Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an ongoing pandemic. In the absence of universally available approved vaccines and evidence-based pharmacological therapy for the disease,Reference Wiersinga, Rhodes, Cheng, Peacock and Prescott1 it is crucial that governments and clinical practitioners identify vulnerable populations and establish specific prevention and treatment strategies. To date, older age, obesity, smoking, cardiovascular disease, diabetes, chronic obstructive pulmonary disease and hypertension have been identified as risk factors for COVID-19.Reference Zhou, Yu, Du, Fan, Liu and Liu2–Reference Grasselli, Greco, Zanella, Albano, Antonelli and Bellani4 However, there is little epidemiological evidence on the effect of mental disorders, despite raised concerns about increased risk of COVID-19 among people with mental illness.Reference Druss5,Reference Yao, Chen and Xu6
People with mental disorders may be more vulnerable to viral or bacterial infection than those without these disorders, since various risk factors, such as unhealthy lifestyle and poor socioeconomic background, are often more prevalent in this population.Reference Seminog and Goldacre7,Reference Hughes, Bassi, Gilbody, Bland and Martin8 These individuals’ low cognitive ability and poor awareness of risk overall may also increase the infection risk.Reference Yao, Chen and Xu6 Moreover, once infected, mentally ill patients may also have a higher risk of severe adverse outcomes, because of their communication difficulties and physicians’ discrimination against or negative attitude towards them. These problems may delay medical interventions for COVID-19, worsening prognosis.Reference Momen, Plana-Ripoll, Agerbo, Benros, Børglum and Christensen9,Reference Bailey, Wirtalla, Sharoky and Kelz10 Furthermore, such individuals tend to be highly susceptible to stress, and excessive stress caused by restriction of social activities and fear of the epidemic may lead to suppressed immune responses.Reference Segerstrom and Miller11
Aims
Given that mental disorders are highly prevalent worldwide (pooled prevalence estimate: 17.6% across 59 countries),Reference Steel, Marnane, Iranpour, Chey, Jackson and Patel12 investigating the relationship between mental disorders and SARS-CoV-2 infection and severity is important for public health. Here, we assessed the association between mental disorders and the risk of SARS-CoV-2 positivity. We also evaluated the risk of death and severe events (defined as intensive care unit (ICU) admission, use of mechanical ventilation and acute respiratory distress syndrome) among confirmed COVID-19 patients with mental disorders, using a nationwide cohort of people who underwent testing for SARS-CoV-2.
Method
Study design and data source
We conducted a population-based cohort study using the National Health Insurance claims data from the Health Insurance Review & Assessment service (HIRA) linked to the Korea Disease Control and Prevention Agency (KDCA) data. Data were collected up to 15 May 2020 and included demographic and clinical information and a 3-year medical history of people who underwent COVID-19 screening during the pandemic. Information on confirmed COVID-19 patients and those who died from COVID-19 was retrieved from the KDCA data to improve the internal validity of the database. Clinical information included disease diagnosis, procedures, in-patient medication orders and prescriptions from all medical institutions in Korea. Disease information was recorded according to ICD-10. Information on the use of these anonymised data can be obtained from https://hira-covid19.net/.
Study population
We constructed two study cohorts: (a) a cohort of people with or without mental disorders who underwent testing for SARS-CoV-2 from 1 December 2019 to 15 May 2020, to investigate the risk of testing positive for SARS-CoV-2 and (b) a subcohort of confirmed COVID-19 patients from among the first cohort, to assess the risk of mortality and severe events following COVID-19. Laboratory confirmation of SARS-CoV-2 was based on diagnostic reverse transcription polymerase chain reaction (RT-PCR) tests, as recommended by the World Health Organization.
Assessment of mental disorders
We classified people who had a diagnostic code for mental and behavioural disorders (ICD-10 codes: F00–F99) at least once within 6 months prior to the first date of COVID-19 testing as individuals with mental disorders, and the rest as individuals without mental disorders. We selected a 6-month period to examine whether having psychiatric symptoms or mental disorder characteristics at the point of potential exposure to the virus affected the risk of infection or severity of outcomes. Individuals with mental disorders were matched with four controls without mental disorder diagnoses, according to age (±2 years), gender and Charlson Comorbidity Index (CCI), which was assessed over 1 year before the first COVID-19 testing. This matching was conducted separately for the overall cohort and the subcohort.
