The compound 3,4-methylenedioxymethamphetamine (MDMA), popularly known as ‘ecstasy’, is the most commonly misused controlled drug after cannabis among young people in Europe. 1 Its use poses a significant public health problem due to its known acute toxicity (hyperthermia and related medical complications). Reference Hall and Henry2 However, there has also been concern about the possible long-term neurotoxic effects of MDMA on brain serotonin neurons.
The neuronal serotonin transporter (SERT) is present in the serotonin (5-HT) synapse as well as along the 5-HT axons. Reference Zhou, Tao-Cheng, Segu, Patel and Wang3 Serotonin transporter is considered to be one of the markers of the integrity of serotonin neurons and has been validated in animal models of MDMA neurotoxicity. Reference Battaglia, Yeh, O'Hearn, Molliver, Kuhar and De Souza4–Reference Szabo, McCann, Wilson, Scheffel, Owonikoko and Mathews6 Studies in rodents and non-human primates suggest long-lasting reductions in SERT availability after single and multiple administrations. Reference Easton and Marsden7–Reference Hatzidimitriou, McCann and Ricaurte9 In humans, imaging studies using either positron emission tomography (PET) or single photon emission computed tomography (SPECT) have also shown lowered brain SERT binding in current MDMA users but it is uncertain for how long this abnormality might persist following abstinence. The aim of the present study was to investigate SERT binding, using PET in conjunction with [11C]DASB, a highly specific radioligand for the human SERT, in former MDMA users who had been abstinent from the drug for at least 1 year.
Method
Participants
Three groups of male volunteers were recruited via newspaper or magazine advertisement and word of mouth. The groups comprised 12 former MDMA users who had taken the drug on a regular basis (at least 25 occasions) previously but who had not taken it for at least 1 year, although they continued to use other recreational drugs (the MDMA group), 9 polydrug users who were using a range of recreational drugs matched with the former MDMA users but who reported never having taken MDMA (the polydrug group), and 19 drug-naive controls who reported no history of illicit drug use (the drug-naive group). We chose to study a polydrug (i.e. minus MDMA) user group so that in the event of a change of 5-HT transporter (5-HTT) binding in the MDMA group, the contribution of concomitant polydrug use could be examined.
Potential participants were diagnostically assessed by experienced clinical psychiatrists (S.S., Z.B.) using the Structured Clinical Interview for DSM–IV Axis I Disorders. Reference First, Spitzer, Gibbon and Williams10 Inclusion criteria for all groups were male, aged 25–60, no history of prescribed psychotropic medication, no history of major psychiatric or neurological problems, no history of drug dependence, not having drunk more that three units of alcohol in the 24 h prior to testing and no use of recreational drugs for at least 3 days prior to testing. All participants filled in questionnaire measures for depression (Beck Depression Inventory, BDI), Reference Beck, Ward, Mendelson, Mock and Erbaugh11 anxiety (Spielberger Anxiety Inventory, STAI), Reference Spielberger, Garsuch and Lushene12 neuroticism (Eysenck score) Reference Eysenck and Eysenck13 or premorbid IQ (Spot-the-Word Test). Reference Baddeley, Emslie and Nimmo Smith14 All participants gave full written informed consent for the study and were paid an honorarium for their participation. The study was approved by the research ethics committee at Hammersmith Hospital, London, and the Administration of Radioactive Substances Advisory Committee, UK.
A semi-structured drug-use history interview schedule was completed by all participants. The MDMA and polydrug groups were well matched in terms of drug and alcohol use (Table 1). Each participant from the MDMA and polydrug groups gave a urine sample on the day of the PET scan that was screened for MDMA, cocaine, amphetamine, opiates or benzodiazepines. Individuals with a positive urine screen for any of these substances were excluded from the study. Participants also gave hair samples that were tested for MDMA, cocaine and amphetamine; individuals testing positive for MDMA (n=2) were subsequently excluded from the analysis. The average hair sample covered a period of 2.7 months (s.d.=0.9). Matched drug-naive individuals were rigorously screened at interview for drug and alcohol history by experienced psychiatrists and a random 50% provided urine and hair samples that were tested to confirm no illicit drug use.
