Introduction
The role of emerging market countries in global health governance has attracted increasing scholarly attention. Studies have investigated the influence of Brazil, Russia, India, China, and South Africa (BRICS) on reforms in the World Health Organization (WHO),Footnote 1 discussed whether the rise of the BRICS is leading to a paradigm shift in global health,Footnote 2 and assessed the potential of coalitions among these countries to shape the global politics of access to medicines.Footnote 3 In this literature – as in the wider International Relations scholarship on the rise of the BRICSFootnote 4 – the focus has almost exclusively been on how BRICS states shape world politics. Little attention has been paid to the question of how the rise of companies from these and other emerging markets has affected global political dynamics. This is surprising not only because the importance of business in global politics is now widely recognised,Footnote 5 but also because the rise of the BRICS is driven largely by the spectacular growth of their economies and share in world trade, both of which is carried by companies from these countries.
This article analyses the involvement of pharmaceutical companies from emerging markets in global health governance. It shows that they play a key role as low-cost suppliers of medicines and vaccines and, increasingly, also of new technologies. The article shows that, in so doing, pharmaceutical companies from emerging markets not only have become central to the political economy underlying global health governance, but that they have also enabled governments to implement a key goal of global health policy: widening access to medicines and vaccines. The article therefore presents an argument that sits between those highlighting an increasing role of nonstate actors in global health governanceFootnote 6 and those emphasising the continuing importance of the state in formulating and implementing global health policies.Footnote 7 As Garrett W. Brown shows in his contribution to this Special Issue, national governments and political leadership shape whether and how global health policies are implemented at the local level. Yet, as the present article highlights, the interests and capabilities of companies can significant influence which policy goals can feasibly be pursued and implemented.
Existing studies on the global response to the HIV/AIDS pandemic have illustrated the important role played by pharmaceutical companies from emerging markets.Footnote 8 In the 1980s and 1990s, the international community tried to address the spiralling HIV/AIDS pandemic in low- and middle-income countries through improved technical coordination and prevention. Antiretroviral medicines (ARVs) were available to patients in high-income countries, but donors rejected the idea of subsidising treatment in low- and middle-income countries because of the high costs of medicines.Footnote 9 In the early 2000s, however, a policy change occurred, and in 2005, G8 leaders endorsed the goal of providing ‘as close as possible to universal access to treatment for AIDS by 2010’.Footnote 10 An important factor for the change of heart among donors was the emergence of pharmaceutical companies from middle-income countries on the scene. They helped bring the price of ARVs down from approximately US $10,000 to less than US $150,Footnote 11 which made large-scale subsidies and, hence, the policy goal to provide universal access possible.Footnote 12
Since then, widening access to pharmaceuticals has become a key policy goal of global health governance not only with regard to HIV/AIDS but also with regard to many other diseases, including malaria, tuberculosis, and neglected tropical diseases, and, most recently, antibiotics. This has had a significant effect on the institutional structure of global health governance leading to a range of public-private partnerships to facilitate the procurement and development of medicines and vaccines. Global health partnerships are now a key feature of global health governance, including the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM), the GAVI Alliance, and the Drugs for Neglected Diseases Initiative (DNDi), for instance.Footnote 13 Access policies have also yielded some impressive results. Today, approximately ten million people living with HIV/AIDS in low- and middle-income countries receive ARVs,Footnote 14 more than 11.2 million people have been treated for tuberculosis,Footnote 15 and more than 440 million children in low- and middle-income countries have been vaccinated against diseases such as diphtheria, tetanus, pertussis, hepatitis B, haemophilius influenzae type b, meningitis, polio, Japanese encephalitis, diarrheal diseases, pneumococcal diseases, and, most recently, cervical cancer.Footnote 16
There are indications that companies from emerging markets play an important role in the political economy underlying access policies, not only with regard to medicines for HIV/AIDS. Systematic evidence is, however, scarce.Footnote 17 This article analyses the involvement of pharmaceutical companies from emerging markets in global health governance by examining their contribution to some of the largest public-private partnerships for financing, procurement, and drug development. It shows that these companies are key suppliers and increasingly important partners in the development of new medicines and vaccines. This places them in a crucial position for the implementation of what has become a central goal of global health governance, widening access to pharmaceuticals.