Outcome ascertainment
Confirmed COVID-19 patients were ascertained from the KDCA database. To measure COVID-19 severity, we identified two end-points: death (primary end-point) and severe events (secondary end-point). Death cases were also identified using the KDCA variable, which defined a death case as all-cause death in a patient with laboratory-confirmed SARS-CoV-2 infection. We designated three health outcomes after infection as severe events: ICU admission (National Procedure Codes (NPC): AH190, AH290, AH390, AH110, AH210, AJ001, AJ003, AJ004, AJ005, AJ006, AJ007, AJ008, AJ009, AJ100, AJ200, AJ101, AJ102, AJ201 and AJ202), use of mechanical ventilation (NPC: M0850, M0857, M0858, M0860, M5830, M5850, M5857, M5858, M5860, MM360 and MM400) and acute respiratory distress syndrome (ICD-10: J80 and P22).
Potential confounders
Participants’ demographic information (type of insurance and residential area) and clinical baseline characteristics (comorbidities and use of medications) during 1 year prior to the first date of SARS-CoV-2 testing were considered as potential confounders, for which adjustments were made in a statistical model. We selected comorbidities that may be associated with mental disorders and that could be risk factors for infection or worse prognosis, as follows: diabetes (E10–E14), hypertension (I10–I15), heart failure (I11.0, I13.0, I13.2, I42 and I50), stroke (I60–64), myocardial infarction (I21–22 and I252), asthma (J45–J46), chronic obstructive pulmonary disease (J41–J44), renal disease (I12, I13.1, N03.2–N03.7, N05.2–N05.7, N18, N19, N25.0, Z49.0–Z49.2, Z94.0 and Z99.2), liver disease (B15–19, K70, K71.3–K71.5, K71.7, K72.1, K72.9, K73, K74 and K76), cancer (registration codes: V193, V194 and V027) and pneumonia (J12–J18). The use of medications that were likely to exert confounding effects was considered: angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, calcium channel blockers, thiazide diuretics, anticoagulants, anticonvulsants, digoxin, insulin, non-insulin glucose-lowering agents, non-steroidal anti-inflammatory drugs, acetaminophen and narcotic analgesics.
Statistical analysis
Continuous variables are described as mean and standard deviation (s.d.) and categorical variables are described by frequency and percentage. We calculated the standardised difference to compare the distribution of baseline characteristics between the group with and the group without mental disorders. Standardised differences greater than 0.1 were considered as imbalances of covariates.
We computed the percentages of individuals who were SARS-CoV-2 positive among individuals with and without mental disorders, using a cohort of individuals who underwent a SARS-CoV-2 test before and after matching. Odds ratios (ORs) for SARS-CoV-2 infection and their 95% confidence intervals (CIs) were calculated using a logistic regression model for the unmatched cohort and a conditional logistic regression model for the age-, gender- and CCI-matched cohorts, with adjustment for insurance type (health insurance or medical aid), residential area (metropolitan, urban or rural), comorbidities and the use of medications.
To examine the effect of mental disorders on COVID-19 severity, we calculated the percentages of individuals who died or experienced severe events among patients with a positive SARS-CoV-2 test. We analysed data obtained before and after matching, according to the presence or absence of a mental disorder. Likewise, logistic regression and conditional logistic regression analyses were used for the unmatched and matched cohorts respectively to estimate ORs for mortality and severe events. We adjusted ORs for the same variables used in the analysis of the risk of a positive SARS-CoV-2 test.
Subgroup analyses were conducted to estimate the risk of SARS-CoV-2 infection and severe COVID-19 in individuals with two pre-specified categories of ICD-10 mental disorder (schizophrenia, schizotypal and delusional disorders (F20–F29) and mood disorders (F30–F39)) as compared with individuals without mental disorders. These two conditions were selected as severe mental disorders; the lifestyles and symptoms of individuals with these diseases may have different effects on the risks. Additionally, we also classified and analysed individuals by recent use (within 30 days prior to their first SARS-CoV-2 test) of antipsychotics, since some antipsychotics exert immunomodulatory effects that could negatively affect COVID-19 prognosis.Reference Pollmächer, Haack, Schuld, Kraus and Hinze-Selch13 For subgroup analyses on the risk of severe outcomes among confirmed COVID-19 patients, we adjusted for only some variables as covariates in the model, to avoid problems of multicollinearity and overfitting, particularly when the number of potential confounders is large with respect to the study size.Reference Chen, Nian, Zhu, Talbot, Griffin and Harrell14,Reference Greenland, Daniel and Pearce15 We selected variables that were regarded as clinically important, remained unbalanced between the two groups after matching and were more prevalent in patients with than in those without mental disorders (insurance type, diabetes, pneumonia, use of beta-blockers and anticonvulsants).
