There has been much debate Reference Hollander, Braun and Simeon1–Reference Bienvenu, Samuels, Wuyek, Liang, Wang and Grados5 regarding the optimal diagnostic classification of obsessive–compulsive disorder (OCD) in relation to the recently published DSM-5. 6 This revision has seen OCD removed from the broad category of anxiety disorders and placed at the centre of a new separate chapter – obsessive–compulsive and related disorders (OCRDs) – including body dysmorphic disorder (BDD), trichotillomania (hair-pulling disorder), as well as excoriation (skin picking) and hoarding disorder as new diagnoses. Based upon available evidence, it has been argued that OCD shares a stronger commonality with these disorders in terms of its core phenomenological, neurobiological and treatment characteristics. Reference Bartz and Hollander2,Reference Lochner and Stein7–Reference Pallanti and Hollander10 However, by implication, the notion that OCD has less in common with anxiety disorders or that anxiety is less relevant to OCD remains contested. Reference Storch, Abramowitz and Goodman3 Multivariate twin studies are particularly well-suited for addressing whether co-occurring mental disorders, such as OCRDs and anxiety disorders, demonstrate overlap in their genetic and environmental risk factors. Reference Neale and Maes11 Of the few existing studies to have directly examined OCD with other anxiety disorders in adults, OCD was reported to show the highest percentage of specific genetic risk factors (45%), although OCD and anxiety disorders demonstrate a substantial common genetic liability (55%). Reference Tambs, Czajkowsky, Røysamb, Neale, Reichborn-Kjennerud and Aggen12 In a recent study that evaluated dimensional representations of OCD and OCRD symptoms (i.e. symptoms in a normative twin population), two distinct genetic liability factors were identified: one factor primarily representing OCD symptoms, hoarding disorder and BDD symptoms; and a second factor representing trichotillomania and skin picking symptoms. Reference Monzani, Rijsdijk, Harris and Mataix-Cols13 In this same cohort it has been reported that 64% of the total covariance between OCD and BDD was explained by shared genetic factors. Reference Monzani, Rijsdijk, Iervolino, Anson, Cherkas and Mataix-Cols14 In summary, prior twin studies suggest that OCD most likely shares genetic factors with both anxiety disorders and certain OCRDs. However, we highlight that no study to date has directly compared OCD with OCRDs and anxiety disorder symptoms in the same twin population assessed at the same period of time. Such comparisons will be important for addressing whether OCD is more or less aetiologically aligned with OCRDs v. anxiety disorders.
Our primary aim was to therefore more thoroughly assess the structure of genetic and environmental risk factors for dimensional representations of OCD, other OCRD and anxiety disorder symptoms in an adult twin population using classical multivariate twin modelling. Our second aim was to complement this approach with a recently introduced regression-based twin analysis, which allows inferences to be made about potential causal relationships between predictor and outcome variables. Reference Hopper, Bui, Erbas, Matheson, Gurrin and Burgess15 With both approaches we sought to address the debated question of whether OCD symptoms are more or less aetiologically aligned with the symptoms of other OCRDs or anxiety disorders. If OCD is more aligned with certain OCRDs v. anxiety disorders, then evidence for a strong common genetic liability should be minimal in a multivariate analysis that combines OCRD and anxiety disorder domains. Further, if this ‘weak’ pattern of liability is evident, there should be minimal evidence for potential causal influences between OCD and anxiety disorder symptoms.
Method
Participants and measures
A total of 2495 voluntary twin members (18 to 45 years old; mean 34.5 years (s.d. = 7.8) and 33.9 (s.d. = 8) monozygotic (MZ) and dizygotic (DZ) respectively) of the Australian Twin Registry (ATR) were recruited for this email-based online survey (1281 MZ and 1214 DZ twins). Online Fig. DS1 provides a schematic overview of the whole sample. All participants gave informed consent after receiving complete information on the study and before starting to fill out the survey. Full recruitment details are provided in López-Solà et al. Reference López-Solà, Fontenelle, Alonso, Cuadras, Foley and Pantelis16 To address this study's aims, we focused specifically on twins' responses to six validated dimensional self-report measures of OCD, OCRD and anxiety disorder symptoms. To address our particular aims, the inclusion of three symptom domains per diagnostic category was considered optimal with respect to the planned multivariate model fitting analyses. OCD symptoms were estimated with the Obsessive Compulsive Inventory-Revised (OCI-R). Reference Foa, Huppert, Leiberg, Langner, Kichic and Hajcak17 For OCRDs, we assessed hoarding disorder symptoms with the Hoarding Rating Scale-Self Report (HRS-SR) Reference Tolin, Frost and Steketee18 and BDD symptoms with the Dysmorphic Concern Questionnaire (DCQ). Reference Oosthuizen, Lambert and Castle19 For anxiety disorders, we assessed social phobia symptoms with the Social Phobia Inventory (SPIN); Reference Connor, Davidson, Churchill, Sherwood, Foa and Weisler20 panic disorder symptoms with the Anxiety Sensitivity Index (ASI); Reference Reiss, Peterson, Gursky and McNally21 and generalised anxiety disorder (GAD) symptoms with the ‘Stress’ subscale of the Depression, Anxiety and Stress Scale-21 (DASS-21). Reference Antony, Bieling, Cox, Enns and Swinson22
Statistical analysis
To ensure data normality, all questionnaire responses underwent Box–Cox transformations (yλ t = (yλ−1)/λ). Reference López-Solà, Fontenelle, Alonso, Cuadras, Foley and Pantelis16,Reference Box and Cox23 To examine the phenotypic structure of OCRD and anxiety disorder symptoms prior to classical twin modelling analysis, a varimax-rotated principal component analysis (PCA) was performed in SPSS (version 20), adjusting the transformed total scores of each scale for age and gender. In order not to confound the comparison of the OCD symptoms and hoarding disorder symptoms measured by the HRS-SR, estimated total scores on the OCI-R excluded twins' responses to the hoarding subscale of this measure (items 1, 7 and 13).
