Neurological ‘soft signs’ are commonly found in individuals with schizophrenia (e.g. Reference Gupta, Andreasen and ArndtGupta et al, 1995) and appear to have a developmental origin, having been identified in studies of high-risk children (Reference Erlenmeyer-Kimling and CornblattErlenmeyer-Kimling & Cornblatt, 1987), birth cohorts (Reference Jones, Rodgers and MurrayJones et al, 1994), prodromal illness (Reference McGorry, McFarlane and PattonMcGorry et al, 1995), patients with negative symptoms (Reference Malla, Norman and AgullarMalla et al, 1997), and drug-naïve patients (Reference Schroder, Niethammer and GeiderSchroder et al, 1991). One putative aetiological factor is early childhood infectious illness. Viral exposure in utero has been suggested (Reference Mednick, Machon and HuttunenMednick et al, 1988; Reference Rantakallio, Jones and MoringRantakallio et al, 1997), although findings are inconsistent with respect to the presence or identity of viral infection across studies (Reference Done, Johnstone and FrithDone et al, 1991; Reference Westergaard, Mortensen and PedersenWestergaard et al, 1999). We explored associations between common childhood infections and adult schizophrenia, affective psychosis, epilepsy and a range of neurological soft signs in the 1958 UK National Child Development Study (NCDS) cohort. We asked the following: are neurological soft signs in childhood increased in relation to adult psychosis; are infectious illnesses during childhood associated with adult psychosis; and, are there consistent associations between early infection, increased neurological soft signs and adult psychosis?
METHOD
The present study was based on data contained in the 1958 Perinatal Mortality Study, later the NCDS, which included 98% of births in mainland Britain during the week 3-9 March 1958. Five subsequent sweeps traced and gathered data on cohort members at age 7, 11, 16, 23 and 33 years (NCDS 1-5). Data were initially obtained following consent by parents. Consent was sought from individual cohort members in later sweeps and ethical approval was obtained from all responsible regional ethical committees involved in the case-finding exercises.
Neurological ‘soft signs’
Systematic medical examinations were carried out on over 12 000 members of the cohort at the ages of 7 and 11 years, and ‘neurological’ measures were recorded by school medical officers during these examinations, from parents, teachers and a standardised medical examination (Table 1). Some of these measures were coded as ordered categoricals, e.g. question: “Child has poor hand control”; answer “Certainly applies”, “somewhat applies”, and “not at all”. Where possible this information was retained in the analysis.