As a sensitivity analysis, we applied various definitions of individuals with mental disorders: (a) individuals with ≥2 diagnostic codes for mental and behavioural disorders within 6 months prior to their first COVID-19 test; (b) individuals who had a record of a diagnostic code and psychiatric medication within 6 months; (c) individuals with a diagnostic code within 30 days; and (d) individuals with a diagnostic code within 1 year. We also calculated the E-value to assess the robustness of the association between mental disorders and COVID-19 mortality to potential unmeasured confounders. The E-value is the minimum strength of association that an unmeasured confounder would need to have with both the treatment and the outcome on the risk ratio scale to explain away the observed treatment–outcome association.Reference VanderWeele and Ding16 If the calculated E-value is large, strong unmeasured confounders would be needed to explain away an effect estimate fully.
All analyses were conducted using the SAS Enterprise Guide version 6.1 for Windows provided by the HIRA (SAS Institute Inc., Cary, NC, USA). Two-sided P-values <0.05 were considered statistically significant.
Ethics approval
We assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human participants/patients were approved by the Institutional Review Board of the Sungkyunkwan University in Korea (IRB number: SKKU-2020-05-012). The need to obtain informed consent from participants was waived owing to the nature of this observational study.
Results
Characteristics of the study population
A total of 230 565 individuals had undergone a laboratory test for SARS-CoV-2 as of 15 May 2020. Among them, 33 653 (14.6%) had mental disorders and 196 912 (85.4%) did not (Fig. 1). The mean ages of those with and without mental disorders were 62.4 years (s.d. = 21.4) and 44.5 years (s.d. = 20.6) respectively (supplementary Table 1, available at https://doi.org/10.1192/bjp.2020.251). For 24 558 individuals with mental disorders, 97 966 controls were matched by age, gender and CCI. In the matched cohort, the mean age of the two groups was about 55 years, and 45.7% were male (Table 1). We identified 7077 people with confirmed COVID-19 in the first study cohort. Of these COVID-19 patients, 928 (13.1%) had mental disorders and 6149 (86.9%) had no mental disorders. After matching, there were 734 patients in the mental disorder group and 2817 in the reference group. The baseline covariates of the matched cohort were relatively well-balanced compared with those of the unmatched cohort.

Fig. 1 Flowchart of study population selection from Korea's nationwide coronavirus disease 2019 (COVID-19) database from 1 December 2019 to 15 May 2020.
Matching, each individual with a mental or behavioural disorder was matched by age, gender and Charlson Comorbidity Index with up to four individuals without mental disorders; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1 Baseline characteristics of individuals with and without mental disorders who underwent tests for SARS-CoV-2 and those who were diagnosed with COVID-19 after age, gender and Charlson Comorbidity Index matching

SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; aSD, absolute standardised difference; COPD, chronic obstructive pulmonary disease; ACE, angiotensin-converting enzyme inhibitors; NSAIDs, non-steroidal anti-inflammatory drugs.
a. Hospital admission and treatment for COVID-19 were assessed from the cohort entry date to the end of the study period.
b. Comorbidities and the use of medications were assessed for the year before the cohort entry date.
The risk of SARS-CoV-2 positivity in individuals with mental disorders
In the unmatched cohort, 2.76% (928) individuals with mental disorders tested SARS-CoV-2 positive, compared with 3.12% (6149) without mental disorders (Table 2). The adjusted OR for a SARS-CoV-2-positive test in patients with mental disorders was 0.99 (95% CI 0.92–1.07) compared with those without mental disorders. After matching, the percentage of SARS-CoV-2-positive patients in both groups was approximately 3.0%, and the fully adjusted OR was 0.95 (95% CI 0.87–1.04).