Table 1 Mean (s.d.) and range of drug use reported by former MDMA users and polydrug controls

| MDMA group, n=12 | Polydrug group, n=9 | |||
|---|---|---|---|---|
| Mean (s.d.) | Range | Mean (s.d.) | Range | |
| 3,4-methylenedioxymethamphetamine (MDMA) | ||||
| Time since last use, years | 2.74 (1.46) | 390-1825a | - | - |
| Age of first use, years | 18.08 (1.9) | 14-22 | - | - |
| Length of regular use, years | 4.33 (2.8) | 1.5-10 | - | - |
| Frequency of use, days per month | 4.75 (3.1) | 1-12 | - | - |
| Number of tablets used in a typical session | 2.75 (1.9) | 1-8 | - | - |
| Lifetime occasions used | 243.75 (244.4) | 60-864 | - | - |
| Cannibis | ||||
| Time since last use, days | 249.14 (446.5) | 3-1460 | 295.89 (620.30) | 3-1825 |
| Age of first use, years | 16.36 (1.9) | 14-20 | 17.78 (4.0) | 12-25 |
| Length of regular use, years | 8.93 (3.4) | 3.8-14 | 14.39 (13.0) | 1.5-38 |
| Frequency of use, days per month | 18.41 (11.00) | 2-30 | 15.44 (11.84) | 1-30 |
| Dose, ounces per month | 0.60 (0.80) | 0.3-5 | 1.12 (1.34) | 0.1-2 |
| Amphetamine | ||||
| Time since last use, days | 2391.82 (1689.2) | 365-6205 | 1744.63 (1529.36) | 30-3650 |
| Age of first use, years | 18.75 (2.49) | 15-24 | 20.88 (5.59) | 16-33 |
| Length of regular use, years | 1.71 (0.76) | 1-3 | 4.75 (7.1) | 1-22 |
| Frequency of use, days per month | 4.57 (6.88) | 1-20 | 7.69 (8.84) | 1-25 |
| Dose, grams per session | 1.64 (1.31) | 1-4.5 | 1.30 (1.93) | 0.1-6 |
| Cocaine | ||||
| Time since last use, days | 886.05 (927.63) | 3-2730 | 684.14 (1316.4) | 4-3650 |
| Age of first use, years | 20.77 (1.69) | 18-23.5 | 22.33 (4.76) | 17-30 |
| Length of regular use, years | 3.60 (1.95) | 1-6 | 4.83 (2.93) | 2-10 |
| Frequency of use, days per month | 5.47 (8.18) | 2-20 | 3.5 (4.20) | 1-12 |
| Dose, grams per session | 2.35 (2.79) | 0.3-6.5 | 0.67 (0.49) | 0.3-1.5 |
| Lysergic acid diethylamide (LSD) | ||||
| Time since last use, days | 1948.2 (1479.71) | 30-4380 | 5108.5 (2295.8) | 2920-8000 |
| Age of first use, years | 17.13 (1.64) | 14-19 | 19.25 (2.99) | 16-23 |
| Length of regular use, years | 1.90 (1.47) | 0.5-4 | 2.67 (2.08) | 1-5 |
| Frequency of use, days per month | 4.90 (3.78) | 1-10 | 1.67 (0.58) | 1-2 |
| Dose, trips per session | 2.10 (0.96) | 1-3.5 | 1.00 (0.00) | 1-1 |
| Alcohol | ||||
| Time since last use, days | 4.9 (5.4) | 1-21 | 2.9 (2.5) | 1-7 |
| Age at first use, years | 14.8 (1.8) | 11-17 | 15.3 (1.9) | 12-19 |
| Length of regular use, years | 10.8 (2.8) | 4.5-15 | 18.4 (9) | 8-31 |
| Frequency, days per month | 8.7 (5.9) | 2-20 | 9.2 (6.6) | 2-20 |
| Dose, units per session | 7.2 (2.5) | 4-12 | 8.7 (4.6) | 2-16 |
Radioligand
The radiotracer [11C]DASB was synthesised as previously described. Reference Wilson, Ginovart, Schmidt, Meyer, Threlkeld and Houle15 The standard DASB and the precursor desmethyl DASB were obtained from Target Molecules Ltd., Southampton (UK). [11C]DASB was injected into an antecubital vein as a smooth bolus over 30 s. The injected radioactivity dose was between 308 and 563.7 MBq (mean 518.3 MBq, s.d=62). The radiochemical purity of the injected [11C]DASB was high and ranged from 95 to 100% with a mean of 97.6% (s.d.=1.4). The specific activity was on average 75530 MBq·μmol–1 (s.d.=12 5821). The injected mass of total cold DASB varied between 0.15 and 12.2 g with a mean value of 3.5 g (s.d.=2.2).
