Childhood maltreatment is a significant risk factor for a wide range of adolescent and adult psychiatric disorders, including anxiety and depression. Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson1,Reference Scott, Smith and Ellis2 Although there is good evidence for alterations in psychological functioning following child abuse in the home Reference Gilbert, Widom, Browne, Fergusson, Webb and Janson1 there remains a paucity of neuroimaging studies that have interrogated its impact on brain function. Reference Carrion, Haas, Garrett, Song and Reiss3-Reference McCrory, De Brito and Viding6 Such studies have the potential to identify prodromal indicators of psychiatric disorder; in other words, pinpoint latent neurobiological risk markers that may prefigure disorder-related patterns of neural activity. A particular advantage of techniques such as functional magnetic resonance imaging (fMRI) is its sensitivity in detecting subtle alterations in neural processing that may not be observable with behavioural indices. Reference Aue, Lavelle and Cacioppo7 To date only one fMRI study with child participants has investigated the impact of maltreatment in the home on emotional processing. Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4 Maltreated children exhibited significantly greater bilateral activation of the anterior insula and of the right amygdala in response to angry faces, suggesting that exposure to abuse in the home is associated with a pattern of altered brain activity to threat comparable with that seen in several anxiety disorders Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4,Reference Etkin and Wager8 and in soldiers exposed to combat. Reference Van Wingen, Geuze, Vermetten and Fernández9 Increased amygdala activation to negative facial cues has also been reported in fMRI studies investigating the impact of institutionalisation, Reference Maheu, Dozier, Guyer, Mandell, Peloso and Poeth10,Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey11 which is a relatively severe form of early adversity, typically associated with a broader and more marked set of developmental impairments. Reference Rutter, Sonuga-Barke and Castle12 Related findings have been observed in event-related potential (ERP) studies of physically abused children. Reference Pollak, Klorman, Thatcher and Cicchetti5,Reference Pollak13,Reference Cicchetti and Curtis14 Childhood adversity, therefore, appears to be associated with increased allocation of attentional resources to consciously processed threat cues.
A separate body of work in non-maltreated individuals with anxiety-related disorders has examined brain response to pre-attentive emotional processing (i.e. processing of stimuli presented outside of conscious awareness). A distributed network of phylogenetically ancient (largely subcortical) brain structures have been implicated in such ‘pre-conscious’ processing of affect cues, although several of these areas, including the amygdala, are also engaged during conscious processing. Reference Tamietto and De Gelder15 There is now convincing evidence that such processing is altered in anxiety-related disorders. For example, adolescents with generalised anxiety disorder show greater amygdala activation when processing pre-attentively presented angry faces. Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 A similar pattern is also observed in adults with anxiety traits Reference Etkin, Klemenhagen, Dudman, Rogan, Hen and Kandel17 and in patients with post-traumatic stress disorder (PTSD). Reference Armony, Corbo, Clément and Brunet18,Reference Bryant, Kemp, Felmingham, Liddell, Olivieri and Peduto19 The current study aimed to investigate functional brain activation in maltreated children during processing of pre-attentively presented facial affect cues Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 using a dot-probe paradigm. Reference Frewen, Dozois, Joanisse and Neufeld20 We hypothesised that, relative to matched peers, they would show heightened neural response to facial expressions of anger but not happiness. It was expected that this selective response to threat cues would be associated with timing and severity of maltreatment experience. Such a pattern would point to differences in the very early stages of threat perception, prior to higher order strategic or regulatory processing, and constitute a possible marker of increased vulnerability to psychiatric disorder.
Method
Participants
Two groups of children were recruited from the London area. Children with documented exposure to physical abuse and/or intimate-partner violence who were in a stable home placement and who did not have any intellectual disabilities (maltreated group, n = 18) were recruited via a community Social Services department. Written informed assent was obtained from each child and written informed consent from a parent. Where there was shared parental responsibility, consent was obtained from the child's biological parent if still contactable and from Social Services.