Table 2 Risk of a positive test for SARS-CoV-2 and severe outcomes following COVID-19 in individuals with mental disorders compared with those without mental disorders

SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; ref. reference.
a. Number of events/number of individuals ×100.
b. Adjusted for type of insurance, residential area, comorbidities and the use of medications.
c. The cohort matched by age, gender and Charlson Comorbidity Index.
d. Defined as intensive care unit (ICU) admission, use of mechanical ventilation and acute respiratory distress syndrome.
The risk of severe COVID-19 in individuals with mental disorders
Among the 928 COVID-19 patients with mental disorders, 56 (6.03%) died and 44 (4.74%) experienced severe events (Table 2). The percentages of COVID-19 patients without mental disorders who died or had severe events were 0.89 and 2.11% respectively. The ORs of death and severe events were 3.93 (95% CI 2.57–6.03) and 1.47 (95% CI 0.99–2.19) respectively. In the matched cohort, 27 (3.68%) of the 734 COVID-19 patients with mental disorders and 49 (1.74%) of the 2817 COVID-19 patients without mental disorders died. Compared with patients without mental disorders, the risk of death was increased in multivariable analysis (OR = 1.99; 95% CI 1.15–3.43). The percentage of patients who experienced severe events was 4.36% (32/734) in the mental disorders group and 3.48% (98/2817) in the reference group. The association between mental disorders and severe events was not statistically significant (OR = 1.15; 95% CI 0.73–1.82).
Subgroup analysis
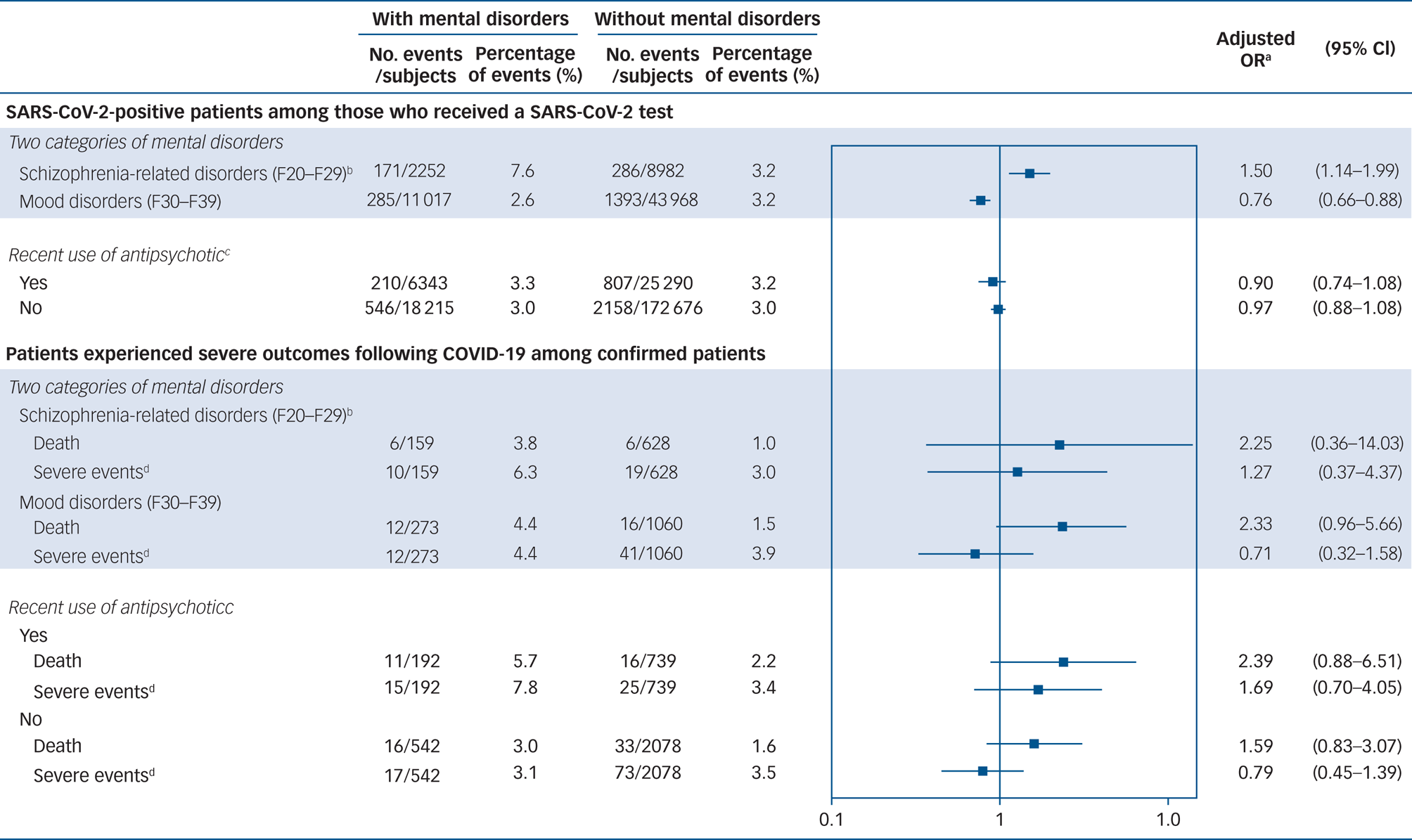
When we repeated the analysis on the subgroup of individuals with schizophrenia, schizotypal or delusional disorders or mood disorders, the adjusted ORs for the risk of SARS-CoV-2 infection were 1.50 (95% CI 1.14–1.99) in those with schizophrenia, schizotypal or delusional disorders and 0.76 (95% CI 0.66–0.88) in those with mood disorders (Fig. 2). The risk of mortality and severe events after SARS-CoV-2 infection was higher in individuals with schizophrenia, schizotypal or delusional disorders than in those without mental disorders, but the effect was not statistically significant (death: adjusted OR = 2.25; 95% CI 0.36–14.03; severe events: adjusted OR = 1.27; 95% CI 0.37–4.37). Compared with those without mental disorders, COVID-19 patients with mood disorders had a higher risk of death (adjusted OR = 2.33; 95% CI 0.96–5.66) but not of severe events (adjusted OR = 0.71; 95% CI 0.32–1.58).

