Psychiatric Central Register. This register contains information on all treatment provided by Danish psychiatric hospitals and departments since 1970. Out-patient treatment has only been registered since 1995. After each contact with the psychiatric system patients receive a diagnosis code. Up to 1993 these were assigned using ICD-8, and thereafter ICD-10 has been used; ICD-9 was never in use in Denmark.
Only psychiatrists responsible for the treatment are allowed to enter diagnoses into the register. They generally have knowledge of prior hospitalisations and diagnoses based on case files and use this information in their evaluation of the patients. There are no private psychiatric facilities in Denmark and treatment is free of charge. Information on patients who died during the study period was retrieved from the Danish Cause of Death Registry. Registrations are person identifiable using the Central Persons Register Number. This is a unique number given to every Danish citizen at birth or at time of migration, and it is used in all official registers in Denmark. Knowledge of this number allows age and gender to be determined.
Analysis
For this study the outcome ‘schizophrenia-spectrum disorders’ was defined as schizophrenia (ICD-10 code F20), schizotypal disorder (ICD-10 code F21) and schizoaffective disorders (ICD-10 code F25). Separate analyses were made for other psychotic conditions. Most patients received several different diagnoses during the course of the follow-up. Treatment episodes after index were therefore put into a hierarchy. Schizophrenia-spectrum disorders were given highest priority, followed by the remaining F20 diagnoses, bipolar disorder, acute and transient psychotic conditions, substance-induced psychotic disorders, and other diagnoses (predominantly mood, anxiety and personality disorders).
In the analysis of timing of onset of schizophrenia-spectrum disorders, the time lapse between index and first referral with such diagnoses was recorded. The average age at first referral with a schizophrenia-spectrum disorder among patients with a history of cannabis-induced psychoses was compared with that of others who developed a schizophrenia-spectrum disorder but had no recorded history of cannabis-induced cannabis-induced psychotic symptoms.
Statistics
Model checking showed that the distributions for age and number of subsequent referrals were skewed. Log Gaussian regression models were therefore used, and the back-transformed estimates and confidence intervals are reported. The effects of age and gender on risk of subsequent schizophrenia-spectrum disorder were evaluated using Cox regression. A survival curve, showing the time from index to first treatment for a schizophrenia-spectrum disorder, was estimated for each gender using a Cox regression model adjusting for age. Because of unequal variance of age between the patients and the comparison group, Satterthwaite approximated degrees of freedom were used in the t-tests comparing age at first episode of various psychotic conditions. Stata Statistical Software release 8.0 was used for all analyses.
RESULTS
Patient characteristics
A total of 803 patients received treatment for cannabis-induced psychotic disorders in Danish psychiatric hospitals between 1 January 1994 and 1 July 1999. With a national population of approximately 5.4 million people (Danmarks Statistik, 2004), this results in an average incidence ratio of 2.7 per 100 000 person-years. Prior treatment for psychotic symptoms was recorded for 240 patients, and in 28 cases the diagnosis was changed into a schizophrenia-spectrum disorder before discharge. These cases were excluded. The remaining 535 cases (66.6%) recorded no history of psychotic symptoms and were included for further analysis.
The mean age at time of first treatment for cannabis-induced symptoms was 27.0 years (s.d.=7.7, 25th percentile=20.9, median=25.5, 75th percentile=31.2); 441 (82.4%) were male. A total of 379 patients were admitted to hospital for a median stay of 13 days (25th percentile=4, 75th percentile=29, mean=30.6) and 156 patients received out-patient treatment only. The mean length of follow-up was 5.9 years (s.d.=1.7). Twenty-two patients died during the follow-up period, 13 within 3 years of index and 9 after. Causes of death were suicide (n=7), accident (n=4), natural causes (n=4) and unknown (n=7). Of the 13 who died within 3 years, 7 had been diagnosed with a schizophrenia-spectrum disorder. Of the remaining 9 patients, 6 received such diagnoses before death.