PET scanning
All PET scans were performed on the high-sensitivity Siemens/CTI scanner ECAT EXACT3D, as described previously. Reference Bhagwagar, Murthy, Selvaraj, Hinz, Taylor and Fancy16 The 90-minute three-dimensional dynamic emission scan following injection of radiotracer was acquired in list mode. In the post-acquisition frame rebinning, 28 time frames of increasing length were generated (30 s background frame prior to the injection, then one 15 s frame, one 5 s frame, one 10 s frame, three 30 s frames, three 60 s frames, three 120 s frames, three 180 s frames, eight 300 s frames and four 450 s frames).
The arterial plasma input function for each scan was derived from continuous online whole blood monitoring (initial 28 min) and ten discrete blood samples, in eight of which the fraction of unmetabolised parent compound was determined. Reference Hinz, Selvaraj, Murthy, Bhagwagar, Taylor and Cowen17 Calculations were performed using Matlab (The MathWorks Inc., Natick, Massachusetts, USA) on Sun Ultra™ 10 workstations (Sun Microsystems, Inc., Santa Clara, California, USA).
Magnetic resonance scans and definition of volumes of interest
All volunteers had a structural T1 magnetic resonance imaging (MRI) scan performed on either 0.5 T (0.5 Apollo system, Marconi Medical Systems, Cleveland, Ohio) (repetition time (TR)=30 s, echo time (TE)=3 s, flip angle=30°, number of signal averages (NSA)=1, voxel dimensions 0.98 mm × 1.65 mm × 1.6 mm, acquisition time=13 min) or 1.5 T (1.5 Eclipse system, Marconi Medical Systems, Cleveland, Ohio) scanners (TR=30 s, TE=3 s, flip angle=30°, NSA=1, voxel dimensions 0.98 mm × 1.6 mm × 1.6 mm, acquisition time=13 min) or 3 T (3T Intera Philips Medical Systems) scanners (TR=9.6 s, TE=4.6 s, flip angle=8°, NSA=1, voxel dimensions 0.94 mm × 0.94 mm × 1.2 mm). The scans were inspected by an independent neuroradiologist and found to be normal.
Magnetic resonance images were resliced to a voxel size of 1 mm × 1 mm × 1 mm; centred on anterior commissure (AC) and aligned to the AC–PC (posterior commissure) line. The MRIs were co-registered to the individual summated PET images using SPM2, which adopts a rigid body transformation using a normalised mutual information method. Regions of interest (ROI) were chosen on the basis of a previous [11C]DASB study by McCann et al; Reference McCann, Szabo, Seckin, Rosenblatt, Mathews and Ravert18 they were: amygdala, caudate, anterior and posterior cingulate cortex, dorsal raphe, frontal cortex, hippocampus, insula, putamen, thalamus and cerebellum (the latter as a reference region). As a result of the difficulties in reliable quantification of [11C]DASB in neocortical regions (relatively low signal:noise ratio), the analysis was restricted to the frontal cortex only. Regions of interest were defined on the co-registered MRI with the help of a probabilistic brain atlas template Reference Hammers, Koepp, Free, Brett, Richardson and Labbé19 except for the dorsal raphe. The dorsal raphe region was manually defined as a fixed size region (900 mm3) on the summed PET images of each individual. Standard MNI T1 template (available in the SPM2) was normalised to the co-registered individual MRI and the deformation parameters were applied to the probabilistic atlas. This normalised brain atlas was then resliced to PET space and segmented to obtain PET data from grey matter only. Cerebellar grey matter was used to obtain estimates of the free and non-specific radioligand binding because of the very low density of 5-HTT in the cerebellum. Reference Kish, Furukawa, Chang, Tong, Ginovart and Wilson20 Dynamic PET scans were sampled by applying the individual ROI object maps and regional time–activity curves were created. Time–activity curves were manually quality checked in all the scans.