Classical multivariate twin modelling
Structural equation models were conducted on transformed continuous variables fitted by maximum likelihood. Because univariate twin modelling of this data indicated the presence of genetic gender differences, Reference López-Solà, Fontenelle, Alonso, Cuadras, Foley and Pantelis16 all multivariate twin models were performed using standardised residual values for each symptom domain adjusted for age and gender. Reference Zeegers, Rijsdijk and Sham24 Although controlling for the influence of gender in this manner has been a useful approach in multivariate twin studies, this is not the same as testing for multivariate genetic gender differences in which the variance–covariance structure of the model is allowed to be different for males and females. We chose to control rather than test for multivariate genetic gender differences (a) because of an absence of specific hypothesis regarding gender-related multivariate heterogeneity; and (b) because of the acknowledged additional complexity in fitting such models. Reference Neale, Røysamb and Jacobson25
MZ and DZ cross-twin–within/cross-symptom correlations were estimated for each symptom domain by fitting the data to a constrained saturated model. The structure of genetic and environmental influences on OCRD and anxiety disorder symptoms was then estimated using classical multivariate twin models. Reference Neale and Maes11 Model 1 corresponded to a fully saturated Cholesky decomposition that estimated 1A (additive genetic), 1C (shared environment) and 1E (non-shared environment) factors for each phenotype, making no assumptions about the nature of their underlying covariance. Model 2 corresponded to an independent pathway model, which seeks to estimate a set of common Ac, Cc, and Ec factors hypothesised to directly influence all phenotypes v. specific As, Cs and Es factors that may explain remaining phenotypic variance. Model 3 corresponded to a common pathway model, which estimated whether the covariance among phenotypes was influenced via one latent factor taking into account the shared contribution of common A, C and E factors. The Akaike information criterion (AIC) value was used to provide a measure of the goodness of fit of these models. Reduced submodels were systematically tested to derive the most parsimonious model fitting results. Classical twin modelling was carried out in R (www.R-project.org/) using the OpenMx package. Reference Boker, Neale, Maes, Wilde, Spiegel and Brick26
Identifying potential causal influences
Inference on causation from examination of familial confounding (ICE FALCON) is a regression-based approach for analysing twin pair data on a continuously or dichotomously distributed outcome and a familial predictor measured for both the twin and his or her co-twin. Reference Hopper, Bui, Erbas, Matheson, Gurrin and Burgess15 The underlying statistical model allows one to make inferences about (but of course does not ‘prove’) the existence of causal relationships between predictor and outcome variables via the elimination of familial confounding. Reference Dite, Gurrin, Byrnes, Stone, Gunasekara and McCredie27 This can also be thought of as using the co-twin as a ‘negative control’. If the predictor is familial – that is, it is strongly correlated in twins – and there is at least in part a causal relationship between the predictor and the outcome, the association between the predictor of twin A with the outcome of twin B will decrease in absolute strength towards the null after including the predictor of twin B in the model. Reference Dite, Gurrin, Byrnes, Stone, Gunasekara and McCredie27 In other words, this model considers the evidence for confounding because of genetic and/or environmental factors shared by both twins (Ac, C and Ec factors) v. non-shared factors (As and Es). Accordingly, when evidence consistent with a ‘casual’ association is identified, the role of participant-specific factors is emphasised, although the relative contribution of specific genetic v. environmental influences is not determined. If the associations between the outcome of twin A and the predictors of both the twin A and co-twin B are the same before and after adjusting for each other, then, under this model, there is no evidence consistent with a potential causal relationship. On the other hand, if there is a significant attenuation of the cross-trait cross-pair association after conditioning on twin A, there is evidence ‘consistent with’ some causation. In our analyses, we focused on the relationship between OCD symptoms and each of the OCRD and anxiety disorder symptom domains. A step-by-step explanation of this approach is provided in online Fig. DS2 and online supplement DS1.
Results
Correlation and PCA
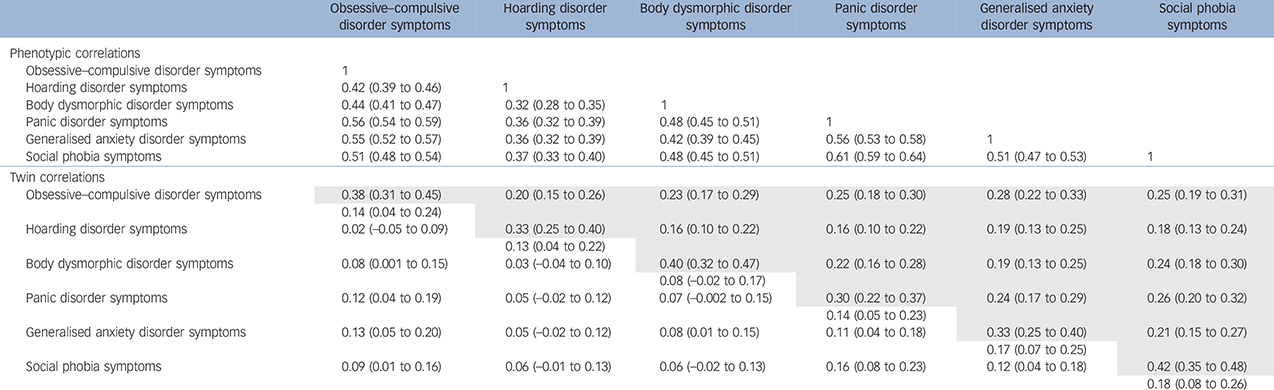
Moderate-strength phenotypic correlations were observed across all symptom domains. The strongest correlations with OCD were found with anxiety disorder symptoms; the weakest associations were observed between hoarding disorder and BDD symptoms, and hoarding disorder and anxiety disorder symptoms (0.32–0.37). The pattern of cross-twin cross-trait correlations in MZ compared with DZ twins supports a relevant genetic component to the liability of each domain and their co-occurrence (Table 1). PCA retained one phenotypic factor with an eigenvalue greater than 1 and explaining 55.6% of the total variance. When forcing it to retain 2 factors, the total explained variance increased to 67.8%, and hoarding disorder symptoms emerged as a distinct factor (see online Table DS1).