There were 2721 patients in the comparison group. In this group the mean age at onset of schizophrenia-spectrum disorders was 33.8 years (s.d.=13.9, 25th percentile=23.5, median=30.7, 75th percentile=41.2); 1560 (57.3%) were male.
Subsequent psychotic conditions
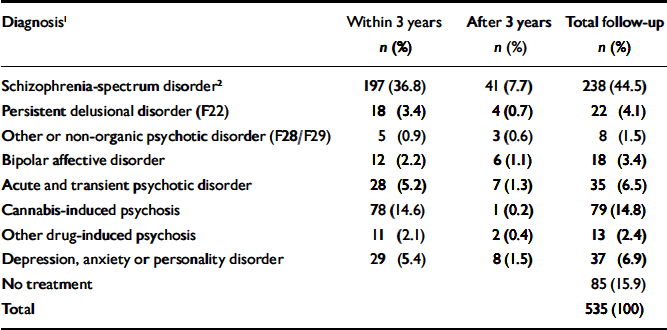
Table 1 lists the most severe diagnoses recorded during follow-up. A total of 238 people (44.5%) diagnosed with cannabis-induced psychotic symptoms later developed a schizophrenia-spectrum disorder. This proportion increases with length of follow-up. Of the patients admitted to treatment in 1994, nearly half (48.9%) received this diagnosis, whereas the proportion was 35.7% for patients admitted between 1 July 1998 and 1 July 1999. Another 9.0% developed persistent delusional disorders, other or unspecified non-organic psychoses, or bipolar affective disorder at some point, raising the total estimate of patients developing persistent psychotic conditions to 53.5%. In total, 77.2% experienced new psychotic episodes after index, if transient psychotic conditions and substance-induced psychoses are included. Only 15.9% remained out of psychiatric care throughout the follow-up period.
Table 1 Patients treated for mental or behavioural disorders after index point (n=535)
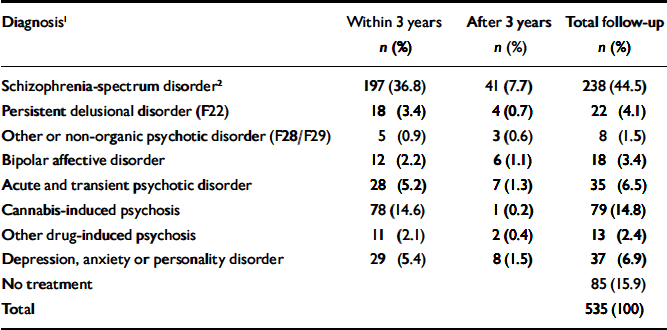
| Diagnosis1 | Within 3 years n (%) | After 3 years n (%) | Total follow-up n (%) |
|---|---|---|---|
| Schizophrenia-spectrum disorder2 | 197 (36.8) | 41 (7.7) | 238 (44.5) |
| Persistent delusional disorder (F22) | 18 (3.4) | 4 (0.7) | 22 (4.1) |
| Other or non-organic psychotic disorder (F28/F29) | 5 (0.9) | 3 (0.6) | 8 (1.5) |
| Bipolar affective disorder | 12 (2.2) | 6 (1.1) | 18 (3.4) |
| Acute and transient psychotic disorder | 28 (5.2) | 7 (1.3) | 35 (6.5) |
| Cannabis-induced psychosis | 78 (14.6) | 1 (0.2) | 79 (14.8) |
| Other drug-induced psychosis | 11 (2.1) | 2 (0.4) | 13 (2.4) |
| Depression, anxiety or personality disorder | 29 (5.4) | 8 (1.5) | 37 (6.9) |
| No treatment | 85 (15.9) | ||
| Total | 535 (100) |
Men had an increased risk of developing schizophrenia-spectrum disorders compared with women (47.6% v. 29.8%). Using a Cox regression model controlling for age, the hazard ratio was 1.82 (95% CI 1.21-2.74, P<0.002). Young age was also related to increased risk. Controlling for gender, the proportional hazard ratio when increasing the age by 1 year was 0.97 (95% CI 0.95-0.99, P<0.003).