Fig. 2 Forest plot summarising the results of subgroup analyses by two categories of mental disorder and the recent use of antipsychotics after age, gender and Charlson Comorbidity Index matching.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019. a. The risks of a positive test for SARS-CoV-2 were adjusted for the type of insurance, residential area, comorbidities and the use of medications. The risks of death and severe events were adjusted for the type of insurance, a medical history of diabetes and pneumonia, and use of beta-blockers and anticonvulsants. b. Schizophrenia, schizotypal and delusional disorders. c. Antipsychotic use within 30 days prior to a first SARS-CoV-2 test. d. Defined as intensive care unit (ICU) admission, use of mechanical ventilation and acute respiratory distress syndrome.
In the analysis based on the recent use of antipsychotics, no increased risk of SARS-CoV-2 infection was observed in either group: adjusted OR = 0.90 (95% CI 0.74–1.08) in individuals with mental disorders who used antipsychotics recently; adjusted OR = 0.97; 95% CI 0.88–1.08 in individuals with mental disorders who did not use antipsychotics. In terms of severe outcomes, the risks of death were higher, regardless of the use of antipsychotics, although these risks were not statistically significant: adjusted OR = 2.39 (95% CI 0.88–6.51) in COVID-19 patients who used antipsychotics; adjusted OR = 1.59 (95% CI 0.83–3.07) in those who did not. Additionally, patients with mental disorders who used antipsychotics were also likely to have a higher risk of severe events (adjusted OR = 1.69; 95% CI 0.70–4.05), whereas the risk was not higher among those who did not use antipsychotics (adjusted OR = 0.79; 95% CI 0.45–1.39).
Sensitivity analysis
The results of analyses using the four different definitions of individuals with mental disorders were mostly consistent with those from the main analysis (supplementary Fig. 1). No increased risk of SARS-CoV-2 infection was observed, but the risks of death and severe events were consistently higher in patients with than in those without mental disorders, regardless of the definition used. The E-value obtained from the estimate of mortality risk was 3.39.
Patient and public involvement
Patients and the public could not be involved in the design, conduct, reporting or dissemination plans of our research, since we used de-identified patient data.
Discussion
Evidence from this nationwide cohort study suggested that mental disorders are not associated with the risk of SARS-CoV-2 infection but are likely to be associated with a higher mortality risk on infection. As regards severe events, we found no evidence of increased risk among confirmed COVID-19 patients with, compared with those without, mental disorders. The risk of a SARS-CoV-2-positive test was higher in individuals with schizophrenia, schizotypal or delusional disorders than in those without mental disorders. The COVID-19-related mortality risk was higher regardless of the two pre-specified types of mental disorder (schizophrenia, schizotypal and delusional disorders; mood disorders) and recent use of antipsychotics, although not statistically significant.