Comparison children (non-maltreated group, n= 23) comparable on age, self-reported Tanner stage, gender, handedness, cognitive ability, socioeconomic status and ethnicity were recruited from
Table 1 Background characteristics and questionnaire data for non-maltreated and maltreated group a
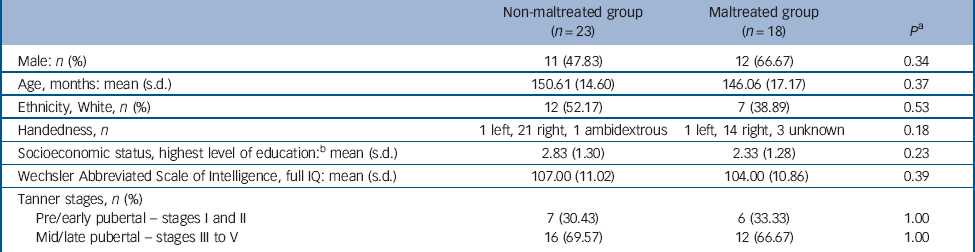
| Non-maltreated group (n = 23) | Maltreated group (n = 18) | P a | |
|---|---|---|---|
| Male: n (%) | 11 (47.83) | 12 (66.67) | 0.34 |
| Age, months: mean (s.d.) | 150.61 (14.60) | 146.06 (17.17) | 0.37 |
| Ethnicity, White, n (%) | 12 (52.17) | 7 (38.89) | 0.53 |
| Handedness, n | 1 left, 21 right, 1 ambidextrous | 1 left, 14 right, 3 unknown | 0.18 |
| Socioeconomic status, highest level of education: b mean (s.d.) | 2.83 (1.30) | 2.33 (1.28) | 0.23 |
| Wechsler Abbreviated Scale of Intelligence, full IQ: mean (s.d.) | 107.00 (11.02) | 104.00 (10.86) | 0.39 |
| Tanner stages, n (%) | |||
| Pre/early pubertal – stages I and II | 7 (30.43) | 6 (33.33) | 1.00 |
| Mid/late pubertal – stages III to V | 16 (69.57) | 12 (66.67) | 1.00 |
a Note, all P were derived from t-tests with the exception of the gender, ethnicity and Tanner stage comparisons, which used the Fisher's exact test and the handedness comparison, which employed a χ2 test.
b The highest level of education attained by the mother or long-term foster mother was taken as an indicator of socioeconomic status and evaluated on a five-point scale (from zero for no formal qualification to five for postgraduate or professional qualification).
secondary/primary schools and via advertisement in local newspapers and on the internet (Table 1). Exclusion criteria included: history of abuse; history of neglect; exposure to intimate-partner violence as reported by the main carer on the Child Bad Experience Questionnaire Reference Dodge, Bates and Pettit21 and the Dunedin Abuse Scales; Reference Magdol, Moffitt, Caspi and Silva22 previous contact with Social Services regarding the child's care. Written informed assent and consent were obtained from the child and a parent(s) respectively. None of the participants reported a history of head trauma, neurological disease, a psychiatric diagnosis, were receiving pharmacological or psychological treatment, or presented with contraindications for MRI. The study was approved by UCL Ethics Committee (0895/002).
Measures
Maltreatment history
Social Services case files were used to obtain an accurate characterisation of a child's maltreatment history. Kaufman and colleagues' coding system Reference Kaufman, Jones, Stieglitz, Vitulano and Mannarino23 were used for this purpose. This measure allows the severity of four maltreatment subtypes (i.e. physical abuse, sexual abuse, neglect and emotional abuse) to be rated on a scale from zero (not present) to four (severe). Number of cases and mean severity scores for each maltreatment subtype on Kaufman's four-point scale are shown in Table 2, and mean estimated age at onset and duration of each form of abuse was calculated for those participants where this information was available. As expected from a community sample, there was a high degree of overlap among abuse categories. Six cases from the maltreatment group were randomly selected and double-rated by a senior social work professional in relation to each form of abuse; there was 83.3% agreement in relation to the presence of physical abuse, neglect and sexual abuse and 100% agreement in relation to emotional abuse.
Child Bad Experience Questionnaire
Main carers were administered a standardised clinical interview protocol that includes probe questions on bullying, accidents, harsh discipline, physical and sexual abuse. Reference Dodge, Bates and Pettit21,Reference Lansford, Dodge, Pettit, Bates, Crozier and Kaplow24
Intimate-partner violence
To screen for exposure to domestic violence in the non-maltreated group, the Physical Abuse Scale of the Dunedin Abuse Scales Reference Magdol, Moffitt, Caspi and Silva22 was used to assess specific abusive behaviours from one intimate partner to the other. Respondents answered questions first about their behaviour towards their current or most recent partner and second about the partner's behaviour towards them. The measure yields separate scores for perpetration and victimisation, for both males and females. The Physical Abuse Scale contains all nine items of Straus's Conflict Tactics Scales Reference Straus, Straus and RJ25 (such as slap, choke, beat up), plus four items describing other physically abusive acts (such as twisting arm, bodily throw).
Cognitive ability
The Vocabulary and Matrix Reasoning subscales of the Wechsler Abbreviated Scales of Intelligence Reference Wechsler26 were used to provide an estimate of full-scale IQ.