Number of subsequent treatment episodes
The average number of treatment episodes caused by schizophrenia-spectrum disorders was estimated for the 238 patients who developed these conditions. The mean number was 1.2 per person-year (95% CI 1.0-1.4) for men and 0.7 per person-year (95% CI 0.4-1.1) for women. Altogether 176 patients (73.9%) received treatment for a schizophrenia-spectrum disorder on three or more separate occasions after index.
Pattern of subsequent diagnoses
Most patients were treated many times during follow-up, and the diagnoses often changed. Table 2 presents the prevalence rates for the entire population of 535 patients. Paranoid schizophrenia was the most common condition, followed by acute and transient psychotic disorders, personality disorders and unspecified schizophrenia. A separate analysis was performed of the last schizophrenia-spectrum diagnosis for each patient within the observation period (results not shown). This revealed that, of the 238 patients who received such diagnoses, 58.0% were treated for paranoid schizophrenia at the most recent contact with a psychiatric department and 20.2% were treated for unspecified schizophrenia. In total, 211 (88.7%) of the patients sought treatment for schizophrenia at their latest contact with the psychiatric care system.
Table 2 Prevalence rates of mental disorders during follow-up (n=535)
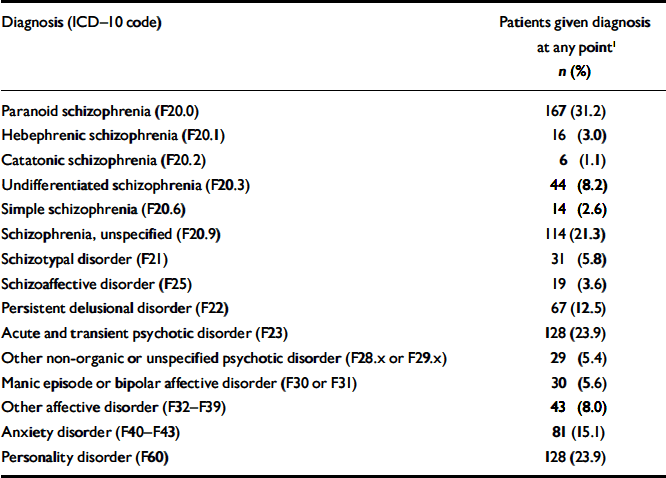
| Diagnosis (ICD–10 code) | Patients given diagnosis at any point1 n (%) |
|---|---|
| Paranoid schizophrenia (F20.0) | 167 (31.2) |
| Hebephrenic schizophrenia (F20.1) | 16 (3.0) |
| Catatonic schizophrenia (F20.2) | 6 (1.1) |
| Undifferentiated schizophrenia (F20.3) | 44 (8.2) |
| Simple schizophrenia (F20.6) | 14 (2.6) |
| Schizophrenia, unspecified (F20.9) | 114 (21.3) |
| Schizotypal disorder (F21) | 31 (5.8) |
| Schizoaffective disorder (F25) | 19 (3.6) |
| Persistent delusional disorder (F22) | 67 (12.5) |
| Acute and transient psychotic disorder (F23) | 128 (23.9) |
| Other non-organic or unspecified psychotic disorder (F28.x or F29.x) | 29 (5.4) |
| Manic episode or bipolar affective disorder (F30 or F31) | 30 (5.6) |
| Other affective disorder (F32–F39) | 43 (8.0) |
| Anxiety disorder (F40–F43) | 81 (15.1) |
| Personality disorder (F60) | 128 (23.9) |
Timing of onset
The first episode of schizophrenia-spectrum disorder occurred after a substantial delay for most of the 238 patients (Fig. 1). It was registered more than 1 year after index for 47.1% of the patients, and 17.2% developed such conditions more than 3 years later.
