The onset of psychosis is mostly preceded by a prodromal phase lasting about 5 years (Reference Häfner, Maurer and LöfflerHäfner et al, 1998). This period is characterised by various mental disturbances such as negative and basic symptoms, attenuated and brief transient frank psychotic symptoms, cognitive impairments, and a marked decline of social functioning and quality of life (Reference Häfner, Maurer and LöfflerHäfner et al, 1998; Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001; Reference Bechdolf, Pukrop and KohnBechdolf et al, 2005; Reference Pukrop, Schultze-Lutter and RuhrmannPukrop et al, 2006; Reference Yung, Stanford and CosgraveYung et al, 2006). Hence, independently from its potential course into psychosis, this syndrome itself already fulfils the criteria for a mental disorder as defined in DSM–IV (American Psychiatric Association, 1994). With no approved treatment existing to date, one major objective of the early intervention studies of the German Research Network on Schizophrenia (GRNS) is the development of therapeutic strategies related to the current clinical state (Reference Häfner, Maurer and RuhrmannHäfner et al, 2004; Reference Ruhrmann, Schultze-Lutter and MaierRuhrmann et al, 2005). The present study aimed to evaluate the short-term symptomatic effects of a needs-focused intervention combined with amisulpride.
METHOD
Participants
Participants were recruited at the early detection centres of the Departments of Psychiatry at the Universities of Cologne, Bonn, Düsseldorf and Munich. They were mainly referred by psychiatrists or psychological therapists in private practice, general practitioners and school or university counselling services, or presented themselves directly. The concept of early detection was introduced into the local health network by numerous workshops and talks. To facilitate risk screening, a 17-item checklist was distributed (Reference Häfner, Maurer and RuhrmannHäfner et al, 2004), which was drawn from the Early Recognition Inventory (ERIraos; Reference Maurer, Horrmann and TrendlerMaurer et al, 2006).
The study adhered to the Guideline for Good Clinical Practice CPMP/ICH/135/95 and CPMP/768/97 (ICH, 1996), the 1996 World Medical Association Declaration of Helsinki and pertinent German legal and regulatory requirements, and was approved by the local ethics committees of the medical faculties of the participating centres. All participants gave their written informed consent and were explicitly informed that they were free to withdraw from the study at any time for any reason, without effect on their medical care. No financial inducement was offered for participation.
Inclusion criteria
Within the GRNS, a two-phase model which differentiates between an early (EIPS) and a late initial prodromal state (LIPS) of psychosis is being evaluated. The late prodromal state is defined by the presence of attenuated positive symptoms and/or brief limited intermittent positive symptoms within the 3 months preceding the study. Attenuated positive symptoms (APS) are defined by the presence of at least one of the following appearing several times per week for a period of at least 1 week: (a) ideas of reference; (b) odd beliefs or magical thinking; (c) unusual perceptual experiences; (d) odd thinking and speech; and/or (e) suspiciousness or paranoid ideation. Brief limited intermittent positive symptoms (BLIPS) comprise the presence of hallucinations, delusions, formal thought disorder, or gross disorganised or catatonic behaviour, spontaneously resolving within 1 week. APS and BLIPS were assessed by dedicated questions of the ERIraos (Reference Maurer, Horrmann and TrendlerMaurer et al, 2006). The age range of participants was 18–36 years (younger people could not be included because all participating centres were certified to treat adults only and older people were considered to be at low risk for psychosis).
Exclusion criteria
The most relevant exclusion criteria were: (a) any lifetime DSM–IV diagnosis of schizophrenia, schizophreniform, schizo-affective, delusional or bipolar disorder; (b) any lifetime DSM–IV diagnosis of brief psychotic episode with a duration of more than 1 week; (c) a DSM–IV diagnosis of delirium, dementia, amnestic and other cognitive disorders, mental retardation, mental disorders due to a general medical condition or mental disturbances due to psychotropic substances; (d) abuse of alcohol or drugs within the past 3 months or the past 4 weeks for cannabis (if prodromal symptoms did not appear before any drug abuse, they had to persist after a period of at least 3 months free of hallucinations or amphetamines lasting or after 4 weeks free of cannabis.); (e) any lifetime continuous treatment with high-potency antipsychotics for more than 1 week or any use of antipsychotics during the 6 months prior to the study; (f) any contraindication for amisulpride; (g) women of childbearing risk not using contraception. Additional exclusion criteria were related to somatic disturbances such as pathological electrocardiographic (ECG) aberrations etc.