Table 1 Childhood illnesses and neurological soft signs recorded by age 11 according to adult outcomes
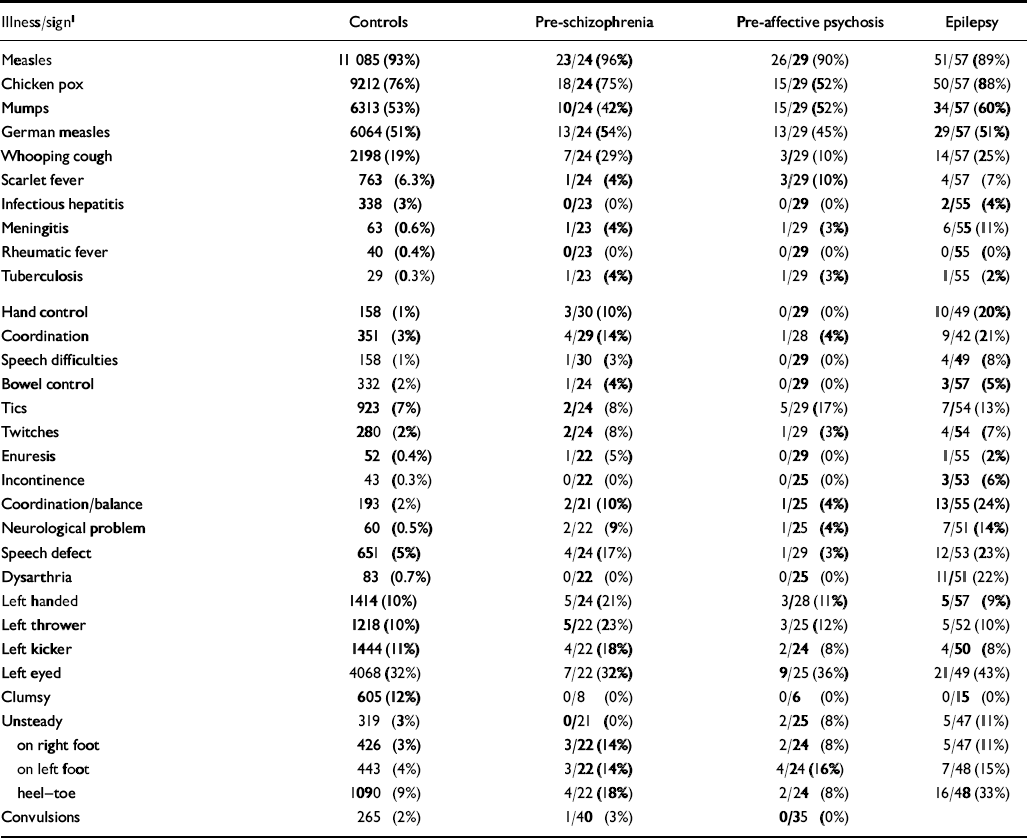
| Illness/sign1 | Controls | Pre-schizophrenia | Pre-affective psychosis | Epilepsy |
|---|---|---|---|---|
| Measles | 11 085 (93%) | 23/24 (96%) | 26/29 (90%) | 51/57 (89%) |
| Chicken pox | 9212 (76%) | 18/24 (75%) | 15/29 (52%) | 50/57 (88%) |
| Mumps | 6313 (53%) | 10/24 (42%) | 15/29 (52%) | 34/57 (60%) |
| German measles | 6064 (51%) | 13/24 (54%) | 13/29 (45%) | 29/57 (51%) |
| Whooping cough | 2198 (19%) | 7/24 (29%) | 3/29 (10%) | 14/57 (25%) |
| Scarlet fever | 763 (6.3%) | 1/24 (4%) | 3/29 (10%) | 4/57 (7%) |
| Infectious hepatitis | 338 (3%) | 0/23 (0%) | 0/29 (0%) | 2/55 (4%) |
| Meningitis | 63 (0.6%) | 1/23 (4%) | 1/29 (3%) | 6/55 (11%) |
| Rheumatic fever | 40 (0.4%) | 0/23 (0%) | 0/29 (0%) | 0/55 (0%) |
| Tuberculosis | 29 (0.3%) | 1/23 (4%) | 1/29 (3%) | 1/55 (2%) |
| Hand control | 158 (1%) | 3/30 (10%) | 0/29 (0%) | 10/49 (20%) |
| Coordination | 351 (3%) | 4/29 (14%) | 1/28 (4%) | 9/42 (21%) |
| Speech difficulties | 158 (1%) | 1/30 (3%) | 0/29 (0%) | 4/49 (8%) |
| Bowel control | 332 (2%) | 1/24 (4%) | 0/29 (0%) | 3/57 (5%) |
| Tics | 923 (7%) | 2/24 (8%) | 5/29 (17%) | 7/54 (13%) |
| Twitches | 280 (2%) | 2/24 (8%) | 1/29 (3%) | 4/54 (7%) |
| Enuresis | 52 (0.4%) | 1/22 (5%) | 0/29 (0%) | 1/55 (2%) |
| Incontinence | 43 (0.3%) | 0/22 (0%) | 0/25 (0%) | 3/53 (6%) |
| Coordination/balance | 193 (2%) | 2/21 (10%) | 1/25 (4%) | 13/55 (24%) |
| Neurological problem | 60 (0.5%) | 2/22 (9%) | 1/25 (4%) | 7/51 (14%) |
| Speech defect | 651 (5%) | 4/24 (17%) | 1/29 (3%) | 12/53 (23%) |
| Dysarthria | 83 (0.7%) | 0/22 (0%) | 0/25 (0%) | 11/51 (22%) |
| Left handed | 1414 (10%) | 5/24 (21%) | 3/28 (11%) | 5/57 (9%) |
| Left thrower | 1218 (10%) | 5/22 (23%) | 3/25 (12%) | 5/52 (10%) |
| Left kicker | 1444 (11%) | 4/22 (18%) | 2/24 (8%) | 4/50 (8%) |
| Left eyed | 4068 (32%) | 7/22 (32%) | 9/25 (36%) | 21/49 (43%) |
| Clumsy | 605 (12%) | 0/8 (0%) | 0/6 (0%) | 0/15 (0%) |
| Unsteady | 319 (3%) | 0/21 (0%) | 2/25 (8%) | 5/47 (11%) |
| on right foot | 426 (3%) | 3/22 (14%) | 2/24 (8%) | 5/47 (11%) |
| on left foot | 443 (4%) | 3/22 (14%) | 4/24 (16%) | 7/48 (15%) |
| heel—toe | 1090 (9%) | 4/22 (18%) | 2/24 (8%) | 16/48 (33%) |