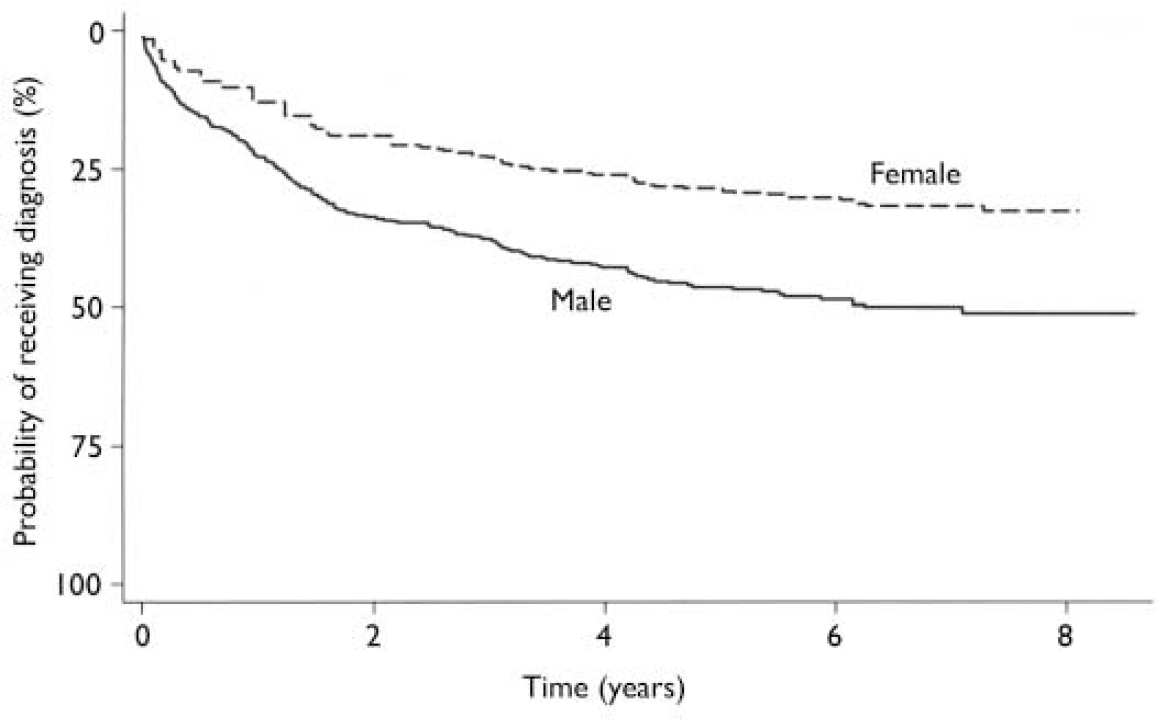
Fig. 1 Time from index to first episode of schizophrenia-spectrum disorder (adjusted for age).
Age at onset
The mean age at first treatment for a schizophrenia-spectrum disorder among the patients was compared with that of a comparison group receiving treatment for the same conditions for the first time in 2002 and 2003, but with no prior history of cannabis-induced psychotic symptoms. Both men and women in the cannabis group were younger at the first time they were diagnosed with schizophrenia (Table 3). For people who developed schizotypal or schizoaffective disorders there was no age difference between the cannabis and comparison groups (results not shown).