The article offers an explanation for this phenomenon by looking at both policy dynamics and company interests. It shows that the focus on access to pharmaceuticals is part of a wider policy shift in global health governance from technical coordination and prevention to fighting specific diseases. This shift has moved access to pharmaceutical treatment and prevention into the centre of global health policy. Drawing on literature from medical sociology and health security, the article uses the concept of ‘pharmaceuticalisation’ to capture this phenomenon. Importantly, it argues that the pharmaceuticalisation of global health governance has created a dependency of governments and global health partnerships on pharmaceutical companies. In particular, it has created a dependency on pharmaceutical companies that can supply at extremely low prices because the ability to scale up access policies and achieve ‘universal’ access depends on the price that governments and global health partnerships have to pay for medicines and vaccines. It is this price pressure that has led many global health partnerships to turn to pharmaceutical companies from emerging markets as partners in global health governance.
Drawing on insights from the International Business literature, the article shows that emerging market companies are willing to supply at low prices not only because of lower production costs but also to get access to new technologies. Yet, the analysis points out, pharmaceutical companies from emerging markets are – like their counterparts from Western Europe and the US – for-profit organisations. There is evidence to suggest that the continuous drive for lower prices is reducing the interests also of emerging market producers to supply medicines and vaccines at the prices required by global health partnerships.
By studying the role of emerging market companies in global health governance, the article exposes a tension between the policy goal to widen access to pharmaceuticals and the incentives for companies to supply them. In other words, the article exposes a tension between the pharmaceuticalisation of global health governance and the political economy of pharmaceutical production. It argues that this tension can endanger the sustainability of global access policies and even undermine some of the recent successes in expanding treatment and immunisation.
By providing the first analysis of the role that pharmaceutical companies from emerging markets play in global health governance the article contributes to the burgeoning research on the political economy of global health.Footnote 18 More broadly, the article hopes to fuel a nascent interestFootnote 19 in International Relations scholarship about the role of emerging markets firms in world politics. It underlines that the rise of the BRICS and other emerging market countries brings with it a rise of companies that affect not only the structure and workings of the global economy but also that of global politics.
The role of pharmaceutical companies from emerging markets in global health governance
Before turning to the empirical analysis of the role that emerging market companies currently play in global health partnerships, I will briefly explain the data underlying the analysis. Global health partnerships take on a variety of functions, including financing, procurement, product development and capacity-building for companies in low- and middle-income countries. The article looks at the three largest partnerships focusing on the financing of medicines and vaccines, GFATM, Stop TB Partnership Global Drug Facility (GDF), and GAVI, and eight partnerships working on the development of new medicines and vaccines, so called Product Development Partnerships, including Aeras, DNDi, International AIDS Vaccine Initiative (IAVI), Infectious Disease Research Institute (IDRI), Medicines for Malaria Venture (MMV), Programme for Appropriate Technology in Health (PATH)/One World Health (OWH),Footnote 20 Sabin Vaccine Institute (Sabin), and the Global Alliance for TB Drug Development (TB Alliance). In addition, the article looks at a global health partnership aimed at increasing the capacity of pharmaceutical companies from low- and middle-income countries to manufacture vaccines for pandemic influenza, the WHO Global Action Plan for Influenza Vaccines (GAP). The selection of global health partnerships is based on two considerations: (1) their size, which is considered an indicator of their impact on global health;Footnote 21 and (2) the availability of data about the corporate partners they work with.
Table 1. Global health partnerships included in this study

Data has been generated from a variety of sources, including annual reports and other publications made available by the global health partnerships and by individual companies; the Global Fund Price and Quality Reporting Tool,Footnote 22 which is a database recording all orders that GFATM has placed with pharmaceutical companies; reports about global health partnerships published by other organisations, such as Policy Cures, the PDP Funders Group, and BioVentures; websites of global health partnerships, partner companies, and international organisations; and articles from the mainstream press and pharmaceutical trade publications.