Measures
The ERIraos (Reference Maurer, Horrmann and TrendlerMaurer et al, 2006) is an extension of the well-established Interview for the Retrospective Assessment of the Onset and course of Schizophrenia and Other Psychoses' (IRAOS; Reference Hafner, Riecher-Rössler and HambrechtHäfner et al, 1992) and allows prospective follow-up studies. The psychopathological section comprises 110 items with scores ranging from 0 to 3. Assessments were trained by the scale's authors, kappa for interrater reliability ranged between 0.55 and 0.69. To assess treatment effects, a Basic and Positive Psychotic Spectrum Symptoms score (ERI–BAPPSS score) was formed of the 16 items related to full-blown psychotic symptoms (including disorganised thinking and behaviour), of the six items assessing attenuated positive symptoms described above and of the ten items assessing a set of basic symptoms which have been shown to be highly predictive for the development of schizophrenia (Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001). Two sub-scores of the ERI–BAPPSS score were calculated, one for the attenuated and full-blown psychotic positive symptoms (ERI–PPS score) and one for the basic symptoms (ERI–BS). In addition, the positive, negative and general psychopathology sub-scales of the Positive and Negative Syndrome Scale, (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987) were used for assessment. Mood was assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS; Reference Montgomery and AsbergMontgomery & Asberg, 1979), and general level of functioning by the Global Assessment of Functioning scale, (GAF; American Psychiatric Association 1994). For safety evaluation, the Extrapyramidal Symptom Rating Scale (ESRS; Reference Chouinard and MargoleseChouinard & Margolese, 2005) and the UKU Side Effect Rating Scale (UKU; Reference Lingjaerde, Ahlfors and BechLingjaerde et al, 1987) were applied. Blood pressure and heart rate were measured on every visit, body mass index (BMI) was calculated every 4 weeks. Laboratory tests were performed at baseline, after 4 and 12 weeks, and every 3 months thereafter.
Study design and intervention
An open-label, randomised parallel-group study was set up with an observation period of up to 2 years. An open-label design was chosen to achieve best possible acceptance of the study at a time when pharmacological intervention in a pre-psychotic state was a rather new idea. For recruitment, an Inclusion Criteria Checklist was adapted from the ERIraos, allowing an allocation of participants to the EIPS, LIPS or GNRS first episode of psychosis study. If a person consented to participate, study-related diagnostic measures (laboratory tests etc.) were performed and randomisation took place locally at each centre. Baseline psychopathology and safety measures were then assessed before the start of treatment. Both conditions featured a needs-focused intervention, which, in the experimental condition, was combined with the second-generation antipsychotic amisulpride.
The needs-focused intervention went beyond usual clinical management because it could include psychoeducation, crisis intervention, family counselling and assistance with education or work-related difficulties, according to need. Regular psychotherapy was not provided.
Amisulpride is a second-generation antipsychotic which has efficacy against negative and affective symptoms, particularly in the low-dose range (Reference GreenGreen, 2002; Reference Leucht, Pitschel-Walz and EngelLeucht et al, 2002). In the current trial, daily doses could range from 50 to 800 mg, with increments of 50 mg at first step and 100 mg at further steps. As a guideline, it was suggested that the dosage be increased as long as attenuated or brief limited intermittent positive symptoms were present. The interval between such steps should be at least 14 days if brief limited symptoms were absent and the APS score had improved. Participants visited the clinic weekly during the first 4 weeks, biweekly until week 12 and monthly thereafter.
Use of chloral hydrate or short-acting benzodiazepines (lorazepam, temazepam) was allowed to treat agitation or sleep disturbances. Extrapyramidal symptoms could be treated with biperiden. In line with the Personal Assessment and Crisis Evaluation (PACE) study (Reference McGorry, Yung and PhillipsMcGorry et al, 2002), the use of antidepressants (citalopram) was permitted for moderate to severe depression.
Statistical analysis
Acute symptomatic treatment effects as well as tolerability were analysed for the first 12 weeks of intervention. This period was the minimum for assessment of acute effects on negative and affective symptoms and global functioning (Reference Möller, van Praag and AufdembrinkeMöller et al, 1994; Reference SmeraldiSmeraldi, 1998). Randomised participants who completed the baseline assessment were considered eligible for intention-to-treat (ITT) analyses. In case of premature drop-out, last-observation carried-forward analysis was used. As assumptions for repeated measurements analysis of covariance (ANCOVA) were not always fulfilled, group comparisons were performed by ANCOVA with the difference score (week 12 minus baseline) as dependent variable, treatment and centre as factors and baseline score as covariate. Interaction between treatment and centre was kept in the model only if significant. Within-group treatment effects were calculated by paired t-tests. For categorical variables the chi-squared test or Fisher's exact test was used. Effect size (d) was calculated and categorised as ‘small’ (d≥0.20), ‘medium’ (d≥0.50) or ‘large’ (d≥0.80) in accordance with Cohen (Reference Cohen1988). For between-group comparisons, effect size was calculated on the basis of residual values produced by ANCOVAs including baseline and centre as terms (Reference CohenCohen, 1988). For safety evaluations of dimensional variables t-tests or, if not appropriate, non-parametric tests (Mann–Whitney U-test, Wilcoxon test) were used. Owing to marked differences in laboratory methods for prolactin measurement, only relative indices such as percentage elevation from baseline to end-point could be considered for analysis.
Statistical significance was assumed at a two-tailed α≤0.05. Analyses were performed using SPSS for Windows version 12.02.
RESULTS
Sample
Recruitment took place between January 2001 and December 2004. Out of 124 people who consented to participate' 65 were randomised to amisulpride plus needs-focused intervention and 59 to the control group (Fig. 1). Eighteen people dropped out before the baseline assessments (4 in the group with amisulpride and 14 in the control group (χ2=8.9, d.f.=1, P<0.01). The remaining 106 were considered for the safety analysis. Three participants in the group with amisulpride had to be excluded from the analysis of acute effect as treatment had already started before baseline assessment. Another participant in the group with needs-focused intervention had a severe, unstable endocrinological dysfunction which was not detectable by routine laboratory measurement. Hence, 102 patients (58 in the amisulpride group and 44 controls) were eligible for statistical analysis (ITT sample).