Table 3 Age at first episode of schizophrenia (ICD–10, F20.x)
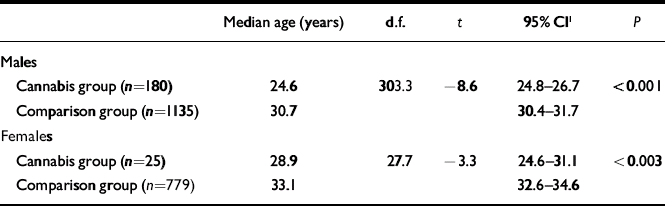
| Median age (years) | d.f. | t | 95% CI1 | P | |
|---|---|---|---|---|---|
| Males | |||||
| Cannabis group (n=180) | 24.6 | 303.3 | –8.6 | 24.8–26.7 | <0.001 |
| Comparison group (n=1135) | 30.7 | 30.4–31.7 | |||
| Females | |||||
| Cannabis group (n=25) | 28.9 | 27.7 | –3.3 | 24.6–31.1 | <0.003 |
| Comparison group (n=779) | 33.1 | 32.6–34.6 |
When more than 5 patients from the cannabis group had a specific type of schizophrenia, analyses were performed for the subgroups. This revealed that male patients treated for paranoid schizophrenia (t=–7.2, d.f.=119, P<0.001), undifferentiated schizophrenia (t=–3.4, d.f.=42, P–0.002) and unspecified schizophrenia (t=–2.2, d.f.=96, P<0.03) were younger at first episode than people from the comparison group. For simple schizophrenia there was no significant difference in age. Female patients from the cannabis group, treated for paranoid schizophrenia, were younger than those from the comparison group (t=–3.1, d.f.=15, P<0.007); there was no significant difference for unspecified schizophrenia.
DISCUSSION
The prognostic significance of cannabis-induced psychoses is not well established. There is general agreement that such conditions are rare (e.g. DSM-IV; American Psychiatric Association, 1994); however, our study is the first to provide an estimate of the incidence rate. Indeed, Hall & Degenhardt (Reference Hall, Degenhardt, Castle and Murray2004) found reports of only 397 cases of ‘cannabis psychosis’ in a review on the topic. This probably explains why the methodological quality of previous investigations has been criticised in several reviews (Reference ThornicroftThornicroft, 1990; Reference ThomasThomas, 1993; Reference Poole and BrabbinsPoole & Brabbins, 1996; Reference JohnsJohns, 2001; Reference Hall, Degenhardt, Castle and MurrayHall & Degenhardt, 2004). Most are case studies or include few participants, and the follow-up intervals have been limited in the longitudinal studies. Some investigations concern patients with pre-existing schizophrenia or other severe mental illnesses, and in many studies the mental status of the patients prior to the cannabis-induced symptoms is uncertain. Our investigation included 535 people with no prior history of psychosis who were followed for at least 3 years. This underlines the significance of the results.
Outcome
It was established that almost half of the patients in our study treated for a cannabis-induced psychotic disorder developed a schizophrenia-spectrum disorder subsequently. A substantial number of patients were diagnosed with paranoid schizophrenia, and the vast majority of those who developed a schizophrenia-spectrum disorder were given this diagnosis at the most recent hospitalisation. New psychotic episodes developed in 77.2% of the entire sample and only 15.9% did not receive psychiatric treatment at any point after index. Male gender and young age were associated with more severe outcome. Most patients were treated on numerous occasions for schizophrenia-spectrum disorders, and in almost half of the cases the first episode occurred with a delay of more than a year. Compared with patients without a history of cannabis-induced psychosis, they developed schizophrenia at a significantly younger age. This effect was most marked for paranoid schizophrenia.