| Convulsions | 265 (2%) | 1/40 (3%) | 0/35 (0%) |
Table 2 Illnesses/signs v. outcomes (odds ratios, 95% CI). Statistically significant results (i.e. 95% CI excludes unity) are shown in bold
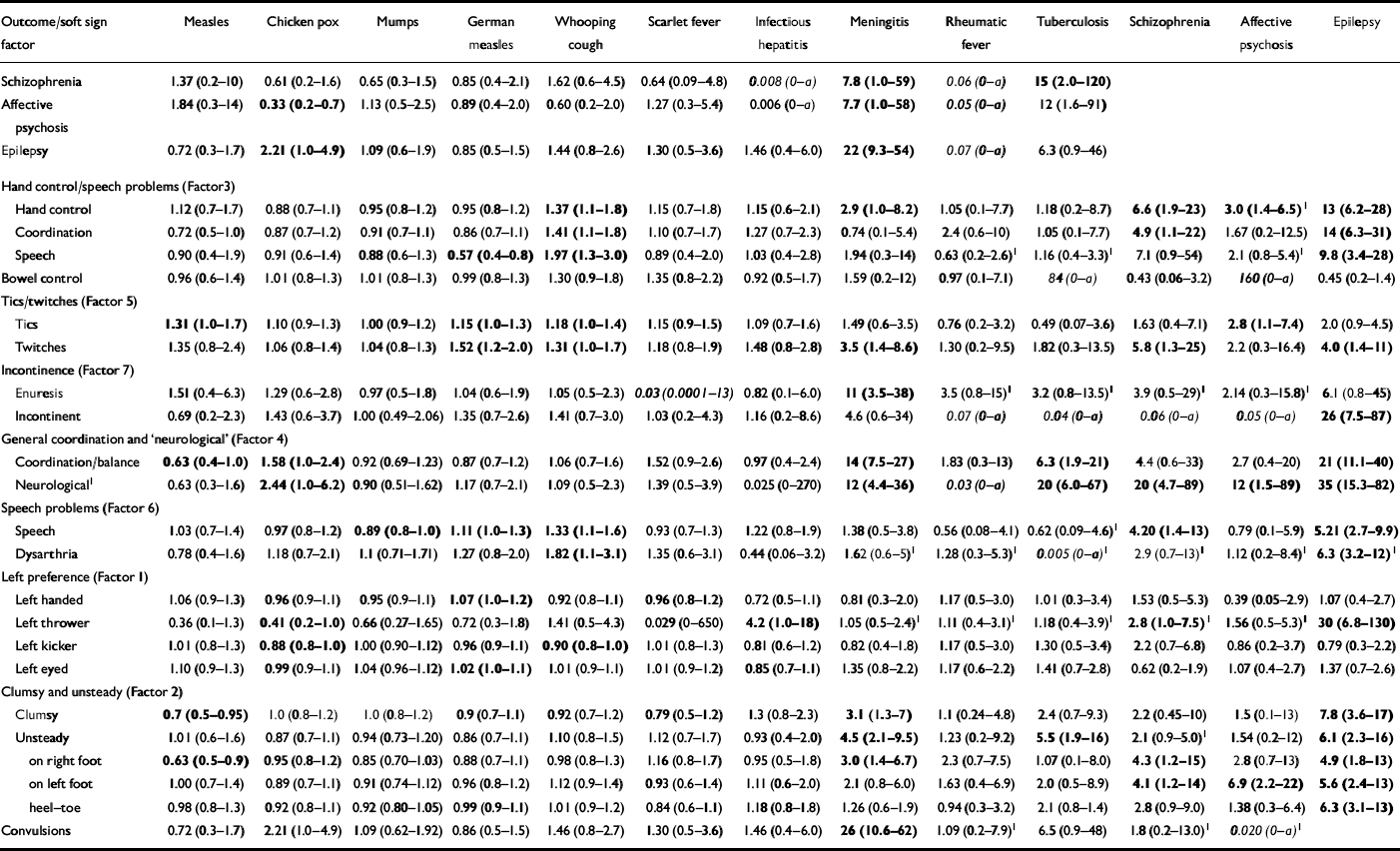
| Outcome/soft sign factor | Measles | Chicken pox | Mumps | German measles | Whooping cough | Scarlet fever | Infectious hepatitis | Meningitis | Rheumatic fever | Tuberculosis | Schizophrenia | Affective psychosis | Epilepsy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | 1.37 (0.2-10) | 0.61 (0.2-1.6) | 0.65 (0.3-1.5) | 0.85 (0.4-2.1) | 1.62 (0.6-4.5) | 0.64 (0.09-4.8) | 0.008 (0-a) | 7.8 (1.0-59) | 0.06 (0-a) | 15 (2.0-120) | |||
| Affective psychosis | 1.84 (0.3-14) | 0.33 (0.2-0.7) | 1.13 (0.5-2.5) | 0.89 (0.4-2.0) | 0.60 (0.2-2.0) | 1.27 (0.3-5.4) | 0.006 (0-a) | 7.7 (1.0-58) | 0.05 (0-a) | 12 (1.6-91) | |||
| Epilepsy | 0.72 (0.3-1.7) | 2.21 (1.0-4.9) | 1.09 (0.6-1.9) | 0.85 (0.5-1.5) | 1.44 (0.8-2.6) | 1.30 (0.5-3.6) | 1.46 (0.4-6.0) | 22 (9.3-54) | 0.07 (0-a) | 6.3 (0.9-46) | |||
| Hand control/speech problems (Factor3) | |||||||||||||
| Hand control | 1.12 (0.7-1.7) | 0.88 (0.7-1.1) | 0.95 (0.8-1.2) | 0.95 (0.8-1.2) | 1.37 (1.1-1.8) | 1.15 (0.7-1.8) | 1.15 (0.6-2.1) | 2.9 (1.0-8.2) | 1.05 (0.1-7.7) | 1.18 (0.2-8.7) | 6.6 (1.9-23) | 3.0 (1.4-6.5) 1 | 13 (6.2-28) |
| Coordination | 0.72 (0.5-1.0) | 0.87 (0.7-1.2) | 0.91 (0.7-1.1) | 0.86 (0.7-1.1) | 1.41 (1.1-1.8) | 1.10 (0.7-1.7) | 1.27 (0.7-2.3) | 0.74 (0.1-5.4) | 2.4 (0.6-10) | 1.05 (0.1-7.7) | 4.9 (1.1-22) | 1.67 (0.2-12.5) | 14 (6.3-31) |
| Speech | 0.90 (0.4-1.9) | 0.91 (0.6-1.4) | 0.88 (0.6-1.3) | 0.57 (0.4-0.8) | 1.97 (1.3-3.0) | 0.89 (0.4-2.0) | 1.03 (0.4-2.8) | 1.94 (0.3-14) | 0.63 (0.2-2.6)1 | 1.16 (0.4-3.3)1 | 7.1 (0.9-54) | 2.1 (0.8-5.4)1 | 9.8 (3.4-28) |
| Bowel control | 0.96 (0.6-1.4) | 1.01 (0.8-1.3) | 1.01 (0.8-1.3) | 0.99 (0.8-1.3) | 1.30 (0.9-1.8) | 1.35 (0.8-2.2) | 0.92 (0.5-1.7) | 1.59 (0.2-12) | 0.97 (0.1-7.1) | 84 (0-a) | 0.43 (0.06-3.2) | 160 (0-a) | 0.45 (0.2-1.4) |
| Tics/twitches (Factor 5) | |||||||||||||
| Tics | 1.31 (1.0-1.7) | 1.10 (0.9-1.3) | 1.00 (0.9-1.2) | 1.15 (1.0-1.3) | 1.18 (1.0-1.4) | 1.15 (0.9-1.5) | 1.09 (0.7-1.6) | 1.49 (0.6-3.5) | 0.76 (0.2-3.2) | 0.49 (0.07-3.6) | 1.63 (0.4-7.1) | 2.8 (1.1-7.4) | 2.0 (0.9-4.5) |
| Twitches | 1.35 (0.8-2.4) | 1.06 (0.8-1.4) | 1.04 (0.8-1.3) | 1.52 (1.2-2.0) | 1.31 (1.0-1.7) | 1.18 (0.8-1.9) | 1.48 (0.8-2.8) | 3.5 (1.4-8.6) | 1.30 (0.2-9.5) | 1.82 (0.3-13.5) | 5.8 (1.3-25) | 2.2 (0.3-16.4) | 4.0 (1.4-11) |
| Incontinence (Factor 7) | |||||||||||||