Quantification of DASB binding
To quantify the binding of [11C]DASB in brain tissue, the graphical analysis of reversible radioligand binding using the plasma input function Reference Logan, Fowler, Volkow, Wolf, Dewey and Schlyer21 was applied to obtain regional estimates of the total volume of distribution V T from the slope of the linear part of the plot. Reference Hinz, Selvaraj, Murthy, Bhagwagar, Taylor and Cowen17 The threshold was set at 35 min, i.e. the transformed data from frames 20 to 28 were fitted to a straight line.
Estimates of the binding potential BP ND in target regions of interest were calculated indirectly from the estimated V T in those regions and the V T estimate in the cerebellum, Reference Innis, Cunningham, Delforge, Fujita, Gjedde and Gunn22

where BP ND refers to the specific binding compared with the non-displaceable uptake. To also assess [11C]DASB binding on a pixel level, spectral analysis was used to generate parametric maps of VT. Reference Cunningham and Jones23 A set of 100 basis functions logarithmically spaced between βmin=0.0007/s and βmax=0.1/s was chosen to obtain unbiased estimates of V T for the specific kinetics of [11C]DASB. Reference Hinz, Selvaraj, Murthy, Bhagwagar, Taylor and Cowen17 Individual V T images were then normalised to the standard MNI T1 template and average V T images for the three cohorts were calculated (online Fig. DS1).
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS Inc., Chicago, Illinois, USA) version 14 for Windows. Our primary hypothesis was that regional 5-HTT receptor binding potential would be lower in the MDMA group compared with the other two groups. We tested this using a repeated measures analysis of variance (ANOVA) with ‘group’ as the between-participants factor, and ‘region’ (brain regions of interest) as the within-participant factor with age as a covariate. Huynh–Feldt correction was applied as appropriate where the assumption of sphericity was violated (uncorrected degrees of freedom are shown for clarity). Other comparisons were made using one-way ANOVA with post hoc t-tests (two-tailed).
Results
The age was significantly different between the groups (ANOVA F=3.76, d.f.=2,39, P=0.03; Table 1). The polydrug group were older than the other two groups (mean age (range): drug-naive group 30.5 (25–45); MDMA group 28.2 (25–36); polydrug group 35.6 (25–50)). Age was therefore used as a covariate in all further analyses.
SERT binding
There were no significant differences in cerebellar volume of distribution V T (F=0.76, d.f.=2,39, P=0.47) (10.63 (s.d.=1.4) (ml plasma)/(cm3 extravascular tissue) in the drug-naive group; 10.43 (s.d.=2.3) (ml plasma)/(cm3 extravascular tissue) in the MDMA group and 11.36 (s.d.=1.7) (ml plasma)/(cm3 extravascular tissue) in the polydrug group. The injected [11C]DASB dose (one-way ANOVA F=2.18, d.f.=2,39, P=0.13), or [11C]DASB specific activity (F=0.07, d.f.=2,39, P=0.93) was not significantly different between the groups.
The repeated measures ANOVA showed a significant main effect of brain region (F=7.84, d.f.=9,324, P=0.002) but no main effect of group (F=1.22, d.f.=2,36, P=0.31) or region × group interaction (F=0.36, d.f.=18,324, P=0.81). There was no significant main effect of age (F=0.18, d.f.=1,36, P=0.66) or age × region interaction (F=0.37, d.f.=9,324, P=0.65). The mean binding potentials of the three groups in all the ROIs studied are shown in Fig. 1. There were no significant correlations observed between any variables of MDMA use and [11C]DASB binding.