Baseline characteristics
Age, gender and distribution of inclusion criteria were not statistically different between groups (Table 1). Early drop-outs (n=18) did not differ from the remaining sample (n=106) with respect to any of these variables. This also applied when comparisons were made separately for the two treatment groups. Regarding baseline psychopathology of the ITT sample (Table 2), only ERI–BS mean scores differed significantly (amisulpride, 8.5, s.d.=6.2; controls, 5.8, s.d.=5.8; t=2.52, d.f.=103.6, P<0.05). Nevertheless, any baseline scores were considered as covariate in the respective analysis.
Table 1 Demographic and clinical characteristics

| Characteristic | Total sample (n=124) | NFI (n=59) | AMI+NFI (n=65) |
|---|---|---|---|
| Age, years: mean (s.d.) | 25.6 (6.3) | 25.1 (6.6) | 26.1 (6.1) |
| Gender, n (%) | |||
| Female: | 54 (43.5) | 28 (52.5) | 26 (40.0) |
| Male | 70 (56.5) | 31 (47.5) | 39 (60.0) |
| Met APS criterion | 116 (93.5) | 56 (94.9) | 60 (92.3) |
| Met BLIPS criterion | 69 (55.6) | 35 (59.3) | 34 (52.3) |
| Met APS criterion only | 55 (44.4) | 24 (40.7) | 31 (47.7) |
| Met BLIPS criterion only | 8 (6.5) | 3 (5.1) | 5 (7.7) |
| Met APS + BLIPS criterion | 61 (49.2) | 32 (54.2) | 29 (44.6) |
Table 2 Psychopathological scores at baseline and end-point (12 weeks, intention-to-treat)

| Scale | AMI + NFI (n=58) | NFI (n=44) | ||||
|---|---|---|---|---|---|---|
| Baseline Mean (s.d.) | End-point Mean (s.d.) | Percentage change | Baseline Mean (s.d.) | End-point Mean (s.d.) | Percentage change | |
| ERI—BAPPSS | 14.2 (8.6) | 5.6 (6.5) | -59.2 | 11.2 (8.3) | 7.9 (8.0) | -29.5 |
| ERI—PPS | 5.4 (3.5) | 1.8 (2.6) | -66.7 | 5.2 (4.7) | 3.4 (4.2) | -34.6 |
| ERI—BS | 8.9 (6.1) | 3.8 (4.8) | -57.3 | 6.0 (4.8) | 4.4 (4.9) | -26.7 |
| PANSS—P | 12.3 (3.8) | 9.7 (3.4) | -21.1 | 12.8 (4.0) | 11.8 (4.5) | -7.8 |
| PANSS—N | 14.7 (5.5) | 12.2 (5.0) | -17.0 | 14.2 (4.4) | 13.5 (5.0) | -4.9 |
| PANSS—G | 31.6 (8.2) | 25.8 (8.7) | -18.4 | 32.0 (7.0) | 29.2 (8.9) | -8.8 |
| MADRS | 18.3 (7.8) | 11.8 (9.0) | -35.5 | 15.9 (6.3) | 12.9 (8.4) | -18.9 |
| GAF | 57.9 (10.0) | 66.8 (14.1) | + 15.4 | 58.3 (9.5) | 60.7 (14.7) | + 4.1 |
Dosage and concomitant medication
The mean daily dose of amisulpride was 118.7 mg (s.e.=10.7, median 98.1), the mean maximum dose 181.9 mg (s.e.=19.0, median 137.5) and the mean dose at end-point 169.5 mg (s.e.=18.5, median 100.0 mg). Additional selective serotonin reuptake inhibitors (SSRIs) were prescribed in seven participants in each group (NS). In three of seven from the amisulpride group pre-study medication was continued. Benzodiazepines were prescribed for six participants: five in the amisulpride group, with one starting before study entry, and one in the control group (NS). One participant in each group took chloral hydrate to prevent sleep disturbances.
Psychopathological outcome measures
The combined treatment produced a significantly superior effect on ERI–BAPPSS scores (F (1,98)=7.49, P<0.01), with significant improvement observed in both groups (amisulpride, t=6.88, d.f.=57, P<0.001; controls t=2.87, d.f.=43, P<0.01). Table 2 provides the pre- and post-treatment scores and Fig. 2 the effect sizes for between- and within-group comparisons. Amisulpride produced a large effect, whereas needs-focused intervention alone produced only a small effect on ERI–BAPPSS scores. The same pattern applied for ERI–PPS scores (F (1,98)=7.42, P<0.001; amisulpride, t=7.35, d.f.=57, P<0.001; controls t=2.57, d.f.= 43, P<0.05) or ERI–BS scores (F (1,98)= 6.30, P<0.05; amisulpride, t=6.88, d.f.= 57, P<0.001; controls, t=2.87, d.f.=43, P<0.01).