Table 1 Phenotypic, cross-twin within-trait (diagonal) and cross-twin cross-trait (off-diagonal) correlations for monozygotic (MD) and dizygotic (DZ) male and female twins with 95% confidence intervals a
| Obsessive–compulsive disorder symptoms |
Hoarding
disorder symptoms |
Body dysmorphic
disorder symptoms |
Panic
disorder symptoms |
Generalised
anxiety disorder symptoms |
Social
phobia symptoms |
|
|---|---|---|---|---|---|---|
| Phenotypic correlations | ||||||
| Obsessive–compulsive disorder symptoms | 1 | |||||
| Hoarding disorder symptoms | 0.42 (0.39 to 0.46) | 1 | ||||
| Body dysmorphic disorder symptoms | 0.44 (0.41 to 0.47) | 0.32 (0.28 to 0.35) | 1 | |||
| Panic disorder symptoms | 0.56 (0.54 to 0.59) | 0.36 (0.32 to 0.39) | 0.48 (0.45 to 0.51) | 1 | ||
| Generalised anxiety disorder symptoms | 0.55 (0.52 to 0.57) | 0.36 (0.32 to 0.39) | 0.42 (0.39 to 0.45) | 0.56 (0.53 to 0.58) | 1 | |
| Social phobia symptoms | 0.51 (0.48 to 0.54) | 0.37 (0.33 to 0.40) | 0.48 (0.45 to 0.51) | 0.61 (0.59 to 0.64) | 0.51 (0.47 to 0.53) | 1 |
| Twin correlations | ||||||
| Obsessive–compulsive disorder symptoms | 0.38 (0.31 to 0.45) 0.14 (0.04 to 0.24) |
0.20 (0.15 to 0.26) | 0.23 (0.17 to 0.29) | 0.25 (0.18 to 0.30) | 0.28 (0.22 to 0.33) | 0.25 (0.19 to 0.31) |
| Hoarding disorder symptoms | 0.02 (−0.05 to 0.09) | 0.33 (0.25 to 0.40) 0.13 (0.04 to 0.22) |
0.16 (0.10 to 0.22) | 0.16 (0.10 to 0.22) | 0.19 (0.13 to 0.25) | 0.18 (0.13 to 0.24) |
| Body dysmorphic disorder symptoms | 0.08 (0.001 to 0.15) | 0.03 (−0.04 to 0.10) | 0.40 (0.32 to 0.47) 0.08 (−0.02 to 0.17) |
0.22 (0.16 to 0.28) | 0.19 (0.13 to 0.25) | 0.24 (0.18 to 0.30) |
| Panic disorder symptoms | 0.12 (0.04 to 0.19) | 0.05 (−0.02 to 0.12) | 0.07 (−0.002 to 0.15) | 0.30 (0.22 to 0.37) 0.14 (0.05 to 0.23) |
0.24 (0.17 to 0.29) | 0.26 (0.20 to 0.32) |
| Generalised anxiety disorder symptoms | 0.13 (0.05 to 0.20) | 0.05 (−0.02 to 0.12) | 0.08 (0.01 to 0.15) | 0.11 (0.04 to 0.18) | 0.33 (0.25 to 0.40) 0.17 (0.07 to 0.25) |
0.21 (0.15 to 0.27) |
| Social phobia symptoms | 0.09 (0.01 to 0.16) | 0.06 (−0.01 to 0.13) | 0.06 (−0.02 to 0.13) | 0.16 (0.08 to 0.23) | 0.12 (0.04 to 0.18) | 0.42 (0.35 to 0.48) 0.18 (0.08 to 0.26) |
a. Cross-twin cross-trait correlations for MZ are given in the area above the diagonal and for DZ twins in the area below the diagonal.
Multivariate twin modelling
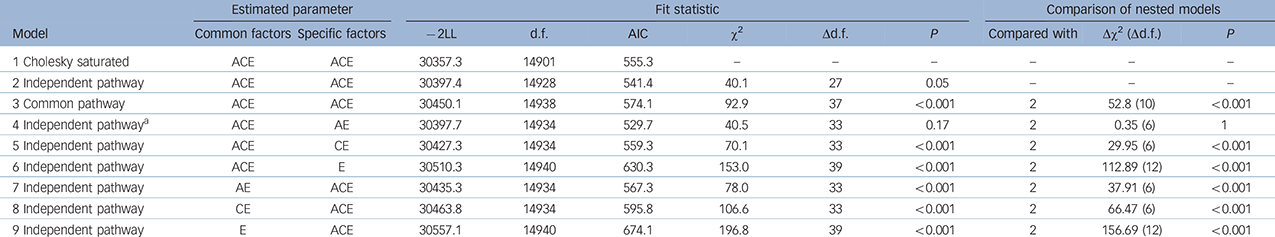
The independent pathway model provided an improved fit, compared with the fully saturated Cholesky with reduced parameters (Table 2). However, the more restrictive common pathway model resulted in a significantly worse fit with an increased AIC value. A series of independent pathway nested submodels were fitted to test the importance of specific parameters compared with the fully saturated Cholesky and the independent pathway model. In these submodels, the genetic and environmental liabilities were either forced to be entirely common/shared (models 4–6) or entirely independent/specific (models 7–9). Model 4 was the best-fitting such that the covariation between phenotypes was explained by a set of common Ac, Cc and Ec factors, and the remaining variance by As and Es effects specific to each phenotype. Removing the Cs factor did not lead to worse fit, suggesting that these factors were less important in explaining individual differences. Removing As +/− Cs (models 5 and 6) and forcing all genetic risk to be common led to significantly worse fit, suggesting that some of the genetic liability is specific to each phenotype. Models 7 to 9 were forced to have an As, Cs and Es specific to each phenotype, and none of them demonstrated good fit, indicating the existence of common liability to all phenotypes.