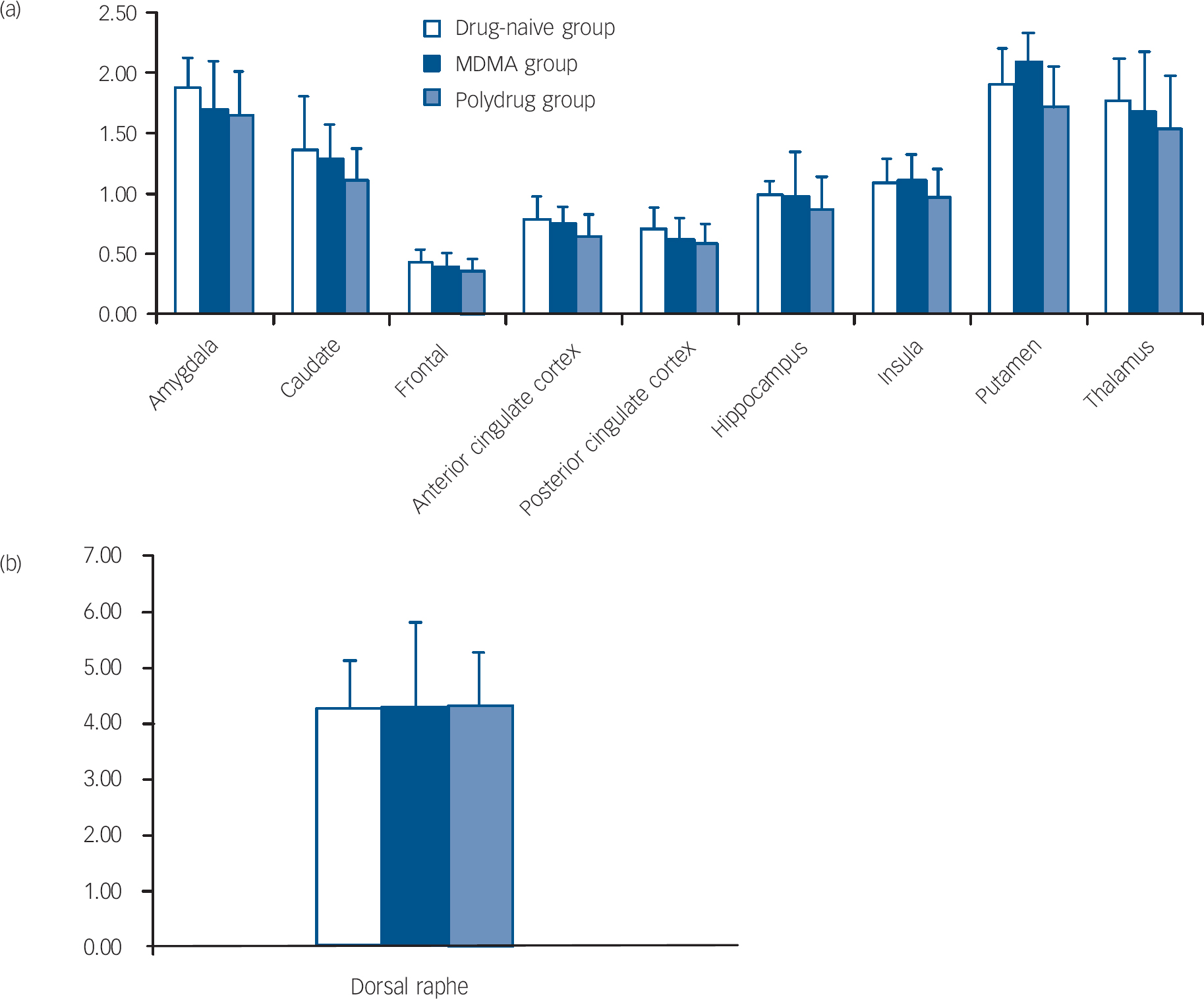
Fig. 1 Binding potential BP ND of [11C]DASB in the drug-naive, MDMA and polydrug groups in different regions of the brain. (a) and (b) error bars represent within-group standard deviation.
We found no significant differences between the MDMA group compared with the polydrug group in any of the illicit drugs studied, with or without Bonferroni correction for multiple comparisons. There were no significant group differences in depression (BDI), anxiety (STAI), neuroticism (Eysenck score) or premorbid IQ (Spot-the-Word Test) (P>0.2).
Discussion
The main finding of this study is that former MDMA users abstinent from MDMA for a minimum of 1 year show no differences in SERT availability compared with drug-naive controls or matched polydrug controls who had never used MDMA. The results can be explained in two ways. Either former MDMA users do not show any difference in SERT binding during MDMA use and this remains the case after abstinence, or a period of prolonged abstinence from MDMA use allows a recovery of SERT availability.