Fig. 1 CONSORT diagram showing participant flow through the study and reasons for exclusion or discontinuation. LIPS, late initial prodromal state; AMI, amisulpride; NFI, needs-focused intervention.
A significant effect of treatment with amisulpride also emerged regarding the PANSS positive sub-scale (PANSS–P) score (F (1,98)=7.83, P<0.01); paired t-tests revealed a significant decrease of baseline scores only in the group with amisulpride (t=5.50, d.f.=57, P<0.001). Across samples, baseline and difference scores of ERI–PSS and PANSS–P showed a significant but moderate correlation (ρ=0.34, P<0.001 and ρ=0.39, P<0.001 respectively).
Analysis of PANSS negative sub-scale (PANSS–N) scores by ANCOVA also yielded a significantly better effect of amisulpride (F (1,98)=4.85, P<0.05). Within-group comparisons revealed a significant effect only for amisulpride (t=4.56, d.f.= 57, P<0.001). General psychopathology improved significantly in the amisulpride group (F (1,98)=4.63, P<0.05; amisulpride: t=5.02, d.f.=57, P<0.001; controls, t=2.11, d.f.=43, P<0.05). A superior effect for amisulpride was also observed for GAF scores (F (1,98)=5.70, P<0.05). Paired t-tests showed a significant change in the amisulpride group only (t=4.56, d.f.=56, P<0.001). In terms of GAF categories, mean scores of the amisulpride group improved from ‘moderate’ to ‘mild’. No significant difference between groups emerged regarding MADRS scores (F (1,98)=2.12, NS) but both treatment conditions produced a significant decrease of scores (amisulpride t=5.39, d.f.=57, P<0.001; needs-focused intervention alone, t=2.39, d.f.=43, P<0.05), with a superior effect size in the amisulpride group.
For a categorical analysis of sustained risk in terms of inclusion criteria, the ERI–PPS score was dichotomised into score 0 v. score ≥1. Chi-squared tests revealed a significantly higher portion of participants with a score of 0 in the amisulpride than in the control group (27/58, 46.6% v. 9/44, 20.5%; χ2=7.46, d.f.=1, P<0.01; ϕ=0.27, P<0.01; Fig. 3).
Safety
The only severe adverse event occurred in the group with needs-focused intervention alone where, despite starting treatment with citalopram, a patient became severely depressed and suicidal. The numbers and reasons for drop-outs are given in Fig. 1. Table 3 lists the frequencies of clinically significant adverse events as assessed by the UKU. The three drop-outs related to adverse events were provoked by prolactin-associated symptoms, i.e. galactorrhoea in two participants and sexual dysfunction in another.
Table 3 Adverse events (UKU side-effects scale) with a severity of at least moderate and a frequency of at least 5%

| Side-effect, % | NFI (n=40) | AMI+NFI (n=61) | Causal relationship to amisulpride rated ‘possible’ or ‘probable’ |
|---|---|---|---|
| Concentration difficulties | 70 | 70.5 | 8.2 |
| Asthenia/lassitude/increased fatiguability | 40 | 65.6* | 42.6 |
| Sleepiness/sedation | 10 | 14.8 | 9.8 |
| Failing memory | 22.5 | 49.2** | 1.6 |
| Depression | 62.5 | 68.9 | 9.8 |
| Tension | 57.5 | 67.2 | 9.8 |
| Increased duration of sleep | 12.5 | 41** | 42.6 |
| Decreased duration of sleep | 30 | 14.8 | 6.6 |
| Increased dream activity | 0 | 26.2*** | 23.0 |
| Nausea/vomiting | 0 | 11.5* | 9.8 |
| Polyuria/polydipsia | 0 | 6.6 | 3.3 |
| Orthostatic dizziness | 0 | 6.6 | 6.6 |
| Palpitation/tachycardia | 10 | 9.8 | 1.6 |
| Increased tendency to sweating | 0 | 19.7** | 16.4 |
| Headache | 15 | 27.9 | 8.2 |
| Menstrual disorders (% of females) | 0 | 9.8 (24.0) | 9.8 (24.0) |
| Galactorrhoea (% of females) | 0 | 8.2 (20.0) | 8.2 (20.0) |
| Breast tenderness/swelling (% of females) | 0 | 6.6 (16.0) | 6.6 (16.0) |
| Diminished sexual desire | 10 | 34.4** | 18.0 |
| Erectile dysfunction (% of males) | 0 | 6.6 (11.1) | 4.9 (8.3) |
| Ejaculatory dysfunction (% of males) | 0 | 3.3 (5.6) | 3.3 (5.6) |
| Orgastic dysfunction | 0 | 6.6 | 4.9 |
Prolactin levels increased significantly more frequently in the amisulpride-treated group (36/44, 81.8% v. 7/34, 20.6%; χ2=29.07, d.f.=1, P<=0.001). The mean relative change from baseline to end-point was 795.4% (s.e.=144.5%, median 658.6%) in the amisulpride group and 47.2% (s.e.=44.6%, median 0.0%) in the group with needs-focused intervention alone (t=4.95, d.f.=50.97, P<0.001). At end-point, the upper limit of normal was exceeded more than twice by 1 of 31 (3.2%) controls and 29 of 40 (75.2%) in the amisulpride group who started in the normal range (Fisher's exact test, P<0.001), with no significant difference in the number of males (16/25, 64.0%) and females (13/15, 86.7%) in the amisulpride group. However, the mean increase relative to baseline was much higher in females than in males (1343.2%, s.e.=338.7, median 784.4 v. 511.4%, s.e.=79.9, median 590.3; t=2.53, d.f.=15.51, P<0.05). The mean and maximum daily or cumulative dose of amisulpride were not significantly correlated with percentage elevation of prolactin, number of participants with increase or number exceeding twice the upper limit of normal. Addition of an SSRI to amisulpride (7/44, 3 males, 4 females) was significantly correlated with larger prolactin elevations (r=0.32, P<0.05); mean values were more than twice as high in the subgroup receiving both drugs (amisulpride: 664.6%, s.e.=790.2%; amisulpride plus SSRI, 1473.3%, s.e.=556.9%; NS), a pattern repeated when males and females were analysed separately. Significant clinical side-effects associated with increased prolactin levels are listed in Table 3. Menstrual disturbances emerged only transiently in four females; another female developed a prolonged cycle and another dropped out later owing to amenorrhoea. Among males, two developed erectile and ejaculatory dysfunction and another decreased sexual desire and erectile dysfunction.