Data was collected about the number of companies from emerging markets that individual global health partnerships work with as suppliers, research and development (R&D) partners, board members, and funders. Subsidiaries of companies where the parent company is headquartered in a high-income country were not included. In addition, the proportion of companies from emerging markets in the total number of suppliers and R&D partners of global health partnerships was calculated. With regard to data on board membership, the proportion of companies from emerging markets in the total number of board members was calculated. Publicly available data on the funders of global health partnerships was insufficient to conduct the same calculation for the proportion of companies from emerging markets in the total number of funders.
There are some limitations to the data, which have implications for the conclusions that can be drawn from the analysis. Firstly, data on the volumes procured from individual companies has not been collected. This limits the conclusions that can be drawn about the relative importance of individual companies as suppliers of medicines and vaccines. Secondly, comprehensive data on the number of relationships that each global health partnership has with individual companies is not publicly available because of confidentiality clauses covering several agreements.Footnote 23 This limits the conclusions that can be drawn about the relative importance of individual companies as R&D partners. Finally, data was collected for the time period of January 2010 to September 2013. Yet, data on suppliers, R&D partners, board members and funders had to be taken from different sources, such as websites and annual reports, for example, which cover different time periods. The information on individual global health partnerships published in this article therefore cover slightly different periods within the last three years.
Emerging market companies as suppliers of medicines and vaccines
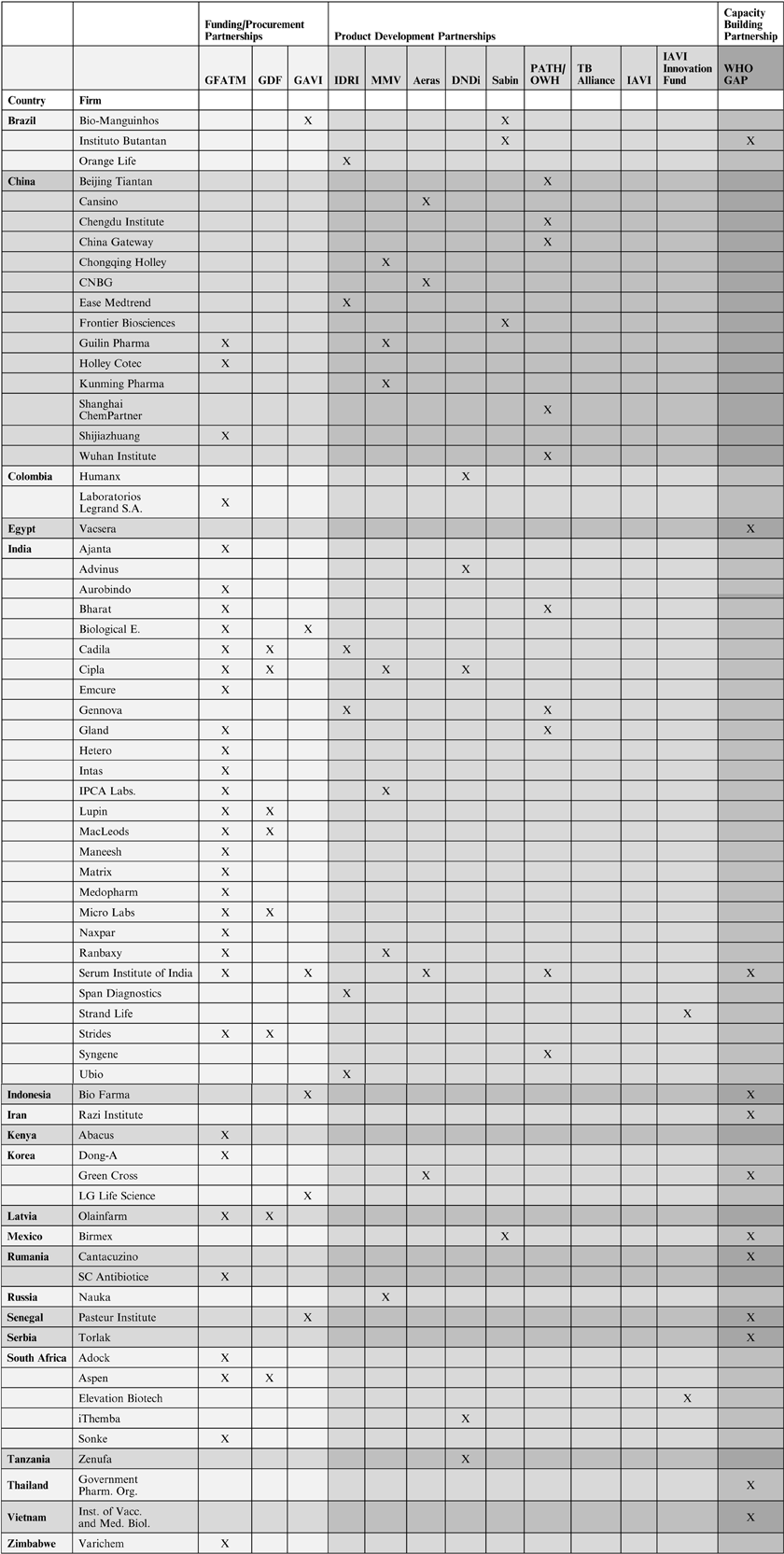
Companies from emerging markets make up between approximately 30–50 per cent of suppliers of medicines and vaccines to global health partnerships.Footnote 24 An analysis of the orders that GFATM placed for ARVs for HIV/AIDS in the period of January 2010 until September 2013 shows that 14 out of 26 (54 per cent) suppliers were from emerging markets, with nine companies from India and three from South Africa. A different study found that between 2003 and 2008, the number of suppliers from India alone had doubled.Footnote 25 Similarly, 12 out of 22 (55 per cent) companies supplying antimalaria drugs to the GFATM between January 2010 and September 2013 were from emerging markets, nine of which were from India and two from China.
The percentage was slightly lower with regard to suppliers of antituberculosis medicines to GFATM. Eleven out of 33 (33 per cent) companies were from emerging markets with seven being from India. Similarly, the number of emerging markets suppliers of antituberculosis medicines to the GDF was about one third or 8 out of 22 (36.4 per cent). Six of them were Indian companies. GAVI worked with 12 suppliers of vaccines, 50 per cent of which are based in Africa, Asia, and Latin America. This is a significant increase from to 2001 when only 1 supplier was from a low-income country (Senegal). In 2014, GAVI announced that it was extending its supply base to China.Footnote 26