The second explanation would clearly seem more likely given the large body of preclinical and some clinical studies consistently showing neurotoxic effects of MDMA on brain serotonin transporters with current MDMA use. Reference Green, Mechan, Elliott, O'Shea and Colado8,Reference Reneman, de Win, van den Brink, Booij and den Heeten24 Thus, our findings support and extend the results of previous studies indicating possible recovery of serotonin transporters following cessation of MDMA use. Reference Buchert, Thomasius, Wilke, Petersen, Nebeling and Obrocki25–Reference Thomasius, Petersen, Buchert, Andresen, Zapletalova and Wartberg28
The power analysis suggests that with this study population we would have been able to detect a change of 15–20% in the binding potential estimates in most of the brain regions studied at 80% power and P<0.05. This suggests that our study was sufficiently powered to detect differences of the order usually reported between current MDMA users and drug-naive controls in ligand imaging studies of the 5-HTT.
As a result of the limitations of retrospective design of the study, it is difficult to control for pre-existing differences in SERT binding between the MDMA and control groups. However, Thomasius et al Reference Thomasius, Zapletalova, Petersen, Buchert, Andresen and Wartberg27 recently followed up a series of both current and former MDMA users twice at 9- to 12-month intervals after original testing assessing 5-HTT binding with PET using [11C](+)McN5652. The former users showed no significant differences in 5-HTT binding compared with control participants on any of the test sessions with no changes in binding in former users over time. However, current MDMA users had reduced SERTat baseline (compared with the drug-naive control group) but showed a slight increase in SERT in mid-brain binding over follow-up, despite the fact they had continued to use MDMA, albeit in reduced quantities. The authors, combining these results, conclude that the effects of MDMA use on SERT availability may be reversible.
However, preclinical research does suggest that MDMA can cause persisting serotonergic damage. For example, Hatzidimitriou et al Reference Hatzidimitriou, McCann and Ricaurte9 investigated SERT densities in primates 7 years after MDMA administration and found that many brain areas, especially the neocortex, hippocampus, amygdala and cingulate cortex still showed reductions in SERT, whereas the globus pallidus had become hyper-innervated and showed greater levels of 5-HT axonal markers than control animals. Fischer et al Reference Fischer, Hatzidimitriou, Wlos, Katz and Ricaurte29 reported similar results, and found that altered re-innervation patterns were more pronounced in primates than in rats.
This contrast between the animal and the human studies could be explained by several factors. It is possible that a subcutaneous dose regimen of 5 mg/kg twice daily for 4 consecutive days, used for example in Hatzidimitriou et al, Reference Hatzidimitriou, McCann and Ricaurte9 has higher neurotoxicity potential than the oral doses used recreationally by the former users in the present study. Although the authors claim interspecies scaling indicates their doses could lie in the range of human recreational doses, a recent study found no changes in serotonergic function in rhesus monkeys that self-administered MDMA Reference Fantegrossi, Woolverton, Kilbourn, Sherman, Yuan and Hatzidimitriou30 at a rate of 2–4 mg/kg three times per week – a dose regime more comparable to the way in which recreational MDMA users ‘self-administer’ the drug. Reference Verheyden, Henry and Curran31 Alternatively, the longitudinal study of Thomasius et al Reference Thomasius, Zapletalova, Petersen, Buchert, Andresen and Wartberg27 could indicate a greater resilience to MDMA-induced serotonergic injury in humans compared with non-human primates.
It is important to note that in this study we included only males. Reneman et al Reference Reneman, Booij, de Bruin, Reitsma, de Wolff and Gunning32 suggest from a small-scale study that females are more susceptible to MDMA-related neurotoxicity than males. It is therefore possible that in female participants we might have detected a persistent effect of MDMA to lower [11C]DASB binding to the 5-HTT. Another limitation is that we only measured SERT binding and normal availability of SERT binding sites might not exclude changes in other aspects of 5-HT function, for example 5-HT synthesis. Finally, it is possible that normal levels of SERT binding might conceal altered patterns of 5-HT innervation consequent upon neuronal damage and regeneration.
In summary, this study supports previous research indicating no differences in [11C]DASB binding to the serotonin transporter in former MDMA users compared with drug-naive controls indicating possible recovery of 5-HT function after cessation of MDMA.
Funding
This study was supported by the Medical Research Council (MRC). R.H. was supported by an MRC studentship.





eLetters
No eLetters have been published for this article.