Liver alanine aminotransferase levels more than twice the upper limit of normal were reported in three participants in the amisulpride group (4.9%).
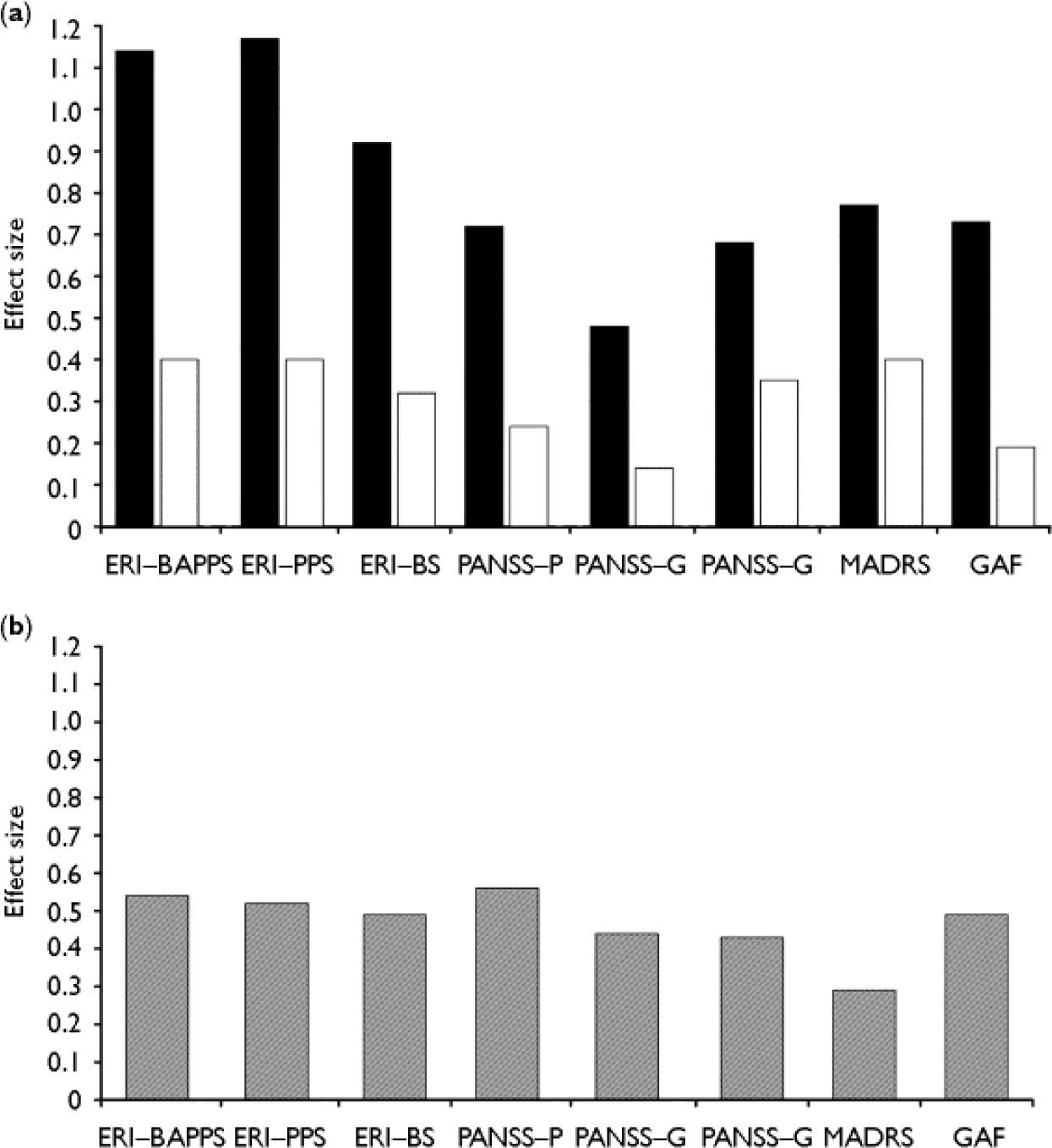
Fig. 2 (a) Within-group comparisons (baseline v. week 12, intention-to-treat) of effect size. (b) Between-group comparisons of effect size. ▪, Amisulpride plus needs-focused intervention (n=59); □, needs-focused intervention alone (n=44). Effect size d≥0.20, ‘small’; d≥0.50, ‘medium’; d≥0.80: ‘large’ (Reference CohenCohen, 1988). ERI, Early Recognition Inventory; BAPPSS, Basic and Positive Psychosis Spectrum Symptoms; PPS, Positive Psychosis Spectrum; BS, Basic Symptoms; PANSS, Positive and Negative Syndrome Scale; P, positive symptoms; N, negative symptoms; G, general psychopathology; MADRS, Montgomery—Åsberg Depression Rating Scale; GAF, Global Assessment of Functioning scale.