Emerging market companies as partners for innovation
The proportion of emerging market companies among corporate R&D partners of global health partnerships is not as high as among their suppliers. In most global health partnerships studied here it varied between approximately 10–30 per cent. IAVI and the TB Alliance did not have R&D collaborations with emerging markets companies. A study by the PDP Funders Group suggests that the role of emerging markets companies as R&D partners has increased significantly since 2007.Footnote 27
Table 2. Developing countries biopharmaceutical companies' involvement in global health partnerships

IDRI lists six companies from emerging markets as R&D partners out of 18 corporate collaborators (33 per cent) overall. Four of the six are from India.Footnote 28 MMV works with 62 pharmaceutical companies on R&D, seven of which are from emerging markets (11 per cent), with three from China, three from India, and one from Russia.Footnote 29 According to Aeras annual report 2010, three out of a total of 15 industry partners were from emerging markets (20 per cent), with one from China, one from India, and one from Korea.Footnote 30 In 2012, Aeras announced it had started to work with another Chinese biotechnology company.Footnote 31 DNDi lists five companies from emerging markets as collaborators out of 24 corporate partners overall (21 per cent); two of them are from India and the others from Brazil, Colombia, and Tanzania.Footnote 32 The Sabin Institute lists five corporate R&D partners on their website, three of which are from emerging markets (60 per cent), with two from Brazil and one from Mexico.Footnote 33 In addition, the PDP Funders Group reports collaboration between Sabin and the Chinese company Frontier Biosciences.Footnote 34
PATH and OWH are affiliated institutions but they provide separate data on their corporate R&D partners. OWH lists ten collaborators from the biopharmaceutical industry on their website, two of which (20 per cent) are based in India.Footnote 35 According to the PDP Funders Group, OWH had research and development agreements with three companies from China and India,Footnote 36 two of which are not listed on the OWH website. PATH does not provide an overview of corporate R&D partners but a look at PATH's various product development programmes suggests that emerging markets companies play an important role. According to John Boslego, director of PATH's vaccine development program, the organisation has expanded collaboration with emerging market companies in recent years.Footnote 37 In the Meningitis Vaccine Project, an Indian company was chosen as the manufacturer of the new vaccine.Footnote 38 In the area of pneumococcal vaccine development, PATH mentions six collaborators from the biopharmaceutical industry, two of which are from emerging markets, China and India.Footnote 39 For its work on a life rotavirus vaccine, PATH mentions collaboration with three biopharmaceutical companies, all of which are from China and India.Footnote 40 PATH is also providing technical assistance to a Chinese biotechnology company for production of oral polio vaccine.Footnote 41 For its Malaria Vaccine Partnership, PATH lists six collaborators from the biopharmaceutical industry, two of which are based in India.Footnote 42