Psychiatric symptoms
The self-report State-Trait Anxiety Inventory for Children (STAIC) Reference Spielberger27 was used to measure state and trait anxiety, and consists of two separate 20-item self-report scales. The Mood and Feelings Questionnaire (MFQ) Reference Angold, Costello and Messer28 , a 33-item self-report measure, was used to assess core depressive symptoms. The Trauma Symptom Checklist for Children – A (TSCC-A), Reference Briere29 a 44-item self-report measure, was used to assess acute and chronic post-traumatic symptomatology and other symptom clusters. It includes five clinical scales (anger, anxiety, depression, post-traumatic stress, and dissociation). The Strengths and Difficulties Questionnaire (SDQ), Reference Goodman30 a 25-item questionnaire, was completed by parents/carers,
Table 2 Abuse subtype severity scores, and estimated mean age at onset and duration in years

| Abuse subtype | Mean (s.d.) |
|---|---|
| Physical abuse (n = 8) | |
| Kaufman score | 1.50 (0.54) |
| Mean age at onset | 3.29 (2.06) |
| Mean duration | 5.00 (3.16) |
| Neglect (n = 16) | |
| Kaufman score | 2.63 (1.20) |
| Mean age at onset | 3.29 (3.02) |
| Mean duration | 7.00 (3.12) |
| Sexual abuse (n = 5) | |
| Kaufman score | 2.00 (1.87) |
| Mean age at onset | 5.00 (0) |
| Mean duration | 2.67 (2.08) |
| Emotional abuse (n = 17) | |
| Kaufman score | 2.88 (1.05) |
| Mean age at onset | 2.64 (3.25) |
| Mean duration | 6.27 (3.47) |
in order to provide an estimate of emotional symptoms as well as levels of hyperactivity symptoms and conduct problems.
Experimental paradigm
The fMRI task used identical parameters to those employed by Monk and colleagues. Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 Briefly, trials started with a 500 ms fixation cross in the centre of the screen (Fig. 1). Next, two photographs of an actor's face appeared side by side for 17 ms (pre-attentively). For the face trials, an angry or a happy facial expression was paired with a neutral facial expression of the same actor, while on neutral trials the two photos were identical and showed the actor with a neutral facial expression. Following the brief presentation of the faces, two scrambled faces (the mask) replaced the two faces for 68 ms. The mask was then replaced by an asterisk in one hemifield (on the same side as the emotional face for congruent trials and on the opposite side for incongruent trials) for 1100 ms. Participants indicated which side of the screen the asterisk was displayed on by pressing buttons with the index (indicating left) or middle finger (indicating right) of their dominant hand. Inter-trial intervals were 2300 ms. Previous studies using these parameters show that participants report minimal awareness of details of the briefly presented face stimuli. Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16,Reference Mogg and Bradley31 Eighty actors were each presented twice for a total of 160 trials (i.e. 32 trials for each of the five conditions: angry-neutral

Fig. 1 Examples of incongruent and congruent masked angry-neutral trials presented with the duration and name of each event for the two types of trials.
Happy–neutral and neutral–neutral trials (not shown) were also presented to participants.
congruent, angry-neutral incongruent, happy-neutral congruent, happy-neutral incongruent, neutral-neutral) for each of the two runs. The order of the face trials was randomly determined for each participant. Forty blank trials of the same length as the face trials were also presented randomly. Performance was measured as reaction time and accuracy on the location of the probe. Prior to scanning, participants were trained on the task, but with different stimuli showing neutral faces only.
Image acquisition and analysis
Participants were scanned using a 1.5 Tesla Siemens (Siemens Medical Systems) Avanto MRI scanner with a 32-channel head coil. A total of 355 multislice T 2*-weighted echo-planar imaging (EPI) volumes with blood oxygen level-dependent (BOLD) contrast were acquired in two runs of approximately 13 min 30 s each. The EPI sequence was optimised to reduce BOLD sensitivity losses in the amygdala because of susceptibility artefacts. Reference Weiskopf, Hutton, Josephs and Deichmann32 Acquisition parameters were: 27 slices per volume; slice thickness 2 mm; gap between slices 1 mm, echo time (TE) = 50 ms; repetition time (TR) = 85.2 ms; slice tilt −30° (T>C); flip angle 90°; field of view 192 mm × 192 mm2; matrix size 64 × 64; voxel size 3 × 3 × 3 mm. Field maps were collected in order to remove distortion caused by magnetic field inhomogeneity. Stimuli were projected centrally onto a screen at the front of the magnet, which participants viewed using a mirror mounted on the head coil (21 × 13° of visual angle of the whole screen).