| Enuresis | 1.51 (0.4-6.3) | 1.29 (0.6-2.8) | 0.97 (0.5-1.8) | 1.04 (0.6-1.9) | 1.05 (0.5-2.3) | 0.03 (0.0001-13) | 0.82 (0.1-6.0) | 11 (3.5-38) | 3.5 (0.8-15)1 | 3.2 (0.8-13.5)1 | 3.9 (0.5-29)1 | 2.14 (0.3-15.8)1 | 6.1 (0.8-45) |
| Incontinent | 0.69 (0.2-2.3) | 1.43 (0.6-3.7) | 1.00 (0.49-2.06) | 1.35 (0.7-2.6) | 1.41 (0.7-3.0) | 1.03 (0.2-4.3) | 1.16 (0.2-8.6) | 4.6 (0.6-34) | 0.07 (0-a) | 0.04 (0-a) | 0.06 (0-a) | 0.05 (0-a) | 26 (7.5-87) |
| General coordination and ‘neurological’ (Factor 4) | |||||||||||||
| Coordination/balance | 0.63 (0.4-1.0) | 1.58 (1.0-2.4) | 0.92 (0.69-1.23) | 0.87 (0.7-1.2) | 1.06 (0.7-1.6) | 1.52 (0.9-2.6) | 0.97 (0.4-2.4) | 14 (7.5-27) | 1.83 (0.3-13) | 6.3 (1.9-21) | 4.4 (0.6-33) | 2.7 (0.4-20) | 21 (11.1-40) |
| Neurological1 | 0.63 (0.3-1.6) | 2.44 (1.0-6.2) | 0.90 (0.51-1.62) | 1.17 (0.7-2.1) | 1.09 (0.5-2.3) | 1.39 (0.5-3.9) | 0.025 (0-270) | 12 (4.4-36) | 0.03 (0-a) | 20 (6.0-67) | 20 (4.7-89) | 12 (1.5-89) | 35 (15.3-82) |
| Speech problems (Factor 6) | |||||||||||||
| Speech | 1.03 (0.7-1.4) | 0.97 (0.8-1.2) | 0.89 (0.8-1.0) | 1.11 (1.0-1.3) | 1.33 (1.1-1.6) | 0.93 (0.7-1.3) | 1.22 (0.8-1.9) | 1.38 (0.5-3.8) | 0.56 (0.08-4.1) | 0.62 (0.09-4.6)1 | 4.20 (1.4-13) | 0.79 (0.1-5.9) | 5.21 (2.7-9.9) |
| Dysarthria | 0.78 (0.4-1.6) | 1.18 (0.7-2.1) | 1.1 (0.71-1.71) | 1.27 (0.8-2.0) | 1.82 (1.1-3.1) | 1.35 (0.6-3.1) | 0.44 (0.06-3.2) | 1.62 (0.6-5)1 | 1.28 (0.3-5.3)1 | 0.005 (0-a) 1 | 2.9 (0.7-13)1 | 1.12 (0.2-8.4)1 | 6.3 (3.2-12) 1 |
| Left preference (Factor 1) | |||||||||||||
| Left handed | 1.06 (0.9-1.3) | 0.96 (0.9-1.1) | 0.95 (0.9-1.1) | 1.07 (1.0-1.2) | 0.92 (0.8-1.1) | 0.96 (0.8-1.2) | 0.72 (0.5-1.1) | 0.81 (0.3-2.0) | 1.17 (0.5-3.0) | 1.01 (0.3-3.4) | 1.53 (0.5-5.3) | 0.39 (0.05-2.9) | 1.07 (0.4-2.7) |
| Left thrower | 0.36 (0.1-1.3) | 0.41 (0.2-1.0) | 0.66 (0.27-1.65) | 0.72 (0.3-1.8) | 1.41 (0.5-4.3) | 0.029 (0-650) | 4.2 (1.0-18) | 1.05 (0.5-2.4)1 | 1.11 (0.4-3.1)1 | 1.18 (0.4-3.9)1 | 2.8 (1.0-7.5) 1 | 1.56 (0.5-5.3)1 | 30 (6.8-130) |
| Left kicker | 1.01 (0.8-1.3) | 0.88 (0.8-1.0) | 1.00 (0.90-1.12) | 0.96 (0.9-1.1) | 0.90 (0.8-1.0) | 1.01 (0.8-1.3) | 0.81 (0.6-1.2) | 0.82 (0.4-1.8) | 1.17 (0.5-3.0) | 1.30 (0.5-3.4) | 2.2 (0.7-6.8) | 0.86 (0.2-3.7) | 0.79 (0.3-2.2) |
| Left eyed | 1.10 (0.9-1.3) | 0.99 (0.9-1.1) | 1.04 (0.96-1.12) | 1.02 (1.0-1.1) | 1.01 (0.9-1.1) | 1.01 (0.9-1.2) | 0.85 (0.7-1.1) | 1.35 (0.8-2.2) | 1.17 (0.6-2.2) | 1.41 (0.7-2.8) | 0.62 (0.2-1.9) | 1.07 (0.4-2.7) | 1.37 (0.7-2.6) |