As mentioned above, the TB Alliance did not collaborate with biopharmaceutical companies from emerging markets. In 2007, however, it worked with the Indian company Lupin on two drug candidates for tuberculosis.Footnote 43 There also seems to be some interest in the growing R&D potential of China. In 2011, the TB Alliance signed a Memorandum of Understanding with the International Scientific Exchange Foundation of China to establish a Global Health R&D Center.Footnote 44 Like the TB Alliance, IAVI did not have any R&D collaborations with pharmaceutical companies from emerging markets.Footnote 45 Yet, IAVI's Innovation Fund has provided grants to two emerging markets companies out of a total of 15 grantees, one from India and one from South Africa.Footnote 46 IAVI's Innovation Fund was established to harness early stage technology in AIDS vaccine development, and to support companies in proof of concept work, which is important to attract commercial investors.
Capacity-building is the focus of the WHO GAP, which WHO has been implementing in collaboration with governments and pharmaceutical companies since 2006. GAP supports vaccine companies in emerging markets in order to increase the global supply of influenza vaccines. Companies in 11 countries, notably Brazil, Egypt, India, Indonesia, Iran, Korea, Mexico, Romania, Serbia, Thailand, and Vietnam, have received grants so far. The grants are awarded under the condition that, in the case of a pandemic, the companies sell 10 per cent of their vaccine production to United Nations agencies for distribution in low- and middle-income countries.Footnote 47 An important contributor to the GAP has been the US Biomedical Advanced Research and Development Authority (BARDA). BARDA manages the procurement and advanced development of medical countermeasures for chemical, biological, radiological, and nuclear agents, and for emerging infectious diseases on behalf of the US government. Since 2005, the US has provided more than US $50 million to advance influenza vaccine development in low- and middle-income countries.Footnote 48 The funding has been channelled primarily through GAP. In 2009, BARDA established a US $7.9 million cooperative agreement with PATH to support the final developmental processes for an influenza vaccine at a Vietnamese manufacturer.Footnote 49 The considerable engagement of the US in these global partnerships suggests that the government believes it can best protect its population from future influenza pandemics by intercepting them in the countries that they likely to spread in first, namely low- and middle-income countries.
Emerging market companies as board members of global health partnerships
Compared to their role as suppliers and R&D partners, the involvement of emerging market companies as board members of global health partnerships is negligible.Footnote 50 For this study, board members were categorised according to whether they are likely to represent the perspectives of companies from emerging markets. Sometimes, board members are selected as representatives of a specific organisation or government. In these cases, the categorisation did not present any difficulty. Sometimes, board members are selected because of the expertise and contacts they have accumulated in the course of their careers. In these cases, it was examined whether they had significant work experience in companies from low- and middle-income countries.