Our data showed an increased relative mortality risk among individuals with mental disorders who have had COVID-19. This finding is in line with a study that found a significant relationship between mental disorders and cause-specific mortality, including death related to infectious diseases.Reference Plana-Ripoll, Pedersen, Agerbo, Holtz, Erlangsen and Canudas-Romo17 The positive association between mental disorders and excess mortality from medical conditions is known to stem from multiple factors. Since mental illness is generally associated with an unhealthy lifestyle (e.g. smoking and alcohol consumption) and a low socioeconomic status,Reference Bellos, Skapinakis, Rai, Zitko, Araya and Lewis18–Reference McLaughlin, Costello, Leblanc, Sampson and Kessler20 these factors may also have contributed to a worse prognosis of COVID-19. Furthermore, health inequality in people with mental illness has been suggested as an important contributor to poor physical health outcomes.Reference Lawrence and Kisely21,Reference Wang, Hsieh and Wang22 Stigma and discrimination towards mental illness and poor communication skills of people with mental disorders may hamper proper and timely provision of medical intervention for COVID-19 and therefore cause more severe health outcomes.
Additionally, the use of antipsychotics may increase the risk of death or severe events. Some antipsychotic medications have immunomodulatory effects:Reference Pollmächer, Haack, Schuld, Kraus and Hinze-Selch13 chlorpromazine and clozapine can affect the production of cytokines, resulting in suppression of the immune response. However, in the subgroup analysis, we found a higher risk of death in COVID-19 patients with than in those without mental disorders, irrespective of the use of antipsychotics. Although the effects of antipsychotics cannot be completely excluded, our findings suggest that having a mental disorder alone may increase the risk of mortality following COVID-19.
Previous studies found a positive association between severe mental illness and microbial or viral infection (pneumonia, human immunodeficiency virus, hepatitis B and hepatitis C).Reference Seminog and Goldacre7,Reference Hughes, Bassi, Gilbody, Bland and Martin8 In our study, individuals with schizophrenia-related disorders were at higher risk of SARS-CoV-2 infection, although no association was observed among individuals with mental disorders overall. People with schizophrenia-related disorders may be more vulnerable to SARS-CoV-2 infection than those with other mental disorders because they are more likely to live in shared accommodation such as psychiatric hospitals. Unlike patients in general hospitals, those in psychiatric hospitals commonly participate in group activities, creating conditions favourable for virus transmission. Additionally, teaching such patients to follow personal control measures would be difficult, given their impaired cognitive ability. In contrast, we found a decreased risk of infection in people with mood disorders compared with those without mental disorders. One possible explanation may be that people with mood disorders tend to engage in fewer social activities than would healthy individuals, reducing the chance of exposure to the virus. Indeed, COVID-19 has spread among young and healthy people owing to the highly contagious nature of the virus, although the fatality rate is low.Reference Ashour, Elkhatib, Rahman and Elshabrawy23,Reference Stone24
Strengths and limitations
Our study had several strengths. We used a nationwide COVID-19 database that includes medical histories of all COVID-19 patients in Korea, which strengthens the generalisability of our results. Moreover, we enhanced the internal validity of our findings by using information on patients with confirmed COVID-19 and those who died from COVID-19 obtained from the KDCA. The Korean government has strictly managed people with confirmed and suspected COVID-19. According to the government's response system, all inbound travellers are monitored and tested if they present with fever or respiratory symptoms. If a person tests positive for SARS-CoV-2, all primary contacts of that person are identified by epidemiological investigation and receive a test if they exhibit symptoms during a 14-day self-quarantine period. COVID-19 patients must be admitted to infectious disease hospitals or accommodated in residential treatment centres, depending on their disease severity, and receive regular check-ups until they meet the criteria for discharge. Therefore, underestimation of the number of patients with confirmed COVID-19 and the number who died would be trivial in this study. Furthermore, owing to the strict patient management system in Korea, confounding effects by differences in access to medical facilities or support between patients with and without mental disorders would be minor. People with mental disorders may have different medical behaviours than those without such disorders, which may cause a difference in medical access; however, the difference would not exist in the study cohort since all confirmed COVID-19 patients were placed in quarantine facilities and managed by the government.