Table 2 Model-fitting results
| Estimated parameter | Fit statistic | Comparison of nested models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Common factors | Specific factors | −2LL | d.f. | AIC | χ2 | Δd.f. | P | Compared with | Δχ2 (Δd.f.) | P |
| 1 Cholesky saturated | ACE | ACE | 30357.3 | 14901 | 555.3 | – | – | – | – | – | – |
| 2 Independent pathway | ACE | ACE | 30397.4 | 14928 | 541.4 | 40.1 | 27 | 0.05 | – | – | – |
| 3 Common pathway | ACE | ACE | 30450.1 | 14938 | 574.1 | 92.9 | 37 | <0.001 | 2 | 52.8 (10) | <0.001 |
| 4 Independent pathway a | ACE | AE | 30397.7 | 14934 | 529.7 | 40.5 | 33 | 0.17 | 2 | 0.35 (6) | 1 |
| 5 Independent pathway | ACE | CE | 30427.3 | 14934 | 559.3 | 70.1 | 33 | <0.001 | 2 | 29.95 (6) | <0.001 |
| 6 Independent pathway | ACE | E | 30510.3 | 14940 | 630.3 | 153.0 | 39 | <0.001 | 2 | 112.89 (12) | <0.001 |
| 7 Independent pathway | AE | ACE | 30435.3 | 14934 | 567.3 | 78.0 | 33 | <0.001 | 2 | 37.91 (6) | <0.001 |
| 8 Independent pathway | CE | ACE | 30463.8 | 14934 | 595.8 | 106.6 | 33 | <0.001 | 2 | 66.47 (6) | <0.001 |
| 9 Independent pathway | E | ACE | 30557.1 | 14940 | 674.1 | 196.8 | 39 | <0.001 | 2 | 156.69 (12) | <0.001 |
−2LL, minus twice the log-likelihood; Δd.f., change in degrees of freedom between the submodel and the full model; Δχ2, difference in goodness-of-fit statistic between the submodel and the full model; A, additive genetic factor; C, shared environmental factor; E, non-shared environmental factor.
a. The best-fitting model based on the lower Akaike information criterion (AIC).
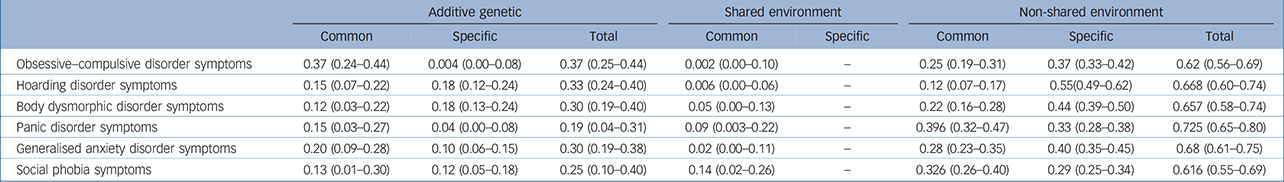
Parameter estimates for the best-fitting model are presented in Table 3 and the independent pathway model in Fig. 1(a). Although the Cc factor could not be dropped from the model, it accounted for a minor fraction of the overall variance (0.2–14%). Figure 1(b) shows that OCD symptoms were the only phenotype to share almost all of its additive genetic influence with the remaining OCRD and anxiety disorder symptoms, suggesting that OCD symptoms are aetiologically related to all of these phenotypes. For hoarding disorder and BDD symptoms, 55% and 61% of the total genetic variance, respectively, was as a result of As, suggesting more specific genetic risk factors for these symptoms v. other anxiety disorder and/or OCRD symptoms. For these reasons, we conducted a separate multivariate analysis with two Ac latent factors: one loading on all symptom domains and another loading only on OCD, hoarding disorder and BDD symptoms. Similar to the former model, we observed that the majority of the genetic variance for OCD symptoms still loaded onto the first Ac factor sharing genetic effects with other anxiety disorders and OCRDs. Only 9% of the total genetic variance of OCD was as a result of genetic factors shared with other OCRDs. For BDD symptoms 50% of its total genetic variance loaded onto the specific genetic factor (As), whereas 1.4% loaded onto shared genetic factors with the other OCRDs. A total of 37.5% of the total genetic variance of hoarding symptoms loaded onto shared genetic factors with the other OCRDs, with 31.3% as a result of specific genetic influences. Results of the two-factor independent pathway model are given in online Table DS2.