GAVI is the only global health partnership analysed in this study that has an almost equal balance of corporate board members from low- and middle-income countries and high-income countries. The organisation reserves one seat for vaccine producers from low- and middle-income countries and one for producers from high-income countries. In addition, there are two independent members on GAVI's governing board with a background in businesses from low- and middle-income countries and 3 with a background in business from high-income countries. The only other global health partnership that has a board member with a business background from an emerging market country is MMV (out of three board members overall with a business background). WHO GAP is an exception as it is part of WHO, where only states can be members. Also GAP's advisory board has only members from the public sector.
Overall corporate representation on the governing boards of the global health partnerships analysed in this study varies greatly. GDF does not have any representatives from the business sector on its governing board, while at IDRI almost 80 per cent of board members (seven out of nine) have a business background. Other global health partnerships with comparatively high proportions of board members with a business background are Aeras with 58 per cent and the TB Alliance with 54 per cent. On IAVI's governing board, approximately one third of the members have a background in business, and at the governing boards of GAVI and MMV the proportion is approximately 25 per cent. The remaining global health partnerships have corporate representations of between 5–15 per cent.
Emerging market companies as funders of global health partnerships
The picture that emerges with regard to the role that emerging market companies play as funders of global health partnerships is similar to that of their role as board members.Footnote 51 From publicly available data on the funding base of global health partnerships two key insights emerge. First, most funding seems to originate from the public sector, notably from governments in North America and Western Europe. The second major funder is the Bill and Melinda Gates Foundation. Funding from the commercial sector is significantly smaller. The insight that ‘the private sector has not generally met the initial … expectations that it would become the principal patron of these partnerships’ is not new.Footnote 52 Perhaps less well known is that emerging market companies hardly feature at all as funders of global health partnerships. Among the global health partnerships analysed for this research, only PATH names companies from emerging markets among its funders. Out of the 27 corporate funders listed by PATH, 1 is a biopharmaceutical company from China and 1 is from India.Footnote 53
In sum, pharmaceutical companies from emerging markets contribute significantly to the work of global health partnerships as suppliers and R&D partners, but their representation at the decision-making level is weak. In other words, pharmaceutical companies from emerging markets have become important partners in the implementation of global policies to widen access to pharmaceuticals, but not in the design of these policies. How can we explain this?
Emerging market companies: Partners in policy implementation – but not policy design
Let us turn first to the rise of emerging market companies as partners in the implementation of access policies. In the last 15 years, the fight against specific diseases, notably HIV/AIDS, malaria, tuberculosis, neglected tropical diseases, and pandemic influenza, has emerged as a key feature of global health governance. With it has come a focus on access to medicines and vaccines as a key policy goal. This focus is manifest at both the discursive and the institutional levels of global health governance.
With regard to HIV/AIDS, the Declaration of Commitment on HIV/AIDS passed by United Nations General Assembly in 2001Footnote 54 made access to medication for HIV/AIDS and the development of a HIV vaccine a key goal of global health governance. The Declaration recognises ‘that access to medication in the context of pandemics such as HIV/AIDS is one of the fundamental elements to achieve progressively the full realization of the right of everyone to the enjoyment of the highest attainable standard of physical and mental health’.Footnote 55 In addition, the Declaration commits to ‘increase investment in and accelerate research on the development of HIV vaccines’.Footnote 56 In the following years, a consensus emerged that global access to ARV treatment was possible.Footnote 57