Extrapyramidal symptoms were analysed with respect to the ESRS total score (range 0–225) and for the sub-scales ‘parkinsonism’ (range 0–96), ‘akathisia’ (range 0–9), ‘dyskinesia’ (range 0–42) and ‘dystonia’ (range 0–60) according to Chouinard & Margolese (Reference Chouinard and Margolese2005). Within-group comparisons revealed no statistically significant change from baseline to end-point in either group. At end-point, total scores ranged from 0 to 5 in the control group and from 0 to 19 in the amisulpride group, with 36 of 61 (59.0%) in the amisulpride group showing no symptoms and 21 of 61 (34.4%) exhibiting scores from 1 to 5. No statistically significant differences emerged between groups with regard to change in scores (baseline v. end-point) or scores at end-point, except for the akathisia end-point scores (amisulpride mean 0.5, s.d.=1.3; controls, mean 0.2, s.d.=0.8; Mann–Whitney U=1140.5, P<0.05); only 4 of 61 and 1 of 43 participants from the amisulpride and control groups respectively crossed the threshold for ‘presence of akathisia’ (score≥3). Biperiden was prescribed for 3 of 51 amisulpride-treated participants. The daily mean, maximum and end-point doses of amisulpride in these participants were 239.4, 408.3, and 333.3 mg respectively.
The BMI increased slightly but significantly in the amisulpride group (mean end-point minus baseline=0.63 (2.6%), s.e.=0.14, Z=–3.71, P<0.001); mean group changes differed significantly (U=389.0, P=0.001). Diastolic blood pressure increased slightly but significantly in the group with needs-focused intervention alone (+3.49 mmHg, s.e.=1.64, Z=2.12, P<0.05) but no significant group difference emerged. Systolic blood pressure or heart rate in the sedentary position did not change significantly in either group; ECG recordings revealed no pathological changes.
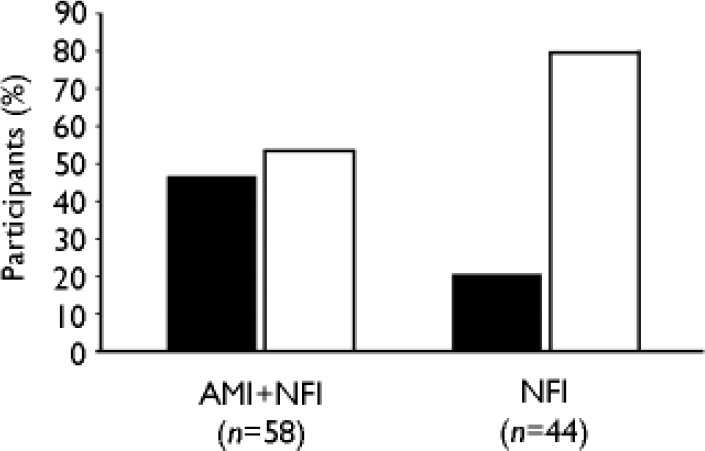
Fig. 3 Percentage of participants with complete (▪, score=0) or incomplete (□, score≥1) remission of attenuated or full-blown psychotic symptoms after 12 weeks of treatment (intent-to-treat, last-observation-carried-forward) as assessed with the Early Recognition Inventory sub-scale for attenuated and full-blown psychotic symptoms (ERI–PPS). AMI, amisulpride; NFI, needs-focused intervention.