Table 3 Standardised parameters for the best-fitting Model 4 (with 95% confidence intervals)
| Additive genetic | Shared environment | Non-shared environment | ||||||
|---|---|---|---|---|---|---|---|---|
| Common | Specific | Total | Common | Specific | Common | Specific | Total | |
| Obsessive–compulsive disorder symptoms | 0.37 (0.24–0.44) | 0.004 (0.00–0.08) | 0.37 (0.25–0.44) | 0.002 (0.00–0.10) | – | 0.25 (0.19–0.31) | 0.37 (0.33–0.42) | 0.62 (0.56–0.69) |
| Hoarding disorder symptoms | 0.15 (0.07–0.22) | 0.18 (0.12–0.24) | 0.33 (0.24–0.40) | 0.006 (0.00–0.06) | – | 0.12 (0.07–0.17) | 0.55(0.49–0.62) | 0.668 (0.60–0.74) |
| Body dysmorphic disorder symptoms | 0.12 (0.03–0.22) | 0.18 (0.13–0.24) | 0.30 (0.19–0.40) | 0.05 (0.00–0.13) | – | 0.22 (0.16–0.28) | 0.44 (0.39–0.50) | 0.657 (0.58–0.74) |
| Panic disorder symptoms | 0.15 (0.03–0.27) | 0.04 (0.00–0.08) | 0.19 (0.04–0.31) | 0.09 (0.003–0.22) | – | 0.396 (0.32–0.47) | 0.33 (0.28–0.38) | 0.725 (0.65–0.80) |
| Generalised anxiety disorder symptoms | 0.20 (0.09–0.28) | 0.10 (0.06–0.15) | 0.30 (0.19–0.38) | 0.02 (0.00–0.11) | – | 0.28 (0.23–0.35) | 0.40 (0.35–0.45) | 0.68 (0.61–0.75) |
| Social phobia symptoms | 0.13 (0.01–0.30) | 0.12 (0.05–0.18) | 0.25 (0.10–0.40) | 0.14 (0.02–0.26) | – | 0.326 (0.26–0.40) | 0.29 (0.25–0.34) | 0.616 (0.55–0.69) |
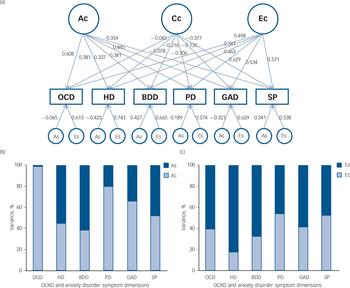
Fig. 1 (a) Independent pathway (best-fitting) model. (b) and (c) The percentage of the variance accounted for by common and specific genetic and non-shared environmental factors.
(a) Ac (symptom-common genetic influence), Cc (symptom-common shared environmental influence) and Ec (symptom-common non-shared environmental influence). The breakdown of the genetic and non-shared environmental variance into common and specific factors is shown in (b) A (Ac and As) and (c) E (Ec and Es), respectively. OCD, obsessive–compulsive disorder symptoms; HD, hoarding disorder symptoms; BDD, body dysmorphic disorder symptoms; PD, panic disorder symptoms; GAD, generalised anxiety disorder symptoms; SP, social phobia symptoms; OCRD, obsessive–compulsive and related disorders.
Potential causal influences
As depicted in Fig. 2 and Table 4, there was evidence for significant causal influences between OCD and anxiety disorder symptom domains. Specifically, OCD symptoms demonstrate a significant causal influence on GAD and panic disorder symptoms, respectively (P<0.0001). In other words, it can be inferred that there is a high probability of observing changes in the severity of these latter domains when a person's OCD symptom severity changes, but not vice versa. By comparison, we observed a significant causal influence of social phobia symptoms on OCD symptoms (P = 0.03), suggesting that OCD symptoms themselves show some dependency on social phobia symptoms. Social phobia was the only symptom domain to demonstrate a causal influence on OCD symptoms.
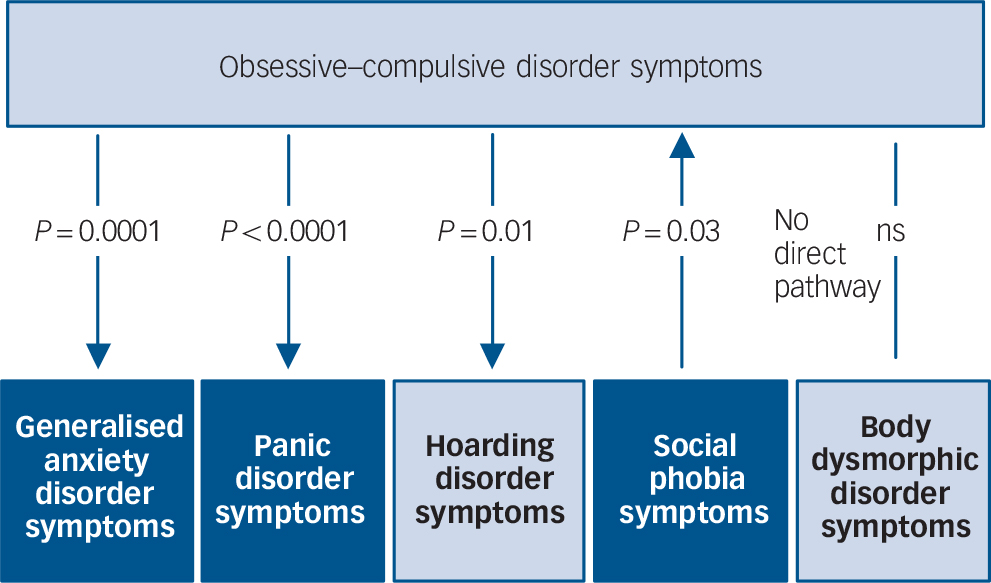
Fig. 2 Causal modelling with inference on causation from examination of familial confounding (ICE FALCON).