The images were pre-processed and subsequently analysed using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8), implemented in MATLAB 7.5 on Windows XP. After discarding the first six functional volumes of each session to allow for T 1 equilibrium, EPI images were spatially realigned to the first volume of the first run to correct for motion artefacts. These images were also corrected for geometric distortions caused by susceptibility-induced field inhomogeneities. The field maps were processed for each participant's run using the FieldMap toolbox implemented in SPM8 to produce a voxel displacement map indicating the field distortions. The EPI images were then unwarped using the voxel displacement maps, normalised into standard anatomical space defined by the Montreal Neurological Institute (MNI) with a resampled voxel size of 3 × 3 × 3 mm, and smoothed with an 6 mm full-width at half maximum Gaussian kernel filter. In line with Monk et al, Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 trials with incorrect behavioural responses or responses that were less than 200 ms or greater than 1100 ms were removed from fMRI analysis. The maltreated group had a mean of 7.1% (s.d. = 5.4%) of incorrect trials, whereas the comparison group had a mean of 4.4% (s.d. = 5.6%) of incorrect trials. Groups did not differ in the number of incorrect trials, t(39) = −1.57, P = 0.12.
After pre-processing, the smoothed, normalised functional imaging data were entered into a voxel-wise participant-specific general linear model (GLM) with five regressors for the masked faces (angry–neutral congruent, angry–neutral incongruent, happy–neutral congruent, happy–neutral incongruent, neutral–neutral) and one regressor for the blank trials. Trials within each of these six regressors were modelled as stick functions based on trial onset, convolved with a canonical haemodynamic response function. In addition, to correct for residual effects of head motion, six participant-specific movement parameters (derived from the realignment phase of pre-processing) were included as regressors of no interest. An additional regressor of no interest was included to model nuisance trials (i.e. trials that contained incorrect responses, null responses or responses that were too fast or too slow). For 22 participants (11 in the maltreated group and 11 in the non-maltreated group) an extra regressor was included to model a small number of corrupted images resulting from motion greater than 1.5 mm (half the size of our voxel size acquisition). These images (≤10% of each participant's data) were removed and the adjacent images interpolated in order to prevent distortion of the between-participant mask. To remove low-frequency drifts, data were high-pass filtered using a set of discrete cosine basis functions with a cut-off period of 128 s.
The parameter estimates were calculated for all brain voxels using the GLM. Preliminary analyses revealed no effects of congruency, and so reported analyses collapse across congruent and incongruent trials for each emotion. Contrast images for ‘angry > neutral’ (i.e. the combination of angry-neutral congruent and angry-neutral incongruent > neutral-neutral) and ‘happy > neutral’ (i.e. the combination of happy-neutral congruent and happy-neutral incongruent > neutral-neutral) were computed in participant-specific fashion. Next, the participant-specific contrast images were entered into separate second-level analyses for each contrast of interest, where group (maltreated group, non-maltreated group) served as a between-participants variable in independent sample t-tests. The interaction between group and emotion for each contrast were then explored. The amygdala, our a priori region of interest, was anatomically defined based on the automated anatomical labelling bilateral mask from the WFU PickAtlas. Reference Maldjian, Laurienti, Kraft and Burdette33 Within the amygdala mask we report results reaching significance at P<0.05, family-wise error (FWE) corrected. Exploratory correlational analyses were conducted in SPSS version 19 on Windows XP to examine potential associations between brain activation and (a) maltreatment indices (severity, duration, age at onset) and (b) psychiatric symptoms for those indices where significant group differences or strong trends (i.e. P<0.1) were observed. Peak voxel data were used as they reflect a weighted average of the surrounding voxels due to smoothing. Parametric and non-parametric correlations were used for normally distributed and non-normally distributed data, respectively. Finally, we also examined trends for group differences at whole brain level using a statistical threshold of P<0.001 (uncorrected) with an extent threshold of k≥4 voxels, calculated according to the theory of Gaussian random fields.
Analysis of behavioural and questionnaire data
Criteria for determining the acceptability of trials in the behavioural analysis were the same as for the fMRI data analysis. Attention bias scores were derived from a standard formula, which involves subtracting for each participant the mean reaction time on trials where the emotion (angry or happy) face and probe appeared on the same side of the screen (congruent trials) from the mean reaction time on trials where the emotion face and probe appeared on the opposite side of the screen (incongruent trials). Positive bias scores (longer reaction times in congruent than incongruent trials) reflect the tendency to monitor the emotional stimulus, whereas negative bias scores reflect the tendency to avoid the emotional stimulus.
Results
Psychiatric symptom scores
The maltreated and non-maltreated groups did not differ on levels of STAIC anxiety and MFQ depression symptoms (Table 3). This was also reflected in the comparable levels of emotional symptoms across groups, reported by parents and carers on the SDQ. The groups differed, however, in relation to conduct problems and hyperactivity symptoms, with the maltreated sample showing on average higher levels of symptoms than their peers. Group differences in relation to levels of TSCC dissociation symptoms and PTSD symptoms were observed at trend level only (Table 3).