DISCUSSION
The early intervention studies of the GNRS follow a unique two-phase approach, differentiating between an early and a late initial prodromal state (Reference Häfner, Maurer and RuhrmannHäfner et al, 2004). The former is characterised by the presence of at least one of a set of basic symptoms (Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001) and/or a combination of functional decline and trait risk factors, whereas the latter comprises emergence of attenuated and/or brief limited intermittent positive symptoms. This approach has the theoretical potential to detect the prodrome of psychosis well in advance of the start of functional decline (i.e. 2–4 years before first admission for overt psychosis; Reference Häfner, Maurer and LöfflerHäfner et al, 1998), and thus much earlier than with imminent or ultra-high-risk criteria (Reference Phillips, Yung and McGorryPhillips et al, 2000; Reference Woods, Breier and ZipurskyWoods et al, 2003). Corresponding to the two-phase model, a two-step treatment approach was developed, offering cognitive–behavioural therapy (CBT) for patients putatively in the early state and antipsychotic treatment for those already in the late state. The definition of LIPS corresponds to the PACE criteria (Reference Phillips, Yung and McGorryPhillips et al, 2000), with the major difference that entry criteria for the LIPS study are restricted to symptoms of the positive psychotic spectrum. Thus in the search for a pharmacological treatment option the investigation of an antipsychotic drug was consistent with the clinical symptoms. Amisulpride was primarily chosen because safety data in studies of schizophrenia indicated a favourable tolerability profile especially in the low-dose range. The incidence of extrapyramidal side-effects has been reported to be similar to placebo in a dose range between 50 and 300 mg, that expected to be prescribed throughout the study (Reference Leucht, Pitschel-Walz and EngelLeucht et al, 2002). Furthermore, weight gain is small (Reference Leucht, Wagenpfeil and HamannLeucht et al, 2004). Moreover, amisulpride has a unique efficacy for positive, negative and depressive symptoms (Reference GreenGreen, 2002; Reference Leucht, Pitschel-Walz and EngelLeucht et al, 2002) and was thus assumed to be particularly suitable for the prodromal phase (Reference Häfner, Maurer and LöfflerHäfner et al, 1998; Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001; Reference Yung, Stanford and CosgraveYung et al, 2006).
Main findings
In the present study, across all measures amisulpride in combination with needs-focused intervention intervention produced superior treatment effects compared with needs-focused intervention alone. The strongest effects were observed for attenuated and brief limited intermittent positive symptoms. As they are assumed to be the most important indicators of imminent risk, it is noteworthy that a complete regression of scores appeared more than twice as often in the amisulpride group. Another strong effect emerged for basic symptoms, which are also closely associated with an enhanced risk for psychosis (Reference Klosterkötter, Hellmich and SteinmeyerKlosterkötter et al, 2001). The future long-term course of the study will have to show whether disappearance of psychopathological risk indicators is associated with lower rates of transition to psychosis.
Since the GNRS model of the prodromal phase called for an instrument integrating both basic symptoms and the ultra-high-risk approach, the ERIraos was used for the assessment of course. The PANSS does not sufficiently assess positive symptoms below the psychotic threshold but was employed in this study as it is widely used in antipsychotic trials and provides an established evaluation of negative symptoms.
Recent findings indicate that low GAF scores are associated with an increased risk for psychosis, especially in combination with attenuated or brief limited intermittent positive symptoms (Reference Yung, Stanford and CosgraveYung et al, 2006). Hence, the improvement of GAF scores in the amisulpride group might also be predictive for a diminished risk. However, as the GAF score does not merely assess the level of functioning but integrates the occurrence and severity of symptoms, in future studies a more specific instrument such as the Social and Occupational Functioning Assessment Scale (SOFAS; Reference Goldman, Skodol and LaveGoldman et al, 1992) might help to further clarify the effect of treatment on functioning and its value as a risk indicator.
Negative symptoms have recently been defined as a separate target of antipsychotic treatment as they are particularly important to functional outcome and quality of life (Reference Kirkpatrick, Fenton and CarpenterKirkpatrick et al, 2006). The use of amisulpride resulted in an improvement in negative symptoms which was not observed with needs-focused intervention alone.
The 3-month study period was obviously sufficient to detect differential effects of treatment on global functioning, affective and negative symptoms, but recent studies suggest that long-term data will show further improvements (Reference Laughren and LevinLaughren & Levin, 2006).
Depressive symptoms improved in both groups, again with an advantage for the amisulpride group. Concomitant SSRIs were prescribed only for a few participants, with numbers nearly equal in both groups. However, a confounding effect on the results cannot be ruled out.
Mean doses of amisulpride were in the expected low dose range, yet it can be assumed that the treatment effects especially on the psychosis spectrum symptoms could have been further increased with somewhat higher doses. However, the low mean dosage may also have been responsible for the good overall tolerability, as demonstrated by the low rate of drop-outs related to adverse events. In line with the literature (Leucht et al, Reference Leucht, Pitschel-Walz and Engel2002, Reference Leucht, Wagenpfeil and Hamann2004), amisulpride showed a most favourable side-effect profile in terms of extrapyramidal symptoms and weight gain, and did not influence blood pressure or heart rate. As a special feature of benzamides, amisulpride markedly increased prolactin levels. In line with recent findings, this effect was not dose-related but was enhanced when SSRIs were combined (Reference Bressan, Erlandsson and SpencerBressan et al, 2004; Reference Kopecek, Bares and SvarcKopecek et al, 2004). In some patients a rise in prolactin levels was associated with side-effects such as galactorrhoea or mostly transient menstrual disorders. However, the related number of drop-outs was fairly low. A temporary decrease in libido occurred in almost all patients. Its origin is often difficult to disentangle, as current mental state itself has to be considered as a major contributing factor. Thus in view of present and other very recent findings (Reference Kopecek, Bares and SvarcKopecek et al, 2004), it seems sensible to recommend monitoring of prolactin levels and clinical side-effects during the use of amisulpride irrespective of dosage. However, increased prolactin levels per se do not seem to call for a change of treatment (Reference Haddad and WieckHaddad & Wieck, 2004). A normalisation of prolactin levels can be expected within 3 months of withdrawal (Reference Schlösser, Gründer and AnghelescuSchlösser et al, 2002).