For the majority of patients, cannabis-induced psychotic symptoms proved to be a first step in the development of a schizophrenia-spectrum disorder or other severe psychopathology. This is inconsistent with findings from previous studies generally reporting complete remission when patients abstain from further cannabis use (Reference Talbott and TeagueTalbott & Teague, 1969; Reference Kolansky and MooreKolansky & Moore, 1971; Reference ThacoreThacore, 1973; Reference Thacore and ShuklaThacore & Shukla, 1976; Reference Rottanburg, Robins and Ben ArieRottanburg et al, 1982; Reference Carney, Bacelle and RobinsonCarney et al, 1984; Reference DrummondDrummond, 1986; Reference Cohen and JohnsonCohen & Johnson, 1988; Reference Chaudry, Moss and BashirChaudry et al, 1991; Reference WylieWylie, 1995; Reference Basu, Malhotra and BhagatBasu et al, 1999), although some have reported that a minority of patients with pre-existing mental problems had a less favourable outcome (Reference BrombergBromberg, 1939; Reference Bernhardson and GunneBernhardson & Gunne, 1972; Reference Tennant and GroesbeckTennant & Groesbeck, 1972; Reference Chopra and SmithChopra & Smith, 1974; Reference Palsson, Thulin and TunvingPålsson et al, 1982). However, patients had generally not been followed after the cannabis-induced psychotic condition remitted, and no study included data on all patients more than 3 months after the end of treatment. Many of the same investigations have reported that cannabis-induced psychoses subside more quickly than psychoses that are not substance-induced (Reference Talbott and TeagueTalbott & Teague, 1969; Reference Bernhardson and GunneBernhardson & Gunne, 1972; Reference Tennant and GroesbeckTennant & Groesbeck, 1972; Reference ThacoreThacore, 1973; Reference Chopra and SmithChopra & Smith, 1974; Reference Thacore and ShuklaThacore & Shukla, 1976; Reference Palsson, Thulin and TunvingPålsson et al, 1982; Reference Rottanburg, Robins and Ben ArieRottanburg et al, 1982; Reference Carney, Bacelle and RobinsonCarney et al, 1984; Reference DrummondDrummond, 1986; Reference Cohen and JohnsonCohen & Johnson, 1988; Reference Chaudry, Moss and BashirChaudry et al, 1991; Reference WylieWylie, 1995; Reference Basu, Malhotra and BhagatBasu et al, 1999). This is consistent with findings in our study. Many patients received out-patient treatment only, and the length of stay was generally short for those who were admitted to hospital. Combining these factors probably explains why the evaluation of the outcome in this study differs so markedly from other studies. The lack of long-term follow-up of the patients means that delayed onset of severe psychopathological symptoms might have been undetected in previous studies.
Existence of ‘cannabis psychosis’
In accordance with our results, some authors have claimed that cannabis-induced psychotic symptoms are often a sign of underlying psychopathology and that the condition is easily confused with schizophrenia (Reference WeilWeil, 1970; Reference ThornicroftThornicroft, 1990; Reference ThomasThomas, 1993; Reference Poole and BrabbinsPoole & Brabbins, 1996). However, data have been lacking in attempts to substantiate this. Criticism has centred on the concept of ‘cannabis psychosis’, and has primarily been based on studies failing to show a symptom profile distinct from other psychoses (Reference Imade and EbieImade & Ebie, 1991; Reference Mathers and GhodseMathers & Ghodse, 1992; Reference McGuire, Jones and HarveyMcGuire et al, 1995). Numerous symptom clusters have been described as characteristic of ‘cannabis psychosis’, and an exact meaning of the concept has never been established (Reference ThornicroftThornicroft, 1990; Reference Hall, Degenhardt, Castle and MurrayHall & Degenhardt, 2004). However, even the critics have accepted that psychotic symptoms can be induced by cannabis, and that such symptoms generally wear off quickly and with complete remission. It has never before been shown that cannabis-induced psychoses are followed by development of long-term psychotic conditions in people with no history of psychosis, and this is the first study to show that such symptoms, in most cases, can be regarded as the first manifestations of long-term psychotic illness. The results do not disprove the existence of ‘cannabis psychosis’, but clearly show that cannabis-induced psychotic symptoms must be regarded as important risk factors for subsequent development of schizophrenia-spectrum disorders.
The question of causality
Recent studies have established that cannabis use increases the risk of developing severe psychotic disorders (Reference Arseneault, Cannon and WittonArseneault et al, 2004), although the findings are not conclusive (Reference Macleod, Oakes and CopelloMacleod et al, 2004). Whether cannabis was causally linked with development of schizophrenia among patients in our investigation cannot be determined since, owing to the study design, it was not possible to control for factors such as hereditary predisposition, other drug use and socio-economic status. However, the fact that patients with cannabis-induced psychotic symptoms developed schizophrenia at a younger age than patients with no recorded history of cannabis-induced psychosis indicates that cannabis use may hasten the pathogenesis. This is in accordance with a recent study showing that the age at onset of schizophrenia is lower among patients using cannabis (Reference Veen, Selten and ven der TweelVeen et al, 2004) and further validates this significant finding.