| Clumsy and unsteady (Factor 2) | |||||||||||||
| Clumsy | 0.7 (0.5-0.95) | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | 0.9 (0.7-1.1) | 0.92 (0.7-1.2) | 0.79 (0.5-1.2) | 1.3 (0.8-2.3) | 3.1 (1.3-7) | 1.1 (0.24-4.8) | 2.4 (0.7-9.3) | 2.2 (0.45-10) | 1.5 (0.1-13) | 7.8 (3.6-17) |
| Unsteady | 1.01 (0.6-1.6) | 0.87 (0.7-1.1) | 0.94 (0.73-1.20) | 0.86 (0.7-1.1) | 1.10 (0.8-1.5) | 1.12 (0.7-1.7) | 0.93 (0.4-2.0) | 4.5 (2.1-9.5) | 1.23 (0.2-9.2) | 5.5 (1.9-16) | 2.1 (0.9-5.0)1 | 1.54 (0.2-12) | 6.1 (2.3-16) |
| on right foot | 0.63 (0.5-0.9) | 0.95 (0.8-1.2) | 0.85 (0.70-1.03) | 0.88 (0.7-1.1) | 0.98 (0.8-1.3) | 1.16 (0.8-1.7) | 0.95 (0.5-1.8) | 3.0 (1.4-6.7) | 2.3 (0.7-7.5) | 1.07 (0.1-8.0) | 4.3 (1.2-15) | 2.8 (0.7-13) | 4.9 (1.8-13) |
| on left foot | 1.00 (0.7-1.4) | 0.89 (0.7-1.1) | 0.91 (0.74-1.12) | 0.96 (0.8-1.2) | 1.12 (0.9-1.4) | 0.93 (0.6-1.4) | 1.11 (0.6-2.0) | 2.1 (0.8-6.0) | 1.63 (0.4-6.9) | 2.0 (0.5-8.9) | 4.1 (1.2-14) | 6.9 (2.2-22) | 5.6 (2.4-13) |
| heel—toe | 0.98 (0.8-1.3) | 0.92 (0.8-1.1) | 0.92 (0.80-1.05) | 0.99 (0.9-1.1) | 1.01 (0.9-1.2) | 0.84 (0.6-1.1) | 1.18 (0.8-1.8) | 1.26 (0.6-1.9) | 0.94 (0.3-3.2) | 2.1 (0.8-1.4) | 2.8 (0.9-9.0) | 1.38 (0.3-6.4) | 6.3 (3.1-13) |
| Convulsions | 0.72 (0.3-1.7) | 2.21 (1.0-4.9) | 1.09 (0.62-1.92) | 0.86 (0.5-1.5) | 1.46 (0.8-2.7) | 1.30 (0.5-3.6) | 1.46 (0.4-6.0) | 26 (10.6-62) | 1.09 (0.2-7.9)1 | 6.5 (0.9-48) | 1.8 (0.2-13.0)1 | 0.020 (0-a) 1 |
Childhood illnesses
Childhood and adolescent health status were based on medical examinations carried out by the school medical officer, and a home interview with the child's main carer (usually the mother) conducted by a health visitor. Data were collected on a wide range of conditions. The reports of well-known relationships between certain childhood health measures and other outcomes, e.g. adolescent health (Reference Power and PeckhamPower & Peckham, 1990) and adult lung function (Reference Johnston, Strachan and AndersonJohnston et al, 1998), confer a degree of internal validity to these records. Furthermore, findings based on these data have appeared in papers on the epidemiology of child ill health (e.g. Reference Power and PeckhamPower & Peckham, 1990; Reference Pollock and GoldingPollock & Golding, 1993), although only a small number have specifically used the childhood viral illness records (Reference Pollock and GoldingPollock & Golding, 1993; Reference Johnston, Strachan and AndersonJohnston et al, 1998). No information was recorded on age at onset, duration or severity of the illnesses.