Other studies
To our knowledge, only one other controlled study on the short-term symptomatic effects in the putatively prodromal state has been published to date. The Prevention through Risk Identification, Management and Education (PRIME) study compared olanzapine (n=30) and placebo (n=29) over 8 weeks (Reference Woods, Breier and ZipurskyWoods et al, 2003). In addition, psychosocial intervention with supportive and psychoeducational components was offered to both groups, which seems to correspond to the needs-focused intervention control condition in the present trial. In a mixed-model analysis, olanzapine significantly improved total, negative, disorganised and positive scores on the SOPS. The effect on the positive scores, however, was not statistically different from placebo and general scores did not significantly change with either treatment. The PANSS positive and general psychopathology scores changed significantly in both groups, but no effect was observed with negative symptoms, MADRS or GAF scores. Compared with the present study, the PRIME study has the clear advantage of a double-blind, placebo-controlled design. However, owing to its smaller sample sizes, the study may have been underpowered as in the last-observation-carried-forward analysis no group difference became statistically significant despite the fact that, for example, the mean SOPS positive score improved about 4.5 times more with olanzapine.
The only other published controlled early intervention study which includes an antipsychotic is the PACE study (Reference McGorry, Yung and PhillipsMcGorry et al, 2002). In an open-label design, a combination of needs-based intervention, CBT and risperidone was tested against an exclusive needs-based intervention. However, comparability with the present study is limited, as transition rate to psychosis was the main outcome measure of the PACE study, and symptomatic effects were thus only reassessed after 6 months. Despite a clearly superior effect of the combination on transition rates, symptomatic improvement was not different between groups, which may in part have been because of adherence in the risperidone group.
Limitations
A limitation of the current study is the lack of masking, which it shares with the PACE study and which may have led to an over-estimation of effects owing to rater bias and/or placebo effects. It seems unlikely, however, that a placebo effect would have produced such marked differences in effect size after 12 weeks of treatment. The results are supported by the PRIME study, in which PANSS positive scores decreased by only 1.6% in the placebo group but by 15.3% in the olanzapine group. Thus the placebo effect might be rather weak in this group of patients. However, the current results justify a placebo-controlled, double-blind trial.
Another limitation is that the needs-focused intervention had some effects on positive psychotic spectrum and basic symptoms. Hence, as in the PACE study and presumably the PRIME study, it is not possible to disentangle the effects of drug and psychosocial support. However, improvement of global functioning and most notably negative symptoms might be predominantly attributable to amisulpride, as the needs-focused intervention alone had no effect on this measure.
Another limitation is the number of early drop-outs in the control group. As the participants did not return, the reasons for drop-out are unknown in most cases. The significant difference in drop-outs between the two conditions might indicate that psychosocial support alone did not meet the subjective needs of at least some participants, but that a combined treatment was more acceptable.
Conclusions
The present trial suggests that an antipsychotic drug treatment provides a marked symptomatic benefit for people in a putatively late initial prodromal state. Confirmation of the results in a placebo-controlled study would be an obligatory prerequisite for any general recommendations. Amisulpride was well tolerated in terms of extrapyramidal symptoms and weight gain. Although prolactin levels were increased more frequently with amisulpride, only a small number of participants developed clinical symptoms. However, in the search for effective acute treatments for prodromal symptoms it seems only reasonable to expect that as in the treatment of overt psychosis no antipsychotic suits all patients. With regard to the ethics of preventive early intervention (Reference McGlashanMcGlashan, 2005), our results suggest that patients in a putatively prodromal state seeking help for their symptoms experience a substantial benefit from treatment, independent of their further course. However, in the light of the emerging findings about the marked disabilities already present in the late prodromal or ultra-high-risk state, it might be helpful to complement the criteria for attenuated and brief limited intermittent positive symptoms with a functional dimension, thus making it clear that early intervention does not only treat single psychopathological symptoms or assumed risks in otherwise healthy people but suffering human beings.
Acknowledgements
The study group thanks Kerstin Eggers, the project's technical assistant for electronic data processing. The LIPS study group comprises Julia Berning (Bonn), Ronald Bottlender (München), Beril Canata (Cologne), Petra Decker (München), Kerstin Eggers (Cologne), Bianca Hoppmann (Cologne), Martin List (Düsseldorf), Ruth Lümkemann (Düsseldorf), Heinz Picker (Cologne), Marcus Sievert (München), Sebnem Simsek-Kraues (Düsseldorf), Markus Streit (Düsseldorf), Sonja Theysohn (Bonn), Stefanie Tschinkel (Cologne), Christian Werner (Düsseldorf), Anke Wieneke (Cologne).
This study is part of the German Research Network on Schizophrenia and was funded by the German Federal Ministry for Education and Research BMBF (grant 01 GI 9935) and Sanofi Synthelabo, Germany.









eLetters
No eLetters have been published for this article.