In the area of neglected tropical diseases, a roadmap by WHO in 2012 identifies preventative chemotherapy, that is, the delivery of medicines, as the key global governance strategy to tackle these diseases.Footnote 58 The roadmap was endorsed by a stakeholder group comprising representatives of governments, the pharmaceutical industry and global health partnerships in the London Declaration on Neglected Tropical Diseases. The first three of the Declaration's seven commitments are about access to medicines. The Declaration's ‘endorsers’ commit to contribute to: (1) ‘Sustain, expand and extend programmes that ensure the necessary supply of drugs and other interventions to help eradicate Guinea worm disease, and help eliminate by 2020 lymphatic filariasis, leprosy, sleeping sickness … and blinding trachoma’; (2) ‘Sustain, expand and extend drug access programmes to ensure the necessary supply of drugs and other interventions to help control by 2020 schistosomiasis, soil-transmitted helminthes, Chagas disease, visceral leishmaniasis and river blindness (onchocerciasis)’; and (3) ‘Advance R&D through partnerships and provision of funding to find next generation treatments and interventions for neglected diseases’.Footnote 59
In the area of pandemic preparedness, the EU adopted a Decision on Serious Cross-border Threats to Health in 2013, and the EU Health Commissioner highlighted, ‘[t]he next milestone for health security under this legislation is the Joint Procurement Framework Agreement … Under this agreement, Member States can … purchase, together, vaccines and other medical countermeasures needed to fight a cross border health threat. This is to ensure that all Member States, big and small, are able to secure vaccines and other medicines for their people and under better conditions than in the past.’Footnote 60 Similarly, the Global Action Plan for Influenza Vaccines of the World Health Organization (WHO) emphasises that ‘[i]nfluenza vaccine development and deployment are critical elements of pandemic influenza preparedness.’Footnote 61
The focus on widening access to pharmaceuticals as a key policy goal in global health governance has become manifest also at the institutional level. Many organisations that make up the organisational landscape of global health governance today are mandated with the financing, procurement and development of pharmaceuticals, and most of them have been created in the period since the late 1990s.
Concerted global efforts to make pharmaceuticals more widely available, especially in low- and middle-income countries, are a relatively new phenomenon. Prior to the late 1990s, international health policies focused largely on coordinating national activities for the events of infectious disease outbreaks and technical guidance through WHO.Footnote 62 Perhaps the most important precedent for a global mobilisation of resources to make pharmaceuticals widely available in low- and middle-income countries is the smallpox eradication programme. Between 1967 and 1979, vaccines were procured and supplied to endemic countries with the help of funding from WHO and especially its largest donor the US.
In order to conceptually grasp the recent focus on widening access to pharmaceuticals in the field of health security policy, Elbe, Roemer-Mahler, and LongFootnote 63 draw on the concept of ‘pharmaceuticalisation’. This concept had originally been developed by sociologists and anthropologists to highlight the proliferation of pharmaceuticals in various areas of social life.Footnote 64 While much of this literature focuses on how the pharmaceutical industry drives pharmaceuticalisation, Elbe et al. apply this concept to investigate government responses to the emergence of bioterrorism and pandemic preparedness as security issues. The authors find that a key element of governments' responses to these new security threats has been the development of medicines and vaccines as ‘medical countermeasures’ and the stockpiling of existing pharmaceuticals. Governments, they argue, are important drivers of pharmaceuticalisation in the field of security because they incentivise the commercial development of medicines and vaccines by providing funds, granting legal protections for pharmaceutical companies, introducing emergency use procedures, and developing systems for mass drug administration. Such incentives are necessary because profit-oriented companies are reluctant to invest in products that may never be needed and for which the number of potential buyers are limited to a few governments.
Pharmaceuticalisation is a useful concept for the present study because it helps understand the role that pharmaceutical companies, in general, and emerging market firms, in particular, have obtained in global health governance in the last 15 years. The concept highlights that during this period global health has become strongly associated with the pharmaceutical treatment and prevention of specific diseases. It points out that, like in the field of security policy, the recent policy shift has been accompanied by efforts to widen access to pharmaceuticals and led to a set of institutional and policy responses aimed at incentivising their development and production, such as grants, technology transfer, advance market commitments, and pooled procurement, for example. Such incentives are required for similar reasons as in the field of security and medical countermeasures: commercial market demand and, hence, profit margins are low.