Epilepsy
The NCDS is one of the few epidemiological studies that has used systematic research criteria for diagnosing epilepsy (Reference Kurtz, Tookey and RossKurtz et al, 1998). Details about diagnostic criteria are provided by Kurtz et al (Reference Kurtz, Tookey and Ross1998).
Psychotic illness
Cohort members discharged from psychiatric hospitals between 1974 and 1986 were identified using information from the now defunct national database, the Mental Health Enquiry. Their case notes were obtained and a Present State Examination (PSE) schedule completed. These were then analysed using the CATEGO computerised diagnostic program from which diagnostic classes were obtained (Reference Wing, Cooper and SartoriusWing et al, 1974). Cases were identified achieving a diagnosis of ‘narrow schizophrenia’ (PSE S+) and affective psychosis (unipolar or bipolar mood disorder with psychotic features; Reference Done, Johnstone and FrithDone et al, 1991).
Data analysis
Logistic regression was performed to explore associations between childhood illnesses, neurological soft signs and adult outcomes in terms of odds ratios. All analyses were performed using the Computer package SPSS for Windows v8.0.
‘Follow-back’ designs such as this use a case-finding model unlikely to be biased by direct effects of the exposures. Likelihood of psychiatric admission as an adult will not be directly affected by illnesses or soft signs recorded in childhood, and the majority of cases of severe mental illness, such as psychosis, will be admitted to hospital. Thus power is limited by the number of cases, but not obviously bias. The calculated power varies with rates in cases and the population. Thus, it was felt that odds ratios with 95% confidence intervals would adequately demonstrate both effect size and uncertainty, as well as inform future power calculations in this field.
Wald statistic-derived odds ratios (with 95% confidence intervals) were felt to be valid in the context of a large sample size (Reference Tabachnik and FidellTabachnik & Fidell, 1996). Given the exploratory nature of this analysis, overall trends were judged to be more informative than any individual test, and any odds ratios the 95% confidence intervals of which excluded unity (95% CI≠1) were examined in terms of the consistency of the size and sign of the effect for that factor. That is, did a given exposure result in similar effects, overall, across outcomes?
For the analysis of neurological soft signs we used factor analysis, as the numerous soft signs were deemed to be manifest measures of a smaller number of latent neurological abnormalities. Data on similar measures of neurological dysfunction gathered at the age of 7 years were used to explore the stability of the factor structure over time. Gender and social class were treated as covariates.
Given the lack of information on duration or onset of the childhood illnesses, no attempt was made to analyse with respect to degree of exposure or development of neurological effects over time. Similarly, a path analysis was not appropriate given that soft signs and ‘illnesses up to this point’ were effectively measured simultaneously. It seemed appropriate to simply explore associations and consistency between the three strata: childhood illness; neurological soft sign; and adult outcome. Associations consistent between these might suggest a causal pathway, or a common underlying deficit with both neurological (soft signs) and immunological (illnesses) markers.
RESULTS
Of the total number of births between 3 and 9 March 1958 in mainland Britain (17 733), data were collected for 98% (17 414). Information on childhood illnesses was gathered at age 11 on 14 501 cases (83%). Using the method described above, 40 cases were identified as achieving a diagnosis of ‘narrow schizophrenia’ (PSE S+); similarly, 35 cases of affective psychosis were identified. Of the 1043 children who at age 7 or 11 reported episodes of loss of consciousness, fits, faints or convulsions, 64 were eventually determined to have epilepsy (Reference Kurtz, Tookey and RossKurtz et al, 1998).
Distribution of illnesses and soft signs for the population and outcomes are shown in Table 1. Note that, for all measures, the numbers of cases that had data in childhood varies. However, this attrition was felt to be independent of measure and outcome, and not to bias the findings.
Associations between total number of soft signs and illnesses were investigated using linear regression and were not significant (standardised β = -0.004, P=0.65). Associations between gender and social class and the total numbers of illnesses and soft signs were small but significant (number of illnesses v. gender Z=- 2.4, P=0.016; v. class χ2=46.3, P<0.001; and number of soft signs v. gender Z= - 13.8, P<0.001; v. class χ2=46.3, P<0.001). Higher social class was associated with a greater number of illnesses. Significantly increased numbers of soft signs were seen for schizophrenia (Z= - 2.04, P=0.042) and epilepsy (Z= - 6.1, P<0.001), but not for affective psychosis (Z=0.89, P=0.37). None of the adult outcomes was associated significantly with the aggregate measure of total number of illnesses recorded.
Given continuing interest in individual illnesses and soft signs, an attempt was made to simplify the data while retaining the real measures.
-
(a) Adjustment for social class resulted in small changes in odds ratios, which in some cases reversed the sign of the effect. Adjusting for gender resulted in only small changes in odds ratios in both directions in the second decimal place, never reversing the sign of the main effect. Adjustment for both gender and social class was little different from the adjustment for social class. Results are reported adjusted, except where the logistic regression model broke down because of small numbers in cells, where unadjusted figures are quoted.
-
(b) Where soft signs were coded as ordered categoricals (i.e. not present, possibly present, or definitely present) this information was retained in the model, although odds ratios are reported only for the definite presence of the soft sign. The exceptions are where insufficient ‘definite’ cases existed to support the model and here the odds ratio for ‘possibly present’ is reported.
-
(c) In tests where no cases had a soft sign by either definite or probable measure, results are given in italics (see Table 2).
None of the common childhood illnesses was associated with adult schizophrenia, although the rare illnesses meningitis and tuberculosis were both associated significantly with odds ratios of 7.8 (95% CI=1.0-59) and 15 (95% CI=2.0-120), respectively. In general, common childhood illness was not associated with affective psychosis, although a reduced risk of chicken pox (OR=0.33, 95% CI=0.2-0.7) is noteworthy. As with schizophrenia, affective psychosis is associated with meningitis and tuberculosis.
For common childhood illnesses, only whooping cough was associated with increased risk of neurological soft signs (small positive effects for seven of the eight significant associations between whooping cough and adult outcomes/neurological soft signs; see Table 2) although even this association disappears as soon as a more conservative criterion for statistical significance (e.g. α≤0.01) is adopted. As expected, meningitis (large positive effects for all 12 significant associations; see Table 2) and tuberculosis (large positive effects for all six significant associations; see Table 2) do show robust associations with neurological soft signs.
Factor analysis of the soft signs yielded seven factors consisting of more than one measure (percentage variance explained in parentheses): (1) ‘left preference’ (12.4%); (2) ‘clumsy and unsteady’ (11.1%); (3) ‘hand control and speech problems’ (8.4%); (4) general coordination and ‘neurological’ (7.5%); (5) ‘tics/twitches’ (7.2%); (6) ‘speech problems’ (6.0%); and (7) ‘incontinence’ (5.8%). These factors do in general represent meaningful clusters of related soft signs, suggestive of common neurological origin (see Table 2).
Associations between these soft sign factors and adult outcomes were investigated using analysis of variance. Children who later developed schizophrenia had increased loading on all soft signs factors, significant for factors 3 and 4. Table 2 also shows a consistent pattern of raised levels of neurological soft signs (i.e. OR>1) in this group. Children who later developed affective psychosis showed a statistically significant increase of soft signs in factor 4 only. Cases of childhood epilepsy, as expected, had increased loadings on all seven soft signs factors, significant for factors 2-5. Logistic regression confirmed that the fit of the outcomes of both schizophrenia and epilepsy on soft signs remained little altered after the effects of tuberculosis and meningitis had been removed.
DISCUSSION
The advantages of studies in a national birth cohort, with prospectively gathered data in unselected populations, when investigating early influences on later illness are clear. Recall bias can confound any attempt to discern effects on serious, rare outcomes. Large sample size allows examination of relatively rare outcomes.
Limitations
Validity and reliability of diagnosis of childhood illness is uncertain. Information regarding the more common viral illnesses was obtained from the parents, diagnoses which even today can confuse health professionals in the absence of serology. For example, more illnesses were found in higher social classes. One possible explanation is that better parental education improves ability to remember or differentiate between common illnesses. The finding contrasts these data — total number of different illnesses noted — with a measure of total exposure to disease, which one would expect to decrease with increasing social class (cf. the number of soft signs). Similarly, ‘meningitis’ cannot be subdivided further, for example into viral or bacterial.
Information on duration, severity and timing of illness is lacking. The lack of data on timing of illnesses could obscure any effects on neurodevelopment that only occur during a critical ‘window’ of development, and the development of any soft signs could precede or follow an illness.
The diagnosis of ‘epilepsy’ was made by age 11, and thus might not include cases of partial epilepsy that commonly might not manifest until young adulthood.
Neurological soft signs measures come from medical, school and parental reports. The measures, while signs of ‘non-localised neurological deficits’, have to be seen in the context of the developmental literature of the time and will only indirectly reflect current concepts (Reference Schroder, Niethammer and GeiderSchroder et al, 1991). However, given the variety of sources of information, exploratory factor analysis of the measures at 7 and 11 years was performed and showed consistency in the factor structure over time, suggesting reliability of the measures as well as validity of the constructs.
Neurological soft signs and adult outcomes
Our findings confirm earlier reports of raised levels of neurological soft signs during childhood in individuals who later develop schizophrenia (Table 1). There is also a significant, but more modest, increased risk in those individuals who later develop affective psychosis. This suggests that some soft signs reflect a more general, ‘latent’ neurological dysfunction. For example, the first and third factors of the factor analysis reinforce the notion of soft signs as markers of disordered neurodevelopment. It is noteworthy that of the ‘significant’ odds ratios (95% confidence intervals that do not include unity) all represent increased frequency of soft signs associated with adult outcomes.
The number of cases of schizophrenia identified lies within confidence limits that could be expected were the case-finding complete (Reference Done, Johnstone and FrithDone et al, 1991). However, the percentage of cases of affective psychosis that are admitted to hospital is going to be a function of accepted practice, social acceptance of psychosis and illness severity, and milder cases are probably not represented. Differential attrition occurring because of measures in the study could include the removal of severe or complicated cases of childhood illness or soft sign (by death), leading to an extreme exposure but no outcome, with a resultant loss of study power.
Attrition at the level of case-finding reflects medical record keeping rather than characteristics of the patient per se. The clear exceptions to this are the tendency of those with chronic psychosis to social and geographical drift, as well as greater inconsistencies in recording personal details (e.g. names). This could lead to under-representation of more severe cases. Overall, the mixing of cases in the control population would decrease the power of the study. Any individual without data at 11 years was excluded from analysis.
‘Narrow schizophrenia’, conforming mainly to the presence of first-rank symptoms, was used as it was felt to reflect the more ‘neurodevelopmental’ features of schizophrenia, and therefore was more likely to be associated with other markers of disordered neurodevelopment. If this is seen as a more ‘genetic’ form of psychosis, a lack of association with infectious illnesses might be expected, unless the diathesis is associated with immunological differences.
Illnesses and soft signs
Viral meningoencephalitis and bacterial meningitis, tuberculosis and more rarely mumps can lead to central nervous system (CNS) involvement. For common viral infections, autoimmune responses to the CNS (e.g. autoimmune encephalitis) can be triggered by chicken pox, measles, scarlet fever and rheumatic fever. Whooping cough can lead to punctate haemorrhages in the brain following explosive coughing, and is sometimes followed by a related ‘post-tussis encephalitis’. However, in this whole population sample it appears that CNS involvement for the more common viral illnesses as reflected in neurological soft signs is negligible, except for a possible small association with whooping cough. For meningitis and tuberculosis, there is evidence of a similar increase in soft signs in the population as a whole.
Overall, then, infections that can directly cause CNS insults are associated with increased soft signs, whereas those mediated through autoimmune mechanisms are not. Whether the infection is viral or bacterial appears secondary to this consideration. Infectious hepatitis serves as a nominal ‘control’ for general effects of illness in these data (infection but no CNS involvement). However, this observation does not extend either in specific illnesses or in total number of illnesses to the adult outcomes, with the exception of epilepsy (see below).
Illnesses and adult outcomes
An association between death rates from bronchopneumonia and dates of birth of individuals with adult schizophrenia in two data-sets has been claimed (Reference O'Callaghan, Sham and TakeiO'Callaghan et al, 1994), as well as an association between schizophrenia and childhood CNS infection (including meningitis) in prospectively gathered data in a Finnish cohort (Reference Rantakallio, Jones and MoringRantakallio et al, 1997). Here the risk for schizophrenia was associated with viral CNS infection and the risk for ‘other psychoses’ associated with bacterial meningitis. Our data do not distinguish viral from bacterial meningitis, but the associations between meningitis and both schizophrenia (OR 7.8, 95% CI 1.0-59) and affective psychosis (adjusted OR 7.7, 95% CI 1.0-58) are clearly compatible with this work but each association is based upon a single case.
The trends of association between tuberculosis, and psychosis and epilepsy are confounded by the association between tuberculosis and meningitis: 5 out of 40 cases of tuberculosis were also recorded as cases of meningitis. At the time of the measures (1969), cases of tuberculosis might have included cases of tuberculous meningitis which were not distinguished in the records.
Although there is a lack of effect of common illnesses on adult outcomes, the ‘protective’ effect (OR=0.33, 95% CI=0.2-07, P=0.004) of chicken pox on adult affective psychosis is unexpected. Although this could be an artefact of multiple comparisons, this finding survives Bonferroni correction.
Is the excess of soft signs in schizophrenia caused by childhood illness?
Although some childhood illnesses are associated with increased rates of neurological soft signs, there are few consistent associations between the childhood illnesses and adult psychiatric disorder.
Our findings show the typical pattern of a highly significant statistical association between neurological soft signs and schizophrenia but no statistically significant association between early infectious illness and schizophrenia. Despite the association between infectious illness and neurological soft signs in the population as a whole, it has to be concluded that neurological soft signs in schizophrenia arise from some cause other than childhood illness.
The link between meningitis and epilepsy is clear but accounts for few cases. This effect (and the similar trend with tuberculosis) is unrelated to social class. The weaker relationships of meningitis with schizophrenia and affective psychosis (each dependent upon a single case) are clearly no explanation for the increase in soft signs in these groups. Thus, the increase in soft signs in psychosis is independent of the effects of these rare illnesses in childhood. Within the limitations of the present data, exposure to common childhood illnesses at any age up to 11 years joins prenatal viral infection and complications of pregnancy and birth as candidate environmental factors in psychosis aetiology of small effect and doubtful significance. The excess of soft signs associated with psychosis must be assumed to reflect on the nature of the deviation in the trajectory of brain development in psychosis and its presumably genetic origin.
CLINICAL IMPLICATIONS
-
▪ Common childhood illnesses are not implicated in the aetiology of adult psychotic illness.
-
▪ Even illnesses such as meningitis rarely occur in the childhoods of individuals who develop psychotic illness.
-
▪ Findings from case—control studies remain vulnerable to selection and recall bias.
LIMITATIONS
-
▪ The diagnoses of childhood illnesses were as reported by parents and might not be reliable.
-
▪ No information was recorded on duration, severity or timing of the illnesses.
-
▪ Epilepsy was identified at age 11, whereas many cases (especially partial fits) will manifest after this age.
Acknowledgements
We gratefully acknowledge constructive suggestions from Professor Peter Jones and Dr Tim Croudace at the University of Nottingham Department of Psychiatry and Community Mental Health, support with the NCDS cohort dataset from Dr Peter Shepherd and the Social Statistics Research Unit at City University, London, and advice on aspects of the epilepsy variable from Dr Pat Tookey (Institute of Child Health, University College London Medical School) and Professor Euan Ross (Department of Community Paediatrics, King's College School of Medicine and Dentistry).





eLetters
No eLetters have been published for this article.