Cannabis use in Denmark
The incidence of cannabis-induced psychotic disorders in Denmark was estimated to be 2.7 per 100 000 person-years. This confirms that such conditions are rare. In the interpretation of this number, it is important to note that in Denmark cannabis is predominantly smoked as hashish, which is more potent than marijuana. A recent publication from the Danish National Board of Health (2003) shows that 40.9% of all Danish citizens aged 16-24 years have used cannabis at some point in their lifetime, and that 19.7% had used the substance in the previous month.
Limitations
The results of this study must be evaluated considering its limitations. Data were not specifically collected for this investigation, so it was not possible to validate the diagnosis of cannabis-induced psychosis. It may be difficult to distinguish between this diagnosis and early signs of schizophrenia. However, many patients only received out-patient treatment for the cannabis-induced symptoms, and for those who were admitted to hospital the length of stay was typically short. Furthermore, there was a substantial delay before schizophrenia-spectrum disorders emerged in most patients. This indicates that the cannabis-induced symptoms were short-lived and that temporary remission was probably achieved in most cases.
It could not be ascertained that the patients fulfilled the diagnostic criteria for schizophrenia-spectrum disorders either. Furthermore, we were not able to control for regular use of cannabis or other drugs, such as stimulants, during the follow-up period. It might be argued that the diagnoses assigned to the patients during follow-up were merely new instances of substance-induced symptoms being misclassified. On the other hand, the fact that 73.9% of the patients were given schizophrenia-spectrum diagnoses on at least three separate occasions is inconsistent with this. In addition, psychiatrists use prior case records upon referral, so previous problems with substance misuse are likely to be detected. Finally, it is standard procedure to enquire about substance use among patients presenting with psychotic symptoms.
Some confounders might have influenced the evaluation of age at first treatment for schizophrenia. It is well established that cannabis use is most common among adolescents and young adults. If we suppose that cannabis use and schizophrenia are unrelated, and the first signs of schizophrenia happen to co-occur with cannabis use, then the symptoms might erroneously be attributed to cannabis. If this were the case, it might lead to bias when evaluating the age at first episode of schizophrenia in the cannabis group. Other factors that we were not able to control for (e.g. socio-economic status and other drug use) might also have influenced these results.
Finally, the patients in our study possibly represent more severe cases of cannabis-induced psychotic symptoms; therefore, the results may not generalise to people who develop psychotic symptoms that are less persistent, or who for other reasons do not present for treatment. Conversely, there is some indication that any occurrence of psychotic symptoms after cannabis intake could constitute a risk for subsequent schizophrenia-spectrum disorders (Reference Verdoux, Castle and MurrayVerdoux, 2004). If this can be corroborated it would have clear implications for the ongoing search for early signs of schizophrenia. This should be further investigated in the future.
Implications
Our study shows that cannabis-induced psychotic symptoms are an important risk factor for subsequent development of severe psychopathological disorder. This is in contrast to previous studies describing the condition as harmless. Although it cannot be determined that cannabis has a causal impact on the subsequent development, the findings have clear implications for clinicians who encounter a patient with cannabis-induced psychotic disorder. The prognosis for the patient is poor, and attention needs to be given to early intervention.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Clinicians should be aware that half of all patients treated for cannabis-induced psychosis will subsequently develop a schizophrenia-spectrum disorder; almost a third will be diagnosed with paranoid schizophrenia.
-
▪ The onset of such psychopathology is substantially delayed in most patients.
-
▪ The first episode of schizophrenia occurs several years earlier in these patients compared with patients with no history of cannabis-induced psychosis.
LIMITATIONS
-
▪ The study was based on register data; consequently, the diagnoses could not be validated.
-
▪ There was limited background information on the patients.
-
▪ Patients seeking psychiatric treatment probably represent the more severe cases. Therefore the results cannot be extrapolated to people developing psychotic symptoms after cannabis intake but not seeking professional help.






eLetters
No eLetters have been published for this article.