The concept of pharmaceuticalisation highlights a set of social and policy dynamics that promote a dependency of societies and governments on pharmaceutical products and, hence, companies. Pharmaceuticals are developed and produced largely in the private, for-profit sector. Hence, by making access to drugs and vaccines a key focus of policy, governments and global health partnerships have made themselves dependent on commercially operating pharmaceutical companies to enable the implementation of this policy. Moreover, in order to be able to provide ‘universal’ access, they depend on commercially operating companies to supply drugs and vaccines at extremely low prices.
Initially, governments, global health partnerships and advocacy groups had approached the world's ‘big pharma’ companies – large European and US companies – as potential suppliers and development partners.Footnote 65 These companies were already producing many of the required medicines and vaccines and had the financial and technological capabilities to develop new products. Yet, big pharma companies have shown limited interest in engaging in global health partnerships and supplying medicines and vaccines at such low price levels.Footnote 66 Opportunity costs are high, profit margins comparatively low, and demand is often difficult to forecast.Footnote 67 There is some evidence to suggest that big pharma companies have substantially invested in partnerships for pharmaceutical development particularly when these investments could be linked to the repurposing of existing products and/or the opening of markets of commercial interest to these companies.Footnote 68
As a result, governments and global health partnerships have increasingly turned to pharmaceutical companies from emerging markets, particularly from India. The Indian pharmaceutical industry has long been the fastest growing pharmaceutical industry in emerging markets, and Indian companies had early on developed the technological capabilities to produce many of the medicines and vaccines required by global health partnerships. Furthermore, until 2005, Indian companies operated under a national intellectual property regime that prevented the patenting of pharmaceutical products.Footnote 69 Finally, Indian manufacturers were able to supply at prices far below those of big pharma companies.Footnote 70
Until recently, however, they did not have the technological and financial capabilities to develop new products. This, however, is changing. In the past decade, the innovative capabilities of companies from emerging markets, particularly Brazil, China and India, has increased substantially.Footnote 71 While innovation currently tends to be incremental rather than for entirely new molecules,Footnote 72 a study on the health biotechnology sectors in Brazil, China, and India identified 165 innovative products within 41 domestic firms in these countries.Footnote 73 Indian companies had the largest share of innovative products with 55 per cent, followed by Chinese firms with 29 per cent, and Brazilian firms with 16 per cent of the total number of products identified.Footnote 74 As a result, governments and global health partnerships have recently intensified collaboration for pharmaceutical development with emerging market producers.
The pharmaceuticalisation of global health governance and the price pressure resulting from the goal to achieve ‘universal’ access has been a key driving force for governments and global health partnerships to turn to pharmaceutical companies from emerging markets as partners for the supply of existing and the development of new medicines and vaccines. Yet, why have these companies invested in these products given extremely low prices and profit margins? While companies from emerging markets are as heterogeneous as companies from high-income countries, and generalisations are therefore difficult, research in International Business has found that companies from emerging markets tend to differ from companies from high-income countries in a variety of ways that may be relevant for this question. Scholars researching the internationalisation strategies of emerging markets companies have argued that their investment decisions can often be explained better by how investments contribute to the capability-building process of the firm than by calculations of short-term returns.Footnote 75 For pharmaceutical companies from emerging markets, access to technology and expertise appears to be of particular importance to build capabilities in areas such as manufacture, scale-up, regulation, and international market penetration.
A Brazilian biotech entrepreneur, who is cited by Rezaie and colleaguesFootnote 76 argued that ‘the increasing attention to neglected diseases by prominent nonprofits … presents Brazilian SMEs [small and medium-sized companies] with an ideal opportunity to build up their international linkages’. A study on Indian biotech companies found that ‘firms are interested in working with [global health partnerships] for access to their expertise and resources in tackling global health issues’.Footnote 77 The Developing Countries Vaccines Manufacturers Network (DCVMN), an alliance of 27 vaccine producers from 14 low- and middle-income countries, names ‘strengthening and enhancement of technology … encourage[ing] continuation of research and development efforts … and facilitate[ing] innovative models of ownership of health related intellectual property’Footnote 78 as key aims of the Network. Indeed, when DCVMN was founded in 2001, ‘[i]t was hoped … that GAVI would support the Network by “push” mechanisms such as facilitating access