P-values refer to the significance of the regression coefficient change between Model II to Model III. The direction of the arrows indicates the direction of estimated ‘causality’. The light and dark colouring symbolise DSM-5 representations of obsessive–compulsive and related disorders and anxiety disorders respectively. ns, not significant.
Table 4 Inference on causation from examination of familial confounding (ICE FALCON) after testing the probability for both directions of causation between each pair of symptoms a
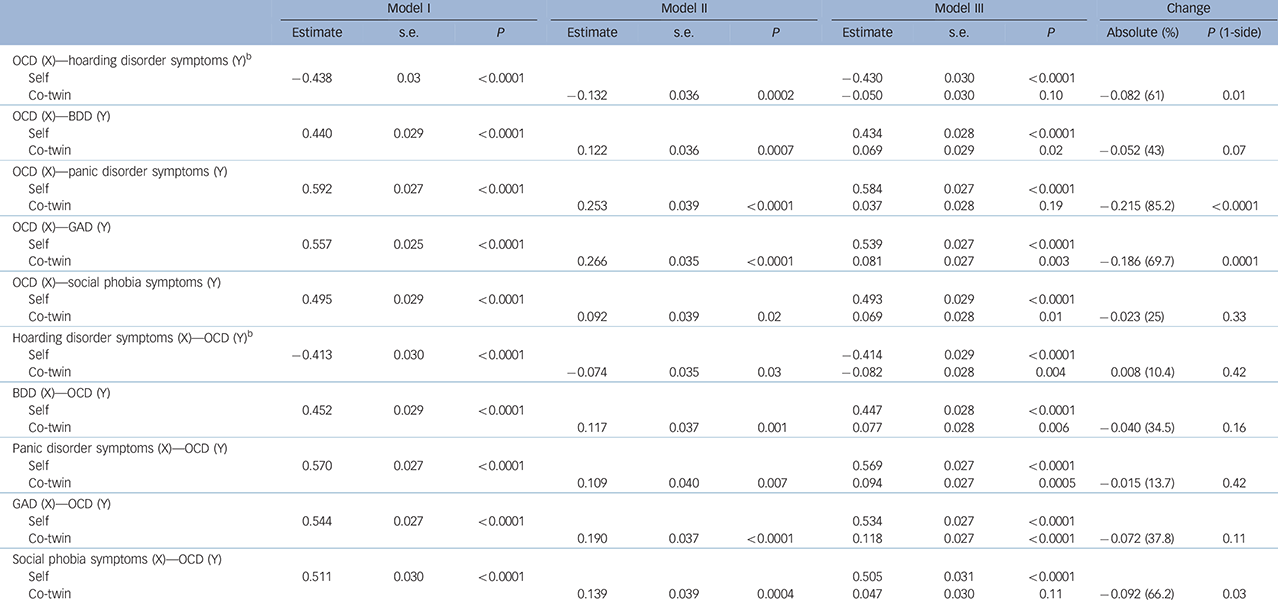
| Model I | Model II | Model III | Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | s.e. | P | Estimate | s.e. | P | Estimate | s.e. | P | Absolute (%) | P (1-side) | |
| OCD (X)–hoarding disorder symptoms (Y) b | |||||||||||
| Self | −0.438 | 0.03 | <0.0001 | −0.430 | 0.030 | <0.0001 | |||||
| Co-twin | −0.132 | 0.036 | 0.0002 | −0.050 | 0.030 | 0.10 | −0.082 (61) | 0.01 | |||
| OCD (X)–BDD (Y) | |||||||||||
| Self | 0.440 | 0.029 | <0.0001 | 0.434 | 0.028 | <0.0001 | |||||
| Co-twin | 0.122 | 0.036 | 0.0007 | 0.069 | 0.029 | 0.02 | −0.052 (43) | 0.07 | |||
| OCD (X)–panic disorder symptoms (Y) | |||||||||||
| Self | 0.592 | 0.027 | <0.0001 | 0.584 | 0.027 | <0.0001 | |||||
| Co-twin | 0.253 | 0.039 | <0.0001 | 0.037 | 0.028 | 0.19 | −0.215 (85.2) | <0.0001 | |||
| OCD (X)–GAD (Y) | |||||||||||
| Self | 0.557 | 0.025 | <0.0001 | 0.539 | 0.027 | <0.0001 | |||||
| Co-twin | 0.266 | 0.035 | <0.0001 | 0.081 | 0.027 | 0.003 | −0.186 (69.7) | 0.0001 | |||
| OCD (X)–social phobia symptoms (Y) | |||||||||||
| Self | 0.495 | 0.029 | <0.0001 | 0.493 | 0.029 | <0.0001 | |||||
| Co-twin | 0.092 | 0.039 | 0.02 | 0.069 | 0.028 | 0.01 | −0.023 (25) | 0.33 | |||
| Hoarding disorder symptoms (X)–OCD (Y) b | |||||||||||
| Self | −0.413 | 0.030 | <0.0001 | −0.414 | 0.029 | <0.0001 | |||||
| Co-twin | −0.074 | 0.035 | 0.03 | −0.082 | 0.028 | 0.004 | 0.008 (10.4) | 0.42 | |||
| BDD (X)–OCD (Y) | |||||||||||
| Self | 0.452 | 0.029 | <0.0001 | 0.447 | 0.028 | <0.0001 | |||||
| Co-twin | 0.117 | 0.037 | 0.001 | 0.077 | 0.028 | 0.006 | −0.040 (34.5) | 0.16 | |||
| Panic disorder symptoms (X)–OCD (Y) | |||||||||||
| Self | 0.570 | 0.027 | <0.0001 | 0.569 | 0.027 | <0.0001 | |||||
| Co-twin | 0.109 | 0.040 | 0.007 | 0.094 | 0.027 | 0.0005 | −0.015 (13.7) | 0.42 | |||
| GAD (X)–OCD (Y) | |||||||||||
| Self | 0.544 | 0.027 | <0.0001 | 0.534 | 0.027 | <0.0001 | |||||
| Co-twin | 0.190 | 0.037 | <0.0001 | 0.118 | 0.027 | <0.0001 | −0.072 (37.8) | 0.11 | |||
| Social phobia symptoms (X)–OCD (Y) | |||||||||||
| Self | 0.511 | 0.030 | <0.0001 | 0.505 | 0.031 | <0.0001 | |||||
| Co-twin | 0.139 | 0.039 | 0.0004 | 0.047 | 0.030 | 0.11 | −0.092 (66.2) | 0.03 | |||
OCD, obsessive–compulsive disorder symptoms; X, independent variable; Y, outcome variable; BDD, body dysmorphic disorder symptoms; GAD, generalised anxiety disorder symptoms.
a. Regression estimates, standard error and P-value from regression models on monozygotic twin pairs.
b. The inverse relationship between OCD and hoarding disorder symptoms was because of the fact that we transformed the raw data using reciprocal function for hoarding disorder symptoms (1/hoarding disorder symptoms0.55) and logarithm function for OCD.
Within the OCRD domain, evidence of a significant causal influence of OCD symptoms was observed on hoarding disorder symptoms (P = 0.01), suggesting that any change in OCD symptom severity would predict a corresponding change in hoarding disorder severity, but not vice versa. There was trend evidence suggesting a causal influence of OCD symptoms on BDD symptoms (P = 0.07), but not vice versa.
Discussion
In summary, our results do not support the contention that OCD symptoms are less aetiologically aligned with the symptoms of anxiety disorders compared with some of the revised DSM-5 OCRDs. On the basis of classical twin modelling, evidence of a genetic commonality between OCD and anxiety disorder symptoms was observed, such that the genetic liability to OCD symptoms was better explained when modelling its shared liability with OCRD and anxiety disorder symptoms compared with when modelling an additional OCRD latent genetic factor. On the basis of causal inference analysis we observed evidence consistent with OCD symptoms being a potential causal risk factor for panic disorder, GAD and hoarding disorder symptoms, in the sense that having OCD symptoms appears to increase the probability of having panic disorder and GAD symptoms, but not vice versa. By comparison, we observed evidence consistent with social phobia symptoms being a potential causal risk factor for OCD symptoms. We could reject the alternate (null) hypothesis that there are no direct causal relations.
A non-specific genetic vulnerability to OCD symptoms
Our twin modelling results indicate that the genetic liability to OCD symptoms was almost entirely shared with the five other symptom domains. Of these domains, panic disorder, GAD and social phobia symptoms also demonstrated greater common vs. specific genetic liabilities. When taken together with previous twin studies, there is now good evidence to suggest that OCD is influenced by moderately heritable genetic factors that are mostly shared with other OCRDs and anxiety disorders. Reference Tambs, Czajkowsky, Røysamb, Neale, Reichborn-Kjennerud and Aggen12–Reference Monzani, Rijsdijk, Iervolino, Anson, Cherkas and Mataix-Cols14,Reference Kirk, Birley, Statham, Haddon, Lake and Andrews28,Reference Taylor29 Thus, it stands to reason that this common liability may partly underlie the co-occurrence of these disorders in terms of their high rates of comorbidity Reference Nestadt, Samuels, Riddle, Liang, Bienvenu and Hoehn-Saric30 and familial aggregation. Reference Bienvenu, Samuels, Wuyek, Liang, Wang and Grados5,Reference Brakoulias, Starcevic, Sammut, Berle, Milicevic and Moses31 Phenomenologically, in addition to the general characteristic of heightened threat estimation, Reference Storch, Abramowitz and Goodman3 there are other underlying features that link OCD and anxiety disorder symptoms. For instance, high levels of self-blaming emotions, such as guilt and shame, appear to be shared between OCD and other anxiety disorders, including social phobia. Reference Pallanti and Quercioli32 Additionally, although most robustly linked to panic disorder, heightened anxiety sensitivity is observed in patients with OCD, GAD and social phobia, which may reflect a common cognitive bias towards ‘over-importance of thoughts’ – a recognised dimension of anxiety sensitivity. Reference Wheaton, Mahaffey, Timpano, Berman and Abramowitz33,Reference Calamari, Rector, Woodard, Cohen and Chik34 Common deficits of attentional control have also been emphasised in relation to OCD and other anxiety disorders, particularly GAD, as a feature that may explain the pervasive negative cognitions (i.e. obsessions, worry) that characterise these disorders. Reference Armstrong, Zald and Olatunji35 Of course, although such features are not characteristic of all patients with OCDs, nor patients with other anxiety disorders, they nonetheless appear to represent important transdiagnostic ‘common threads’ that may in part arise from such estimated common liabilities.
OCD and anxiety disorder symptoms as putative causal risk factors
Extending the classical modelling approach, causal pathway modelling has identified novel and potentially important relationships between OCD and anxiety disorder symptoms. With this approach, the results were more consistent with OCD symptoms being a causal risk factor for panic disorder and GAD symptoms (having OCD symptoms increases the probability of having panic disorder and GAD symptoms, not vice versa), rather than the association between traits being as a result of unmeasured familial factors such as genes and shared environment. In other studies without twins, OCD checking symptoms – one of the most common OCD symptom dimensions – have been linked to the increased probability of comorbid panic disorder and GAD diagnoses. Reference Stasik, Naragon-Gainey, Chmielewski and Watson36 It was proposed that an intolerance of uncertainty, which is present in OCD checking symptoms, but more strongly associated with general worry and anxiety, might explain the link between these domains. Thus, one possibility is that intolerance of uncertainty represents an underlying trait dimension through which OCD aetiologically enhances the risk of panic disorder and GAD symptoms.
By contrast, social phobia symptoms emerged as a potential causal risk factor for OCD symptoms, although this was a more moderate finding compared with the estimated strength of the above associations. Nevertheless, adopting similar logic, one possibility is that a relevant underlying trait factor may explain these findings. In the case of social phobia symptoms, one obvious candidate would be ‘behavioural inhibition’. Although behavioural inhibition has been most strongly characterised as a childhood predictor of social phobia, Reference Rosenbaum, Biederman, Faraone, Hirshfeld and Kagan37 it has also been linked to the development of OCD symptoms in adulthood. Reference Coles, Schofield and Pietrefesa38 Supporting an early aetiological link between these domains, mother-reported levels of inhibition/shyness in preschool-aged twins were reported to show substantial overlap with other anxiety-related behaviours, including OCD-like behaviours. Reference Eley, Bolton, O'Connor, Perrin, Smith and Plomin39 One hypothesis may therefore be that behavioural inhibition, as a core social anxiety trait, partly underlies the development of OCD and potentially represents an important endophenotype related to the co-occurring nature of OCD and anxiety disorders.
Relationships for OCRD symptoms
With respect to the initial multivariate analysis, specific genetic influences were more apparent with regard to hoarding disorder and BDD symptoms. Considering that no multivariate twin studies have examined them together with anxiety disorder symptoms, these results are novel. In a recent study of five OCRD domains, OCD and hoarding disorder symptoms were characterised as sharing more common liability, followed by BDD, then trichotillomania and skin-picking symptoms. Reference Monzani, Rijsdijk, Harris and Mataix-Cols13 In the independent pathway model with two common genetic factors, only a small percentage of the total genetic effect of BDD and OCD symptoms loaded onto the OCRD latent factor, as compared with hoarding disorder symptoms. The greater loading for hoarding disorder symptoms may be explained by the following two points: (a) hoarding disorder demonstrated a ‘weak’ phenotypic correlation with BDD symptoms – the lowest among all domains assessed (see also Monzani et al Reference Monzani, Rijsdijk, Harris and Mataix-Cols13 ); and (b) hoarding disorder symptoms emerged from the exploratory PCA as a second distinct factor, suggesting it contains some unique variance with regard to the other OCRD domains.
Despite these seemingly complex associations, OCD symptoms were identified as a potential causal risk factor for hoarding disorder symptoms. Previous twin studies have documented a close association between both symptom domains, with hoarding disorder symptoms being reported to share a substantial common genetic liability with other major OCD symptom dimensions. Reference Iervolino, Rijsdijk, Cherkas, Fullana and Mataix-Cols40 Some authors have suggested that the characteristic feature of indecision in OCD, Reference Sachdev and Malhi41 which is also observed in hoarding disorder, Reference Frost and Gross42,Reference Samuels, Bienvenu, Pinto, Fyer, McCracken and Rauch43 may be a significant risk factor for hoarding disorder that is genetically transmitted with OCD. Reference Samuels, Bienvenu, Pinto, Fyer, McCracken and Rauch43 Our results potentially add weight to this hypothesis by demonstrating a putative causal link between these symptom domains. Nonetheless, the unique variance estimated for hoarding disorder symptoms at the multivariate level suggests there are likely to be distinct aetiological factors underlying aspects of hoarding disorder that are not present in OCD, such as the inability to discard.
Limitations
There are certain limitations to this study. First, all symptoms were assessed by self-report measures including the DCQ, DASS-21 Stress subscale and ASI, which do not perfectly match the diagnostic criteria for BDD, GAD and panic disorder. For example, although the ASI has shown validity in distinguishing between individuals experiencing panic attacks v. those with panic disorder, Reference Rector, Szacun-Shimizu and Leybman44 it is also predictive of other psychiatric disorders. Reference Calamari, Rector, Woodard, Cohen and Chik34 Second, a reliance on self-report limits the generalisation of these findings to dimensional representations of symptoms rather than disorders, and does not allow one to rule out whether symptoms may be as a result of unmeasured third-party factors, such as other mental or medical conditions. Third, it will be important in future multivariate twin studies to assess relationships between OCRDs, anxiety disorders and depression, tic and somatoform disorder symptoms, given ongoing interest in clarifying the aetiological links between these domains. Indeed, it is highly likely that the aetiological common threads suggested by the current results will extend beyond the specific OCRD and anxiety disorder domains studied here. Finally, extension of the current findings in a prospective longitudinal twin study Reference Stone, Dite, Giles, Cawson, English and Hopper45 will be important for validating inferences of direct causal associations between these domains.
Implications
In conclusion, the current findings suggest that ongoing aetiological (for example molecular genetic) and treatment-focused studies of OCD are likely to benefit from the consideration of a more diverse phenotype that represents its important links with some OCRDs (including tic disorders) but also with certain anxiety disorders. Parallels can be drawn between this sentiment and recent efforts to identify common molecular genetic risk factors that cut across other major psychiatric diagnoses, including schizophrenia, bipolar disorder and major depression, among others. Reference Lee, Ripke, Neale, Faraone, Purcell and Perlis46,Reference Smoller, Ripke, Lee, Neale, Nurnberger and Santangelo47 Importantly, if confirmed by future studies, the observed causal influences identified here may encourage novel approaches to treatment intervention, with potential to reduce the overall burden of these disorders when co-occurring in individual patients.









eLetters
No eLetters have been published for this article.