This study also had some limitations. First, covariates regarding lifestyle (smoking status and alcohol consumption) and socioeconomic status (education and income level) of participants were not included in the analytic model. The National Health Insurance database was constructed on the basis of claims data; therefore, these variables were not available. Although unmeasured confounders are associated with worse outcomes after SARS-CoV-2 infection, the results of the sensitivity analysis suggest that the observed OR of 1.99 could be explained away only by an unmeasured confounder associated with both mental disorder and death that had a 3.39-fold risk ratio. Hence, our findings are robust unless an unmeasured confounder of such magnitude exists. Second, we identified severe events on the basis of diagnostic and procedural codes, which were recorded for administrative purposes; thus, there was potential for misclassification of outcomes. However, a validation study comparing the claims database and in-patients’ hospital medical records reported that the overall agreement of diagnosis was 82.0%.Reference Park, Jang, Jeon, Lee, Lee and Choi25 Procedural codes that are directly related to payment from National Health Insurance are also likely to be highly valid. Third, the risk of SARS-CoV-2 infection might be biased owing to the different probabilities of receiving the test between populations with and without mental disorders. If people with mental disorders were tested more in the absence of any symptoms of COVID-19, for preventive purposes, the risk of SARS-CoV-2 infection may be underestimated. However, it is unlikely that these people would have been included in our study because only people who had an epidemiological link or showed clinical symptoms of COVID-19 could receive a free test for SARS-CoV-2, covered by National Health Insurance in Korea. The percentage of people with mental disorders among people who received a test for SARS-CoV-2 was higher (14.6%) than among the general population in 2019 (6.5%, calculated using national statistics on the number of people who have records of mental and behavioural disorders from the Healthcare Bigdata Hub website of the HIRA). However, this may be due to several outbreaks of cluster infection at nursing homes or psychiatric hospitals, rather than these people being tested more commonly for preventive purposes. Last, although we utilised a database covering overall COVID-19 patients in Korea, the number of patients with mental disorders was not sufficient to evaluate the risk of death and severe events by subgroup analysis based on more subdivided disorder types, such as depression, anxiety and dementia. Further studies are needed to determine whether people with particular mental disorders have a higher risk of SARS-CoV-2 infection and severe COVID-19.
Implications
Although we found no association between overall mental disorders and the risk of SARS-CoV-2 infection among individuals who were tested, the risk was significantly higher in people with schizophrenia-related disorders than in those without mental disorders. Our results also suggest that mental disorders are likely associated with an increased risk of death following COVID-19. Psychiatrists should therefore inform patients and their caregivers about the risk of SARS-CoV-2 infection and guide them to comply with preventive measures. Furthermore, clinicians and healthcare policy makers need to pay more attention to patients with mental disorders during the COVID-19 pandemic and establish preventive strategies for them.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2020.251
Data availability
The data that support the findings of this study are available from the Health Insurance Review & Assessment service (HIRA) in South Korea. Restrictions apply to the availability of these data, which were used under licence for this study.
Acknowledgements
We thank the healthcare professionals dedicated to treating COVID-19 patients in Korea, the Ministry of Health and Welfare, and the HIRA of South Korea for sharing invaluable national health insurance claims data in a prompt manner. We especially thank Do-Yeon Cho and Yujin Lee of the HIRA for executing the analysis software. We also thank Ju Hwan Kim of Sungkyunkwan University and Editage (www.editage.co.kr) for English language editing.
Author contributions
H.-L.J. and J.-Y.S. designed the study. S.-H.P. analysed the data. H.-L.J., S.-H.P. and J.S.K. interpreted the data. H.-L.J. wrote the first draft. J.S.K. and J.-Y.S. critically revised the draft. All authors read and approved the final manuscript. As guarantor and corresponding author, J.-Y.S. had full access to the data, takes responsibility for the integrity of the data and controls the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HI19C1233).
Declaration of interest
J.-Y.S. has received research grants from the Ministry of Food and Drug Safety, Ministry of Health and Welfare, the National Research Foundation of the Republic of Korea, and pharmaceutical companies, including Amgen, Pfizer, Hoffmann-La Roche, Dong-A ST and Yungjin.







eLetters
No eLetters have been published for this article.