Attentional bias on experimental paradigm
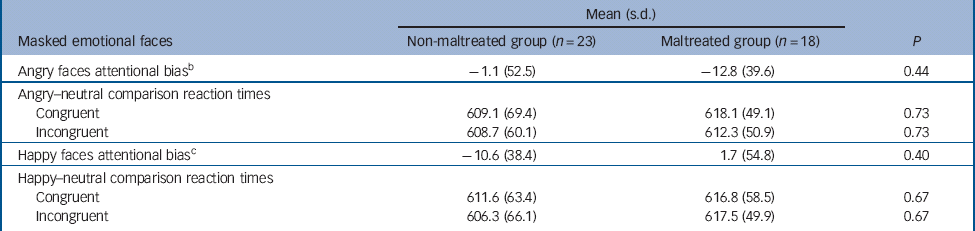
There was no statistically significant group difference in terms of attentional bias and reaction time to either angry or happy trials (Table 4). A repeated measures ANOVA indicated no significant main effects or interactions (group F(1,39) = 0.25, P= 0.62; emotion F(1,39) = 0.22, P= 0.65; congruency F(1,39) = 1.43, P = 0.24; emotion × congruency F(1,40) = 0.10, P = 0.75). Within each group separately there were no significant effects of congruency.
Brain activity
As hypothesised, the maltreated group, compared with the non-maltreated group, exhibited greater activation in the right amygdala (x = +18, y = −1, z = −17; Z = 3.33; k = 2; P = 0.026 FWE corrected) when masked angry and neutral faces were contrasted (Fig. 2a). However, compared with the non-maltreated group, the maltreated group also exhibited greater activation in the right amygdala (x = +21, y = −1, z = −17; Z = 3.20; k = 3; P = 0.039 FWE corrected) when masked happy and neutral faces were contrasted (Fig. 2b). Trends for group differences in activation outside of our amygdala region of interest are shown in Table 5.
Table 3 Psychopathology data for non-maltreated and maltreated groups
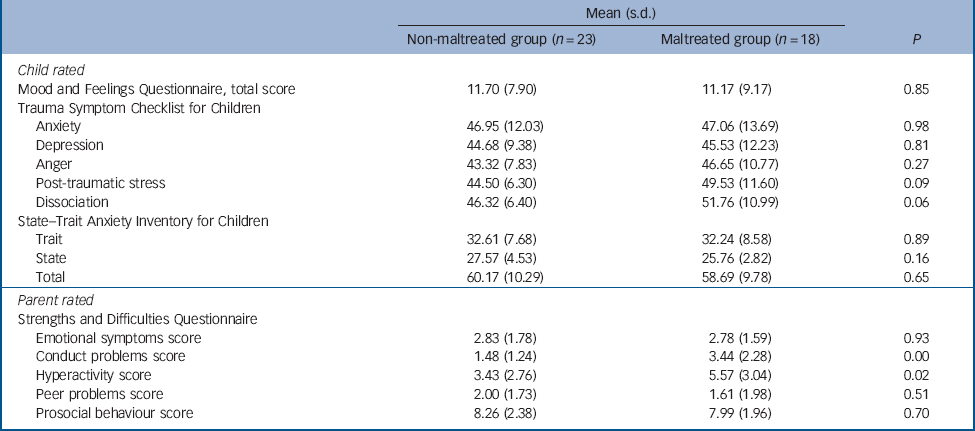
| Mean (s.d.) | |||
|---|---|---|---|
| Non-maltreated group (n = 23) | Maltreated group (n = 18) | P | |
| Child rated | |||
| Mood and Feelings Questionnaire, total score | 11.70 (7.90) | 11.17 (9.17) | 0.85 |
| Trauma Symptom Checklist for Children | |||
| Anxiety | 46.95 (12.03) | 47.06 (13.69) | 0.98 |
| Depression | 44.68 (9.38) | 45.53 (12.23) | 0.81 |
| Anger | 43.32 (7.83) | 46.65 (10.77) | 0.27 |
| Post-traumatic stress | 44.50 (6.30) | 49.53 (11.60) | 0.09 |
| Dissociation | 46.32 (6.40) | 51.76 (10.99) | 0.06 |
| State–Trait Anxiety Inventory for Children | |||
| Trait | 32.61 (7.68) | 32.24 (8.58) | 0.89 |
| State | 27.57 (4.53) | 25.76 (2.82) | 0.16 |
| Total | 60.17 (10.29) | 58.69 (9.78) | 0.65 |
| Parent rated | |||
| Strengths and Difficulties Questionnaire | |||
| Emotional symptoms score | 2.83 (1.78) | 2.78 (1.59) | 0.93 |
| Conduct problems score | 1.48 (1.24) | 3.44 (2.28) | 0.00 |
| Hyperactivity score | 3.43 (2.76) | 5.57 (3.04) | 0.02 |
| Peer problems score | 2.00 (1.73) | 1.61 (1.98) | 0.51 |
| Prosocial behaviour score | 8.26 (2.38) | 7.99 (1.96) | 0.70 |
Table 4 Attentional bias and reaction time data for the dot-probe task, presented by condition and group a
| Mean (s.d.) | |||
|---|---|---|---|
| Masked emotional faces | Non-maltreated group (n = 23) | Maltreated group (n = 18) | P |
| Angry faces attentional bias b | –1.1 (52.5) | –12.8 (39.6) | 0.44 |
| Angry–neutral comparison reaction times | |||
| Congruent | 609.1 (69.4) | 618.1 (49.1) | 0.73 |
| Incongruent | 608.7 (60.1) | 612.3 (50.9) | 0.73 |
| Happy faces attentional bias c | –10.6 (38.4) | 1.7 (54.8) | 0.40 |
| Happy–neutral comparison reaction times | |||
| Congruent | 611.6 (63.4) | 616.8 (58.5) | 0.67 |
| Incongruent | 606.3 (66.1) | 617.5 (49.9) | 0.67 |
a All values are in milliseconds.
b When groups were analysed together, no bias was evident in the angry condition, t(40) = −0.85, P = 0.40.
c When groups were analysed together, no bias was evident in the happy condition, t(40) = −0.73, P = 0.47.
Correlational analyses
No significant correlations were found between amygdala activation and attentional bias scores in either group. However, in the maltreated group several significant associations were observed between amygdala activation and indices of maltreatment experience (Fig. 3). Amygdala response to angry faces was negatively associated with age at onset of emotional maltreatment (r s = −0.70, P = 0.005; Fig. 3a) and age at onset of neglect (r s = −0.72, P = 0.004; Fig. 3b), implying that heightened amygdala response was associated with an earlier onset of these maltreatment subtypes. Consistent with this finding, duration of emotional maltreatment was positively associated with amygdala activation to angry faces (r s = 0.75, P = 0.001; Fig. 3c) and happy faces (r s = 0.72, P = 0.003; Fig. 3d). No significant associations were observed between amygdala activation (for either angry or happy faces) and psychiatric symptoms. However, a few trend-level associations were found (amygdala activation to angry faces and PTSD (r = 0.47, P = 0.06) and dissociation symptoms (r = 0.46, P = 0.06); amygdala activation to happy faces and dissociation symptoms (r = 0.46, P = 0.06)). All significant correlations reported above and depicted in Fig. 3 remained significant when PTSD and dissociation symptoms were included as covariates of no interest in partial correlation analyses.
Discussion
Our aim was to investigate the impact of maltreatment on pre-attentive processing of emotional cues in a community sample of children. Specifically, we used an established paradigm to investigate neural response to angry and happy faces. Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 As predicted, we found that maltreated children, compared with matched peers, showed greater activation in the right amygdala when processing angry faces. However, contrary to our original predictions, we also found elevated right amygdala activation in the maltreated group for happy faces. These findings suggest that maltreatment in the home is associated with alterations even in the earliest stages of affect processing to both positive and negative facial affect.
The increased activation of the amygdala in response to angry faces in the maltreated children is in line with previous ERP and fMRI findings that point to a pattern of ‘hypervigilent’ response to threat-related cues in children exposed to different forms of early adversity. Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4,Reference Pollak13 The right-lateralised amygdala group finding is also in line with most previous effects reported for masked emotional stimuli. Reference Costafreda, Brammer, David and Fu34 The current findings extend previous ERP and fMRI work in maltreated children and point to a pattern of atypical neural processing of threat cues even outside of conscious awareness. However, we also found heightened neural response to happy faces, which appears somewhat at odds with possible adaptation to ‘threat-specific’ cues only. Reference Tamietto and De Gelder15,Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 One possibility is that previous studies have tended to use paradigms in which faces were presented for extended durations; by contrast, the current paradigm, by using pre-attentive presentation of faces, is likely to have indexed earlier stages of processing that are less amenable to higher-order strategic or regulatory influences. Future studies are required to explore whether differential response to pre-attentively presented happy faces has experiential or clinical sequalae.
This pattern of heightened amygdala activation to both happy and angry faces in maltreated children points to broader alterations in affect processing than previously thought. Specifically, these findings suggest that maltreatment heightens neural response to emotional valence (either positive or negative) during the very early stages of facial processing, whereas a selective response to threat (such as anger) may only characterise later stages of processing. Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4 Future studies using magnetoencephalography (MEG) may be helpful in delineating the time course of such processes. Our finding of significant associations between maltreatment experience and neural activation are consistent with the view that heightened amygdala activation to facial affect may represent an adaptation to environmental stress. In relation to emotional abuse, for example, earlier age at onset and longer duration of abuse were associated with greater neural response in the amygdala to angry faces. This finding suggests that
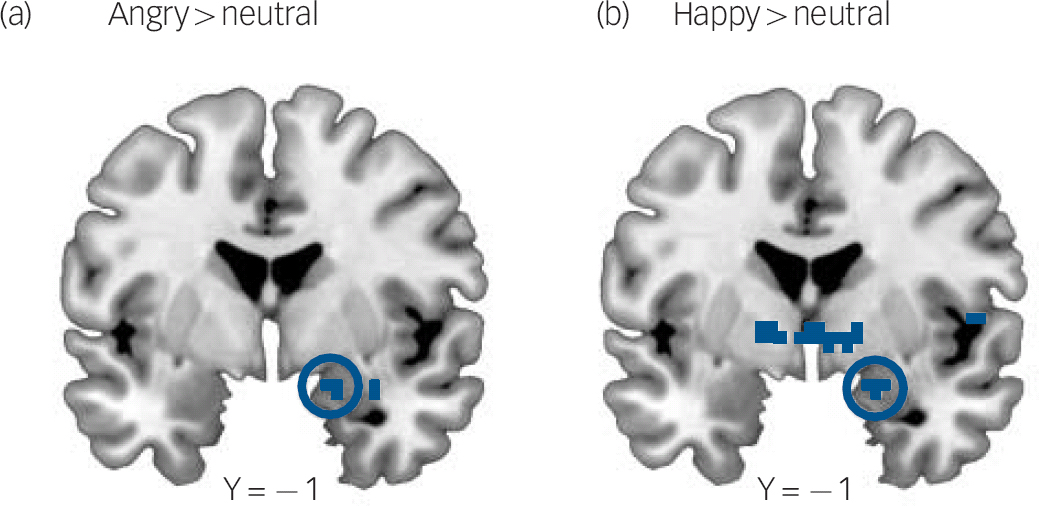
Fig. 2 Greater right amygdala activation to masked angry and masked happy faces relative to neutral faces in the maltreated group.
(a) Statistical parametric map (SPM) showing increased right amygdala activation in maltreated children for the contrast angry > neutral (x, y, z coordinates: 18, −1, −17; Z = 3.33; k = 2; P = 0.026, family-wise error (FWE) corrected). (b) Increased right amygdala activation in maltreated children for the contrast happy > neutral (x, y, z coordinates: 21, −1, −17; Z = 3.20; P = 0.039; k = 3; FWE corrected. SPMs are thresholded at P<0.005 (uncorrected) for visualisation purposes and all coordinates reference the coordinate system of the Montreal Neurological Institute.
Table 5 Whole brain analysis at the uncorrected level, k ≥ 4 voxels, for maltreated > non-maltreated and maltreated < non-maltreated groups for the contrasts angry > neutral and happy > neutral a
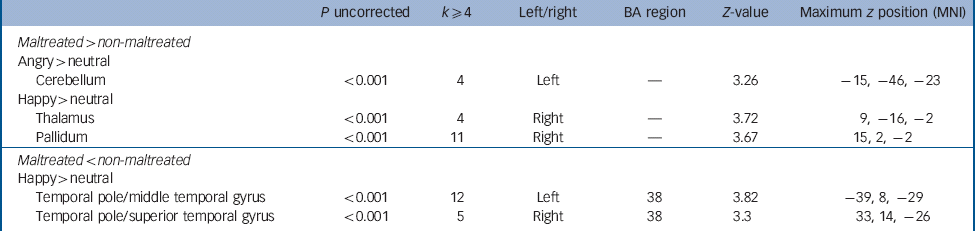
| P uncorrected | k ≥ 4 | Left/right | BA region | Z-value | Maximum z position (MNI) | |
|---|---|---|---|---|---|---|
| Maltreated > non-maltreated | ||||||
| Angry > neutral | ||||||
| Cerebellum | <0.001 | 4 | Left | — | 3.26 | –15, −46, −23 |
| Happy > neutral | ||||||
| Thalamus | <0.001 | 4 | Right | — | 3.72 | 9, −16, −2 |
| Pallidum | <0.001 | 11 | Right | — | 3.67 | 15, 2, −2 |
| Maltreated < non-maltreated | ||||||
| Happy > neutral | ||||||
| Temporal pole/middle temporal gyrus | <0.001 | 12 | Left | 38 | 3.82 | –39, 8, −29 |
| Temporal pole/superior temporal gyrus | <0.001 | 5 | Right | 38 | 3.3 | 33, 14, −26 |
BA, Brodmann area; MNI, Montreal Neurological Institute.
a The comparison maltreated < non-maltreated for the contrast angry > neutral did not produce group differences at P < 0.001 uncorrected.
amygdala activation is to some degree calibrated in line with length of exposure to environmental stress, with exposure during the first 2 years of life appearing particularly influential, consistent with related findings regarding amygdala structure. Reference Tottenham, Hare, Quinn, McCarry, Nurse and Gilhooly35
In considering the research literature more broadly, it has been proposed that early detection of salient emotional cues accords functional and survival advantages Reference LeDoux36 and there is now substantial evidence from human neuroimaging studies for a largely subcortical system that responds to pre-consciously processed emotional signals. Reference Tamietto and De Gelder15 Several studies investigating clinical populations have reported heightened responsiveness of this subcortical system during early stages of affect processing. For example, adult soldiers with PTSD show greater amygdala response to pre-attentively presented threat cues, Reference Rauch, Whalen, Shin, McInerney, MacKlin and Lasko37,Reference Hendler, Rotshtein, Yeshurun, Weizmann, Kahn and Ben-Bashat38 as do children and adolescents with generalised anxiety disorder. Reference Monk, Telzer, Mogg, Bradley, Mai and Louro16 We speculate that such heightened activation may represent an adaptation to environmental adversity conferring short-term functional advantages, for example improving the ability to rapidly detect affective cues in an abusive home environment. However, any such adaptation may incur longer-term costs for the child, limiting attentional resources for mastering age-appropriate skills in social and academic domains. In addition, heightened neural response to affect may increase vulnerability to psychopathology in the longer term. Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4,Reference Shackman, Shackman and Pollak39
Although we have focused on atypical amygdala activation in line with previous studies of childhood adversity, Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4,Reference Maheu, Dozier, Guyer, Mandell, Peloso and Poeth10,Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey11 we also present preliminary evidence for atypical activation in a number of cortical and subcortical regions implicated in pre-attentive processing, including the cerebellum, thalamus and pallidum.
Limitations
A limitation of the current study is the use of a cross-sectional design, which constrains our ability to draw causal inferences between maltreatment exposure and the observed patterns of atypical neural response. Longitudinal studies are required to address issues of causality and whether observed differences remain over time. Another limitation, inherent in studying typically heterogeneous community populations of maltreated children, Reference Dong, Anda, Felitti, Dube, Williamson and Thompson40 is the difficulty in making specific inferences about individual forms of maltreatment. A third limitation relates to potential recruitment bias. Although the final sample included a number of families characterised by severe maltreatment histories, the voluntary nature of the recruitment process may have led to an underrepresentation of more disturbed and chaotic families. Finally, because of our sample size, we were unable to examine the influence of gender, which we know is associated with differential outcomes for boys and girls exposed to early adversity in general Reference Bos, Zeanah, Fox, Drury, McLaughlin and Nelson41 and maltreatment in particular. Reference Lansford, Dodge, Pettit, Bates, Crozier and Kaplow24

Fig. 3 Scatter plots depicting the correlations between the contrast estimates (angry > neutral) from the right amygdala and (a) age at onset of emotional abuse, (b) age at onset of neglect, and (c) duration of emotional abuse. (d) Scatter plot depicting the correlations between the contrast estimates (happy > neutral) from the right amygdala and duration of emotional abuse.
Seventeen maltreated children were identified as having been exposed to emotional abuse, but information about age at onset was only available for 14 participants, whereas information about duration was only available for 15 participants (note that for the plots depicting duration in panels (c) and (d), two data points overlap for 5 years' duration).
Implications
In conclusion, these findings suggest that exposure to maltreatment in the home is associated with an atypical pattern of neural adaptation. Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4,Reference Pollak13 We demonstrate for the first time that childhood maltreatment is associated with increased amygdala activation to pre-attentively presented positive and threatening faces. In other words, maltreated children show a general pattern of atypical affect processing, even outside of conscious awareness. Given the evidence that pre-attentive affect processing is also altered in individuals with anxiety disorders, we suggest that this pattern of heightened amygdala activation in maltreated children may constitute a latent neural risk marker, associated with increased vulnerability to future psychiatric disorder. Reference McCrory, De Brito, Sebastian, Mechelli, Bird and Kelly4
Acknowledgements
We thank the children, parents, carers and social workers who generously participated in this research, and Helen Maris, who helped with the data collection. We also thank Karin Mogg and Brendan Bradley for kindly sharing their stimuli with us.











eLetters
No eLetters have been published for this article.