Obsessive-compulsive disorder (OCD) is a severely impairing and often treatment-refractory neuropsychiatric disorder affecting 1-3% of the population. Reference Mancebo, Greenberg, Grant, Pinto, Eisen and Dyck1-Reference Ruscio, Stein, Chiu and Kessler4 Symptoms are characterised by recurrent, ego-dystonic, intrusive thoughts (obsessions) and stereotyped thoughts or behaviours (compulsions). Reference Williams, Farris, Turkheimer, Pinto, Ozanick and Franklin5-Reference Rachman9 Structural and functional aberrations of the corticostriatal thalamocortical (CSTC) circuit are centrally implicated in the pathophysiology of OCD. Reference Maia, Cooney and Peterson10-Reference Modell, Mountz, Curtis and Greden12 These include abnormalities involving cerebral glucose metabolism, Reference Maia, Cooney and Peterson10,Reference Baxter, Schwartz, Bergman, Szuba, Guze and Mazziotta13 grey and white matter volume, Reference Radua, van den Heuvel, Surguladze and Mataix-Cols14 functional connectivity Reference Chamberlain, Menzies, Hampshire, Suckling, Fineberg and del Campo15,Reference Harrison, Soriano-Mas, Pujol, Ortiz, Lopez-Sola and Hernandez-Ribas16 and increased glutamatergic tone. Reference Pittenger, Bloch and Williams17,Reference Chakrabarty, Bhattacharyya, Christopher and Khanna18 Several studies have reported a dose-dependent relationship between treatment-responsiveness in OCD and improvements of these abnormalities. Reference Baxter, Schwartz, Bergman, Szuba, Guze and Mazziotta13,Reference Rosenberg, Benazon, Gilbert, Sullivan and Moore19,Reference Hansen, Hasselbalch, Law and Bolwig20 Upstream causes of CSTC abnormalities are thought to involve a combination of genetic, Reference Stewart, Mayerfeld, Arnold, Crane, O'Dushlaine and Fagerness21,Reference Stewart, Yu, Scharf, Neale, Fagerness and Mathews22 glutamatergic Reference Pittenger, Bloch and Williams17,Reference Wu, Hanna, Rosenberg and Arnold23 and immunological mechanisms. Reference Abramowitz, Taylor and McKay6,Reference Stein7,Reference Rotge, Aouizerate, Tignol, Bioulac, Burbaud and Guehl24-Reference Frick, Williams and Pittenger26 That both obsessive-compulsive symptomatology and basal ganglia dysfunction are common occurrences among a spectrum of neuropsychiatric disorders with a known (Sydenham chorea Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27-Reference Cunningham31 ) or suspected (paediatric acute-onset neuropsychiatric syndrome (PANS), Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Swedo, Leonard and Rapoport32,Reference Swedo, Leonard, Garvey, Mittleman, Allen and Perlmutter33 Tourette syndrome Reference Singer, Giuliano, Hansen, Hallett, Laurino and Benson34,Reference Kansy, Katsovich, McIver, Pick, Zabriskie and Lombroso35 ) autoimmune aetiology, raises the possibility that primary OCD exists along a continuum with these disorders on the basis of overlapping clinical phenomenology and pathophysiological mechanisms respectively. Reference Fibbe, Cath, van den Heuvel, Veltman, Tijssen and van Balkom36-Reference Swedo, Leonard, Casey, Mannheim, Lenane and Rettew41 With regard to the latter, these disorders are thought to result from a molecular mimicry mechanism whereby group A β-hemolytic streptococcal antibodies cross-react with epitopes expressed on the surface of neurons in the basal ganglia, thereby altering neuronal signaling. Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Kirvan, Swedo, Heuser and Cunningham29,Reference Martino, Church and Giovannoni42-Reference Dale, Church, Surtees, Lees, Adock and Harding45 Evidence supporting this hypothesis derives from clinical studies showing increased odds of anti-basal ganglia antibody (ABGA) seropositivity in these disorders, Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27-Reference Kansy, Katsovich, McIver, Pick, Zabriskie and Lombroso35,Reference Sanchez-Carpintero, Albesa, Crespo, Villoslada and Narbona46 and experimental studies demonstrating the deposition of ABGA and an inducible neuropsychiatric phenotype in rats following exposure either to group A streptococci or the serum of individuals with Sydenham chorea or PANS. Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Yaddanapudi, Hornig, Serge, De Miranda, Baghban and Villar47 ABGA isolated from such experiments exhibit high avidity for discrete sets of antigens possessing variable degrees of proteomic sequence overlap with streptococcal surface proteins including lysoganglioside GM1, Reference Kirvan, Swedo, Heuser and Cunningham29,Reference Kirvan, Swedo, Snider and Cunningham30,Reference Kirvan, Swedo, Kurahara and Cunningham43 tubulin, Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Kirvan, Cox, Swedo and Cunningham28 dopamine receptor 1 (DR1) and DR2, Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Dale, Merheb, Pillai, Wang, Cantrill and Murphy48 and the neuronal glycolytic enzymes: aldolase C, neuron-specific gamma-(γ) enolase, non-neuronal alpha-(α) enolase and pyruvate kinase M1. Reference Kansy, Katsovich, McIver, Pick, Zabriskie and Lombroso35,Reference Dale, Candler, Church, Wait, Pocock and Giovannoni49 Although existing evidence supports the presence of basal ganglia dysfunction in primary OCD and implicates ABGA-mediated basal ganglia dysfunction in neuropsychiatric disorders that frequently manifest with obsessive-compulsive symptoms, the relationship between ABGA and primary OCD remains unclear. We aimed to address this uncertainty by pooling data across studies having evaluated ABGA positivity among individuals with primary OCD relative to various controls or neuropsychiatric disorders previously associated with ABGA.
Method
Methods used to perform this meta-analysis were devised in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement Reference Moher, Liberati, Tetzlaff, Altman and Group50 and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. Reference Stroup, Berlin, Morton, Olkin, Wilkinson and Rennie51
Data sources
We performed a standardised electronic database search for published articles indexed within Ovid MEDLINE (1946-2013), PsycINFO (1806-2013) and/or EMBASE (1974-2013) from inception through to March 2013; there were no search limits or restrictions on language (online Table DS1). To identify unpublished data, we searched BIOSIS previews (1969-2013) and Web of Science (1900-2013), applying search limits for ‘conference proceedings’ and ‘meeting abstracts’ to the latter. We supplemented these searches by manually reviewing the reference lists of content-relevant articles.
Study selection
Eligible studies were delimited to those providing data on ABGA positivity in sera and/or cerebrospinal fluid (CSF) among individuals with primary OCD (cases) compared with one or more comparison groups, to include controls (with any clearly defined inclusion criteria) and/or other neuropsychiatric disorders previously associated with ABGA (henceforth ‘ABGA-associated neuropsychiatric disorders’). We required that OCD was diagnosed using a validated approach and that ABGA positivity was assessed using one of the following immunostaining assay techniques: immunohistochemistry, Western blotting, immunocytochemistry, flow cytometry, enzyme-linked immunosorbent assay (ELISA) or immunoprecipitation. We excluded studies for which cases included participants with OCD in the context of ABGA-associated neuropsychiatric disorders in addition to those with primary OCD, unless we were able to extract outcome data separately for participants with primary OCD. We screened titles/abstracts of each unique record identified by our searches to exclude those that were clearly ineligible. We reviewed the full-texts of each remaining record in detail.
Data extraction and synthesis
After determining our eligible studies, we extracted pre-specified data from each onto a standardised form. Extracted data included characteristics of study sources (eligibility criteria, authors, year of publication, study design, country, publication type, sample size), participants (age, gender, duration of illness, disease severity, diagnostic criteria) and immunostaining assay methodology (techniques, dilutions, tissue preparation). We evaluated study quality using the Newcastle-Ottawa Quality Assessment Scale, Reference Wells, Shea, O'Connell, Peterson, Welch and Losos52 which we adapted to address sources of bias specific to our research question (online Table DS2). Specifically, for each study, we rated the following domains as low, unclear, or high risk of bias: (a) adequate definition of cases; (b) representativeness of cases; (c) adequate definition of controls; (d) comparability of the mean age for case and control groups; (e) comparability of the percentage of females in case and control groups; and (f) masking of outcome assessors to sample case/control status.
The main outcomes were ABGA seropositivity (primary) and ABGA CSF-positivity (secondary), which we defined as specific immunoreactivity (as reported by primary study authors) of sample sera or CSF against basal ganglia homogenate, or when this information was not available, against isolated recombinant forms of one or more putative ABGA antigens (lysoganglioside GM1, tubulin, DR1, DR2, aldolase C, γ/α-enolase, pyruvate kinase M1). We extracted dichotomous event data on the odds of ABGA positivity in each study group. We summarised these data using odds ratios (ORs) and 95% confidence interval (CI) point estimates for each comparison of interest (primary OCD v. controls; primary OCD v. each ABGA-associated neuropsychiatric disorder).
We stipulated immunostaining decision-rules to minimise potential biases related to interstudy variability in immunostaining assay methodology used to assess ABGA positivity. First, for studies using more than one immunostaining assay technique, we preferentially used the results derived from immunohistochemistry. In contrast to Western blotting, immunohistochemistry provides cellular morphological and localisation data and maintains proteins in their natural conformation, which increase the likelihood that any observed immunoreactivity reflects specific neuronal antibody-antigen interactions as they might occur in situ. Reference Fritschy53 Second, for studies using immunohistochemistry that reported zero ABGA positivity events in both cells, we substituted the results from Western blotting. Last, we preferentially used results from Western blotting over ELISA based on evidence that the former has superior sensitivity and specificity for ABGA. Reference Martino, Church and Giovannoni42,Reference Church, Dale and Giovannoni54
Two authors (D.M.P. and H.S.V.) independently assessed study eligibility and performed data extraction. Discrepancies with regard to either process were resolved by consensus. A review author with content expertise in neuropathology (S.N.) reviewed all data extracted on immunostaining methodology for accuracy. We attempted to derive any missing data from studies using indirect calculations and contacted study authors if needed.
Statistical analyses
All statistical analyses were conducted using the Stata Statistical Software package version 12 for Mac. For each comparison of interest, we pooled point estimate ORs and 95% CIs of ABGA seropositivity or CSF-positivity into a combined-effect summary estimate using the random-effects model of DerSimonian & Laird. Reference DerSimonian and Laird55 We tested for the presence of heterogeneity by calculating Cochrane’s Q statistic (P<0.10 denotes significant heterogeneity), and quantified any observed heterogeneity by calculating the I 2 statistic (I 2⩽25%, ⩽50% and ⩽75% were considered indicative of low, moderate and high heterogeneity respectively). Reference Higgins, Thompson, Deeks and Altman56 We performed a funnel plot test to assess the potential for publication bias. Reference Egger, Davey Smith, Schneider and Minder57 We performed sensitivity analyses to assess the influence of decisions made during the review process, including: (a) immunostaining method decision-rules; (b) including sources of unpublished data (meeting abstracts, data obtained from study authors); and (c) including studies with unclear or high risk of bias for each risk of bias domain. Study-level characteristics analysed by subgroup analyses were age strata, disease severity, type of control group, immunostaining technique and antigenic sources. Those analysed by random-effects meta-regression analyses Reference Harbord and Higgins58 were primary OCD groups’ mean age, percent female, Yale-Brown Obsessive Compulsive Scale (Y-BOCS) score Reference Goodman, Price, Rasmussen, Mazure, Fleischmann and Hill59 and mean duration of illness.
Results
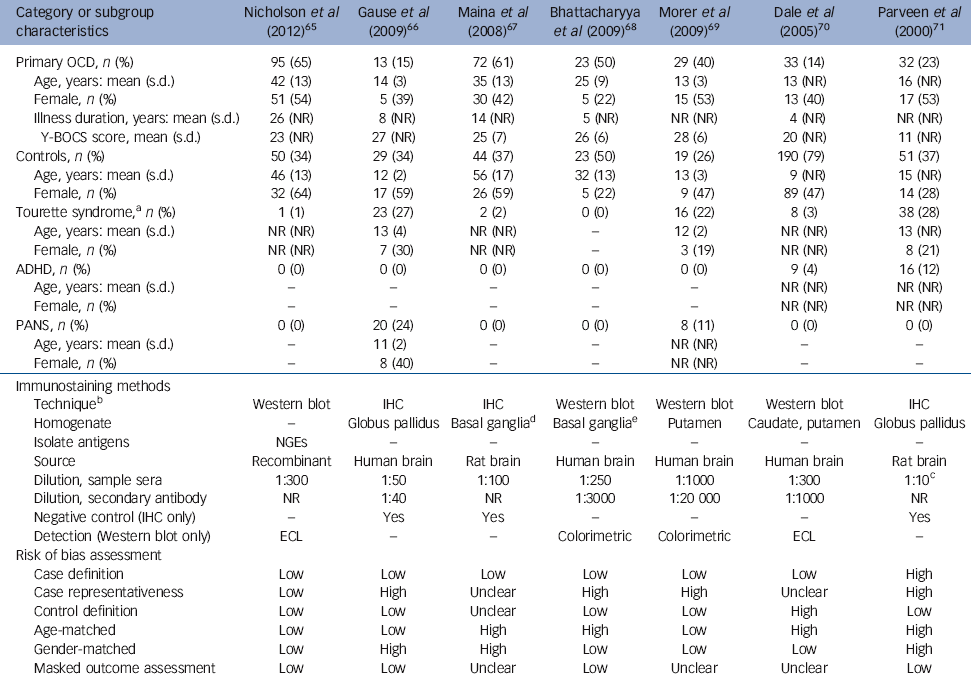
Results of our combined search methods and study selection process are presented in Fig. 1. We excluded 40 records after reviewing their full-texts (online Table DS3 and online supplemental references). Of these, we excluded five potentially eligible studies because they did not provide sufficient data to determine eligibility and attempts to contact the authors for clarifying data were unsuccessful. Reference Kawikova, Grady, Tobiasova, Zhang, Vojdani and Katsovich60-Reference Swedo, Kilpatrick, Shapiro, Mannheim and Leonard64 Seven case-control studies met all eligibility criteria and were included for systematic review and meta-analysis (Table 1). Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65-Reference Trucco, Leckman and Lombroso71 Of these, six Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65-Reference Dale, Heyman, Giovannoni and Church70 were peer-reviewed journal articles and one Reference Trucco, Leckman and Lombroso71 was a meeting abstract. We extracted outcome data directly from these sources with two exceptions, Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68,Reference Trucco, Leckman and Lombroso71 which necessitated contacting the study authors; one Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68 because the original report only provided data on anti-basal ganglia/thalamic antibody seropositivity in aggregate rather than on ABGA alone, and the other Reference Trucco, Leckman and Lombroso71 because it was a meeting abstract that provided insufficient detail. Antigenic substrates used to assess ABGA seropositivity varied from homogenate consisting of putamen alone, caudate alone, or combined caudate, putamen and globus pallidus, to each of the neuronal glycolytic enzymes obtained as commercial recombinant isolated antigens. Primary outcome data were based on immunohistochemistry in three studies, Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67,Reference Trucco, Leckman and Lombroso71 and on Western blotting in four; Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68-Reference Dale, Heyman, Giovannoni and Church70 of the latter group, one study that also utilised immunohistochemistry reported zero events of ABGA seropositivity in both cells. Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 Immunostaining protocols provided variable sample sera and secondary antibody dilutions, immunoreactivity detection techniques and tissue preparation. All studies utilised samples consisting of whole serum rather than isolated immunoglobulin.
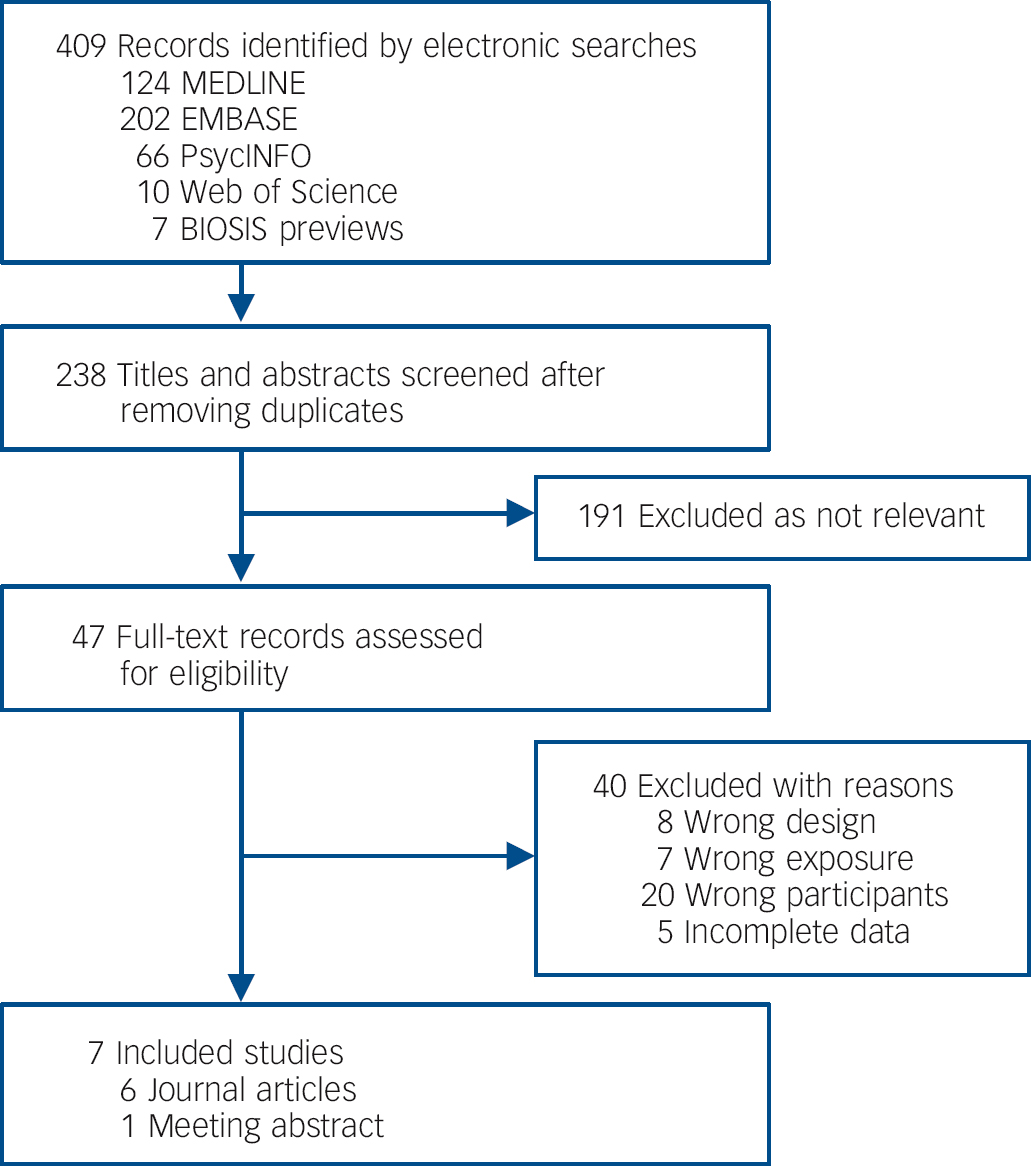
Fig. 1 Study attrition diagram.
Table 1 Characteristics of participants, immunostaining methods and risk of bias assessment in included studies
| Category or subgroup characteristics |
Nicholson et al
(2012) Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65 |
Gause et al
(2009) Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66 |
Maina et al
(2008) Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 |
Bhattacharyya et al (2009) Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68 |
Morer et al
(2009) Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 |
Dale et al
(2005) Reference Dale, Heyman, Giovannoni and Church70 |
Parveen et al
(2000) Reference Trucco, Leckman and Lombroso71 |
|---|---|---|---|---|---|---|---|
| Primary OCD, n (%) | 95 (65) | 13 (15) | 72 (61) | 23 (50) | 29 (40) | 33 (14) | 32 (23) |
| Age, years: mean (s.d.) | 42 (13) | 14 (3) | 35 (13) | 25 (9) | 13 (3) | 13 (NR) | 16 (NR) |
| Female, n (%) | 51 (54) | 5 (39) | 30 (42) | 5 (22) | 15 (53) | 13 (40) | 17 (53) |
| Illness duration, years: mean (s.d.) | 26 (NR) | 8 (NR) | 14 (NR) | 5 (NR) | NR (NR) | 4 (NR) | NR (NR) |
| Y-BOCS score, mean (s.d.) | 23 (NR) | 27 (NR) | 25 (7) | 26 (6) | 28 (6) | 20 (NR) | 11 (NR) |
| Controls, n (%) | 50 (34) | 29 (34) | 44 (37) | 23 (50) | 19 (26) | 190 (79) | 51 (37) |
| Age, years: mean (s.d.) | 46 (13) | 12 (2) | 56 (17) | 32 (13) | 13 (3) | 9 (NR) | 15 (NR) |
| Female, n (%) | 32 (64) | 17 (59) | 26 (59) | 5 (22) | 9 (47) | 89 (47) | 14 (28) |
| Tourette syndrome,Footnote a n (%) | 1 (1) | 23 (27) | 2 (2) | 0 (0) | 16 (22) | 8 (3) | 38 (28) |
| Age, years: mean (s.d.) | NR (NR) | 13 (4) | NR (NR) | - | 12 (2) | NR (NR) | 13 (NR) |
| Female, n (%) | NR (NR) | 7 (30) | NR (NR) | - | 3 (19) | NR (NR) | 8 (21) |
| ADHD, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (4) | 16 (12) |
| Age, years: mean (s.d.) | - | - | - | - | - | NR (NR) | NR (NR) |
| Female, n (%) | - | - | - | - | - | NR (NR) | NR (NR) |
| PANS, n (%) | 0 (0) | 20 (24) | 0 (0) | 0 (0) | 8 (11) | 0 (0) | 0 (0) |
| Age, years: mean (s.d.) | - | 11 (2) | - | - | NR (NR) | - | - |
| Female, n (%) | - | 8 (40) | - | - | NR (NR) | - | - |
| Immunostaining methods | |||||||
| TechniqueFootnote b | Western blot | IHC | IHC | Western blot | Western blot | Western blot | IHC |
| Homogenate | - | Globus pallidus | Basal gangliaFootnote d | Basal gangliaFootnote e | Putamen | Caudate, putamen | Globus pallidus |
| Isolate antigens | NGEs | - | - | - | - | - | - |
| Source | Recombinant | Human brain | Rat brain | Human brain | Human brain | Human brain | Rat brain |
| Dilution, sample sera | 1:300 | 1:50 | 1:100 | 1:250 | 1:1000 | 1:300 | 1:10Footnote c |
| Dilution, secondary antibody | NR | 1:40 | NR | 1:3000 | 1:20 000 | 1:1000 | NR |
| Negative control (IHC only) | - | Yes | Yes | - | - | - | Yes |
| Detection (Western blot only) | ECL | - | - | Colorimetric Colorimetric | ECL | - | |
| Risk of bias assessment | |||||||
| Case definition | Low | Low | Low | Low | Low | Low | High |
| Case representativeness | Low | High | Unclear | High | High | Unclear | High |
| Control definition | Low | Low | Unclear | Low | Low | High | Low |
| Age-matched | Low | Low | High | High | Low | High | High |
| Gender-matched | Low | High | High | Low | Low | Low | High |
| Masked outcome assessment | Low | Low | Unclear | Low | Unclear | Unclear | Low |
ADHD, attention-deficit hyperactivity disorder; ECL, Electrochemiluminescence; IHC, immunohistochemistry; NGEs, neuronal glycolytic enzymes; NR, not reported; OCD, obsessive-compulsive disorder; PANS, paediatric acute-onset neuropsychiatric syndrome; s.d., standard deviation; Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
a. Refers to baseline characteristics data that was provided in aggregate for Tourette syndrome and primary OCD participants.
b. Refers to the immunostaining method that yielded the outcome data included in our meta-analysis.
c. Refers to starting concentration, which was titrated to an endpoint of reactivity at maximum dilution for each sample using two-fold serial dilutions.
d. Not otherwise specified.
e. Caudate, putamen, and globus pallidus.
Studies included 844 total participants, consisting of 297 with primary OCD, 406 controls and 141 with ABGA-associated neuropsychiatric disorders. Primary OCD cohorts included children, Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69,Reference Dale, Heyman, Giovannoni and Church70 adults Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 or both. Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68,Reference Trucco, Leckman and Lombroso71 Percent female ranged from 22 to 54. Mean duration of illness ranged from 4 to 26 years. Mean Y-BOCS scores ranged from 11 (mild) to 28 (severe). Control participants were divided into four mutually exclusive subgroups: healthy (n = 162), Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68-Reference Trucco, Leckman and Lombroso71 psychiatric (n = 94), Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 neurological (n = 100) Reference Dale, Heyman, Giovannoni and Church70 or autoimmune (n = 50). Reference Dale, Heyman, Giovannoni and Church70 Psychiatric controls consisted of individuals with major depressive disorder, Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 bipolar disorder type I, Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 bipolar disorder type II, Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 and schizophrenia. Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67 Neurological controls consisted of individuals with stroke, metabolic movement disorders, and encephalitis. Reference Dale, Heyman, Giovannoni and Church70 Autoimmune controls consisted of individuals with rheumatic carditis, glomerulonephritis and other autoimmune diseases that explicitly did not have neuropsychiatric manifestations. Reference Dale, Heyman, Giovannoni and Church70 ABGA-associated neuropsychiatric disorders identified among included studies consisted of Tourette syndrome (n = 88), Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65-Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69-Reference Trucco, Leckman and Lombroso71 attention-deficit hyperactivity disorder (ADHD) (n = 25) Reference Dale, Heyman, Giovannoni and Church70,Reference Trucco, Leckman and Lombroso71 and PANS (n = 28). Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69
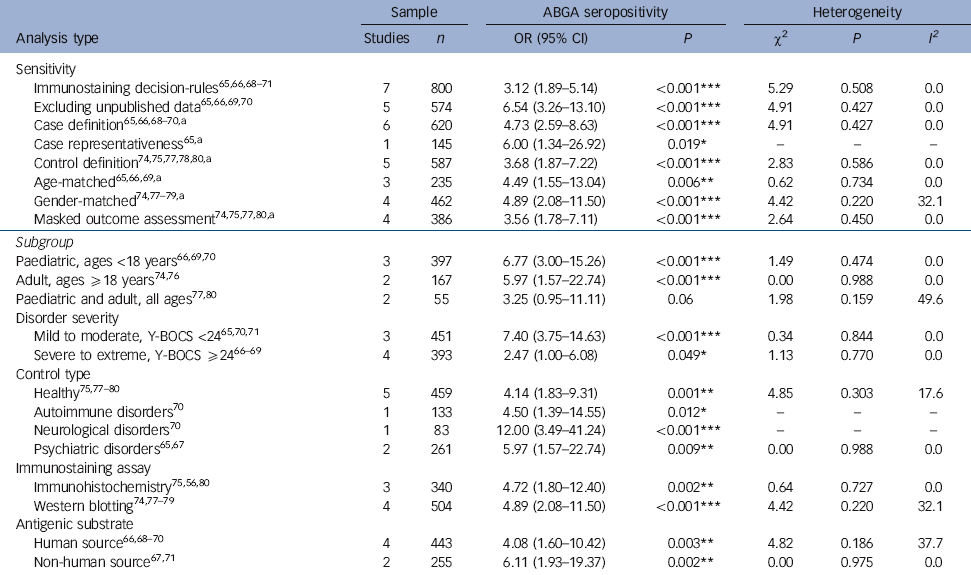
All seven studies provided data on ABGA seropositivity for 297 participants with primary OCD compared with 406 controls, yielding a total sample of 703 participants. Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65-Reference Trucco, Leckman and Lombroso71 Individual study ORs favoured primary OCD in all seven studies, of which this difference of proportions constituted a statistically significant difference in three studies. Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Dale, Heyman, Giovannoni and Church70,Reference Trucco, Leckman and Lombroso71 Pooled analyses showed that a significantly greater proportion of participants with primary OCD were ABGA seropositive compared with various controls (OR = 4.97, 95% CI 2.88-8.55, P<0.00001; Fig. 2), without evidence of heterogeneity (χ2 = 5.05, P = 0.54, I 2 = 0.0%) or publication bias (online Fig. DS1). Results of sensitivity analyses indicated that the direction, magnitude and statistical significance of this effect were independent of publication type, immunostaining methods and inclusion of studies with an unclear or high risk of bias for each of the domains assessed (Table 2). This effect also remained statistically significant for each subgroup analysed with the exception of studies involving both paediatric and adult participants. Random-effects meta-regression analyses showed no significant differences between the magnitude of individual study ORs and any of the study-level characteristics analysed (Table 3). Results from the two studies Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Dale, Heyman, Giovannoni and Church70 relating ABGA seropositivity to participant-level data showed no statistically significant differences between ABGA seropositive and ABGA seronegative participants with regard to age, gender or duration of illness; of these, one study Reference Dale, Heyman, Giovannoni and Church70 documented significantly worse disease severity (i.e. mean Y-BOCS scores) among ABGA seropositive participants. Of the seven included studies, one Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68 provided data on ABGA CSF-positivity, yielding a total sample of 46 participants (23 with primary OCD, 23 healthy controls). A significantly greater proportion of those with primary OCD were ABGA CSF-positive compared with healthy controls (OR = 5.60, 95% CI 1.04-30.20, P = 0.045).
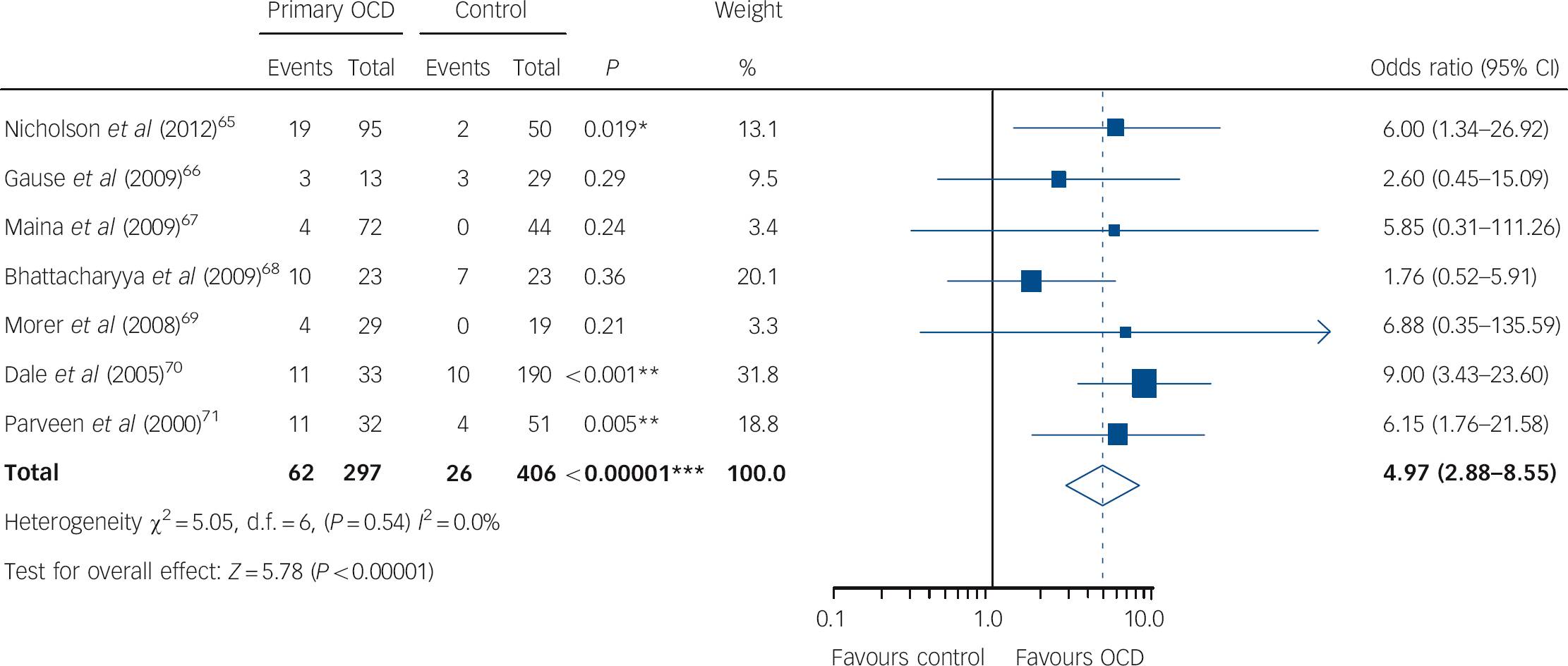
Fig. 2 Forest plot of anti-basal ganglia antibody seropositivity in primary obsessive-compulsive disorder (OCD) compared with controls.
Table 2 Sensitivity and subgroup analyses for anti-basal ganglia antibody (ABGA) seropositivity in primary obsessive-compulsive disorder (OCD) compared with controls
Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
a. Refers to analyses delimited to those studies scored as having a low risk of bias, while excluding those scored as having an unclear or high risk of bias.
* P<0.05
** P<0.01
*** P<0.001.
Table 3 Meta-regression analyses for anti-basal ganglia antibody (ABGA) seropositivity in primary obsessive-compulsive disorder (OCD) compared with controls
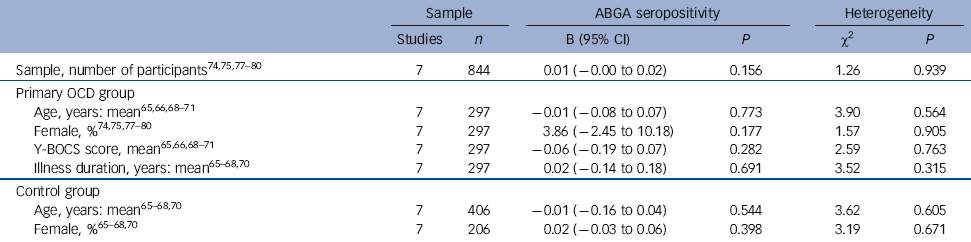
Y-BOCS, Yale-Brown Obsessive Compulsive Scale.
Two studies Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66 provided data on immunoreactivity against the isolated antigens aldolase C, γ-enolase and pyruvate kinase M1 (obtained as commercial recombinant antigens). Of these, the data from one Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65 contributed to the above-reported summary estimate for ABGA seropositivity in primary OCD compared with controls. Data from the other study did not because primary outcome data were also provided based on the results of an assay involving basal ganglia homogenate. A significantly greater proportion of participants with primary OCD were seropositive for γ-enolase antibodies compared with controls (OR = 8.12, 95% CI 1.72-38.47), but not for aldolase C (OR = 2.31, 95% CI 0.61-8.78) or pyruvate kinase M1 (OR = 0.62, 95% CI 0.21-1.88) antibodies. None of the included studies provided data on ABGA positivity, in either sera or CSF, based on assays utilising isolated forms of other putative antigens. In several instances, peak band intensities for primary OCD groups, determined from ABGA seropositive samples by Western blotting using basal ganglia homogenate rather than using isolated antigens, corresponded to putative ABGA antigens (±2 kDa). These included aldolase C (40 kDa), Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 γ/α-enolase doublet (45 kDa), Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 γ-α-enolase heterodimer (98 kDa), Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68,Reference Dale, Heyman, Giovannoni and Church70 tubulin (55 kDa) Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 and pyruvate kinase M1 (60 kDa). Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67,Reference Dale, Heyman, Giovannoni and Church70
Six studies provided data on ABGA seropositivity in primary OCD compared with Tourette syndrome, yielding a total sample of 363 (274 with primary OCD, 88 with Tourette syndrome). Reference Nicholson, Ferdinando, Krishnaiah, Anhoury, Lennox and Mataix-Cols65-Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69-Reference Trucco, Leckman and Lombroso71 The summary estimate was not statistically significant (OR = 0.71, 95% CI 0.36-1.41, P = 0.33; Fig. 3(a)), without evidence of heterogeneity (χ2 = 4.20, P = 0.52, I 2 = 0.0%). Two studies Reference Dale, Heyman, Giovannoni and Church70,Reference Trucco, Leckman and Lombroso71 provided data on ABGA seropositivity in primary OCD compared with ADHD, yielding a total sample of 90 participants (65 with primary OCD, 25 with ADHD). The summary estimate was not statistically significant (OR = 0.89, 95% CI 0.34-2.36, P = 0.81; Fig. 3(b)), without evidence of heterogeneity (χ2 = 0.37; P = 0.54; I 2 = 0.0%). Two studies Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 provided data on ABGA seropositivity in primary OCD compared with PANS, yielding a total sample of 70 participants (42 with primary OCD, 28 with PANS). The summary estimate was not statistically significant (OR = 0.80, 95% CI 0.23-2.86, P = 0.74; Fig. 3(c)), without evidence of heterogeneity (χ2 = 0.49, P= 0.48, I 2 = 0.0%). No studies provided data on ABGA CSF-positivity for participants with ABGA-associated neuropsychiatric disorders.

Fig. 3 Forest plot of anti-basal ganglia antibody seropositivity in primary obsessive-compulsive disorder (OCD) compared with (a) Tourette syndrome, (b) attention-deficit hyperactivity disorder (ADHD), and (c) paediatric acute-onset neuropsychiatric syndrome (PANS).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to assess the relationship between autoimmunity and primary OCD by pooling data from studies having assessed sera and/or CSF samples for ABGA positivity. Our results show that ABGAs are five times more likely to be detected in the serum of individuals with primary OCD compared with control groups consisting of healthy controls as well as those with various psychiatric, neurological and autoimmune disorders. This effect is statistically significant, generalisable to both paediatric and adult forms of primary OCD, and robust to multiple pre-selected sensitivity analyses. This effect derives from seven studies, only three of which reported a statistically significant effect individually, yet all of which reported a consistent effect size as evidenced by the absence of heterogeneity associated with the summary estimates. This suggests that the discordant findings among point estimates of ABGA seropositivity in the extant literature are largely attributable to three inadequately powered studies with Type II error.
In addition, that (a) both studies having assessed immunoreactivity against isolated antigens found significantly increased odds of γ-enolase antibody, but not aldolase C antibody, seropositivity in primary OCD compared with controls, and (b) four studies identified primary OCD group peak bands corresponding to the γ/α-enolase doublet, supports the specific nature of the observed association between primary OCD and autoimmunity. Supporting this interpretation, of the neuronal glycolytic enzymes, γ-enolase exhibits the greatest degree of proteomic sequence overlap with streptococcal pyogenes surface proteins (49%, expected chance identity = 1.0×10-109). Reference Dale, Candler, Church, Wait, Pocock and Giovannoni49 Arguing against this interpretation, however, despite the fact that pyruvate kinase M1 also exhibits considerable homology (38% proteomic sequence overlap) we observed no significant difference in the odds of seropositivity in primary OCD compared with controls in studies utilising recombinant pyruvate kinase as the isolate test antigen. Whether the latter finding is a consequence of Type II error is unknown. Last, existing evidence indicates that those with primary OCD are associated with 5.6-fold increased odds of ABGA CSF-positivity compared with healthy controls. If replicated, these data also favour the hypothesis that primary OCD is autoimmune-mediated in some patients.
Limitations
Results of this meta-analysis should be interpreted in light of several limitations. First, although sensitivity, subgroup and meta-regression analyses indicated that the observed effect of increased odds of ABGA seropositivity in primary OCD compared with controls remains robust to numerous conditionals, the possibility that residual confounding occurred cannot be excluded. Potential confounders that we did not assess include history of streptococcal exposure, autoimmune disease, immunotherapy and psychotropic medication. Second, interstudy variability in the sensitivity and specificity of immunostaining assays due to methodological heterogeneity limits the validity of inferences regarding the absolute odds of ABGA positivity in each study group. Even if this were definitively shown to be the case, however, it would not necessarily affect the ORs relating the proportions of ABGA-positive participants in each group. Last, that none of the eligible studies tested for seropositivity using isolated forms of other putative ABGA antigens (lysoganglioside GM1, tubulin, DR1 or DR2) restricts our ability to comment directly on their relationship with primary OCD. Since antibodies directed against these antigens are known to induce frank chorea in Sydenham chorea, Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27-Reference Kirvan, Swedo, Snider and Cunningham30 our observation that only one included study identified a primary OCD group peak band corresponding to one of these antigens (tubulin, 55 kDa) Reference Morer, Lazaro, Sabater, Massana, Castro and Graus69 may reflect the absence of overt movement sequelae in primary OCD.
Implications
According to Witebsky’s postulates of criteria defining auto-immunity, our results constitute circumstantial evidence of autoimmunity in a subset of primary OCD. Reference Rose and Bona72,Reference Witebsky, Rose, Terplan, Paine and Egan73 To determine whether ABGA are pathogenic, epiphenomena or biomarkers of concomitant autoimmunity secondary to basal ganglia injury (antigen spreading), requires pre-clinical experimental studies designed to provide indirect or direct evidence of autoimmunity. Reference Rose and Bona72,Reference Witebsky, Rose, Terplan, Paine and Egan73 Such studies might be modelled after those supporting the pathogenicity of ABGA in PANS and Sydenham chorea. Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Kirvan, Swedo, Heuser and Cunningham29,Reference Kirvan, Swedo, Snider and Cunningham30,Reference Kirvan, Swedo, Kurahara and Cunningham43 Implicit in the design of these experimental studies is an analysis of which, if any, specific epitopes are pathogenic, and whether ABGA-antigen interactions influence signal transduction. Future studies should evaluate the relationship between ABGA positivity and established abnormalities of the CSTS, Reference Radua, van den Heuvel, Surguladze and Mataix-Cols14 glutamatergic tone, Reference Pittenger, Bloch and Williams17 and other genetic abnormalities among those with primary OCD. Reference Rotge, Aouizerate, Tignol, Bioulac, Burbaud and Guehl24,Reference Fluitman, Denys, Vulink, Schutters, Heijnen and Westenberg74-Reference Poe76 Such studies are likely to clarify whether these abnormalities - largely derived from separate lines of investigation - reflect convergent mechanisms in the pathogenesis of OCD.
The present review also highlights the absence of a standardised immunostaining assay protocol for assessing ABGA seropositivity. Evidenced-based methods that should inform the design of future studies addressing this research question, include the utilisation of:
-
(a) positive and negative laboratory controls Reference Gause, Morris, Vernekar, Pardo-Villamizar, Grados and Singer66,Reference Maina, Albert, Bogetto, Borghese, Cat Berro and Mutani67
-
(b) fresh v. frozen tissue preparation Reference Rippel, Hong, Yoon, Williams and Singer77
-
(c) serial titration of sample dilution to optimise the signal to noise ratio Reference Morshed, Parveen, Leckman, Mercadante, Bittencourt Kiss and Miguel78
-
(d) isolated immunoglobulin v. whole serum Reference Rippel, Hong, Yoon, Williams and Singer77
-
(e) electrochemiluminescence v. colorimetric detection Reference Rippel, Hong, Yoon, Williams and Singer77
-
(f) isolated recombinant forms of each putative ABGA auto-antigen, and homogenate comprising other cortical regions apart from basal ganglia that are implicated in OCD and which have been associated with ABGA (e.g. thalamic nuclei). Reference Bhattacharyya, Khanna, Chakrabarty, Mahadevan, Christopher and Shankar68
Future studies should also evaluate the effect of duration of illness and disease state severity on the likelihood of ABGA positivity, based on prior studies showing these factors influence the pre-test odds of immunoreactivity. Reference Brimberg, Benhar, Mascaro-Blanco, Alvarez, Lotan and Winter27,Reference Dickerson, Stallings, Vaughan, Origoni, Khushalani and Yolken79-Reference Irani, Bera, Waters, Zulliani, Maxwell and Zandi81 In addition, the association between primary OCD and autoimmunity indicated by our results lends support for the rationale to explore the efficacy of rationally based immunomodulatory and anti-inflammatory agents as potential pharmacological treatment approaches; in so doing, clinicians and researchers should aim to identify the subsets of patients for which these approaches would be most appropriate.
Odds of ABGA seropositivity are increased fivefold in primary OCD compared with controls, but are comparable to those associated with disorders previously associated with ABGA, providing circumstantial evidence of autoimmunity in a subset of those with primary OCD. Further experimental studies are needed to ascertain whether this relationship is causal.
Acknowledgements
We thank Drs Bhattacharyya and Morshed for providing us with their data, and thanks to Drs Robin Larson, Natalie Riblet, Kenneth Roberts, Madhavi Lakkaraja, Richard Ferrell and Jeremiah Brown, for their advice concerning systematic review methods as well as how to improve the manuscript. The authors report no biomedical financial interest or potential conflicts of interest.









eLetters
No eLetters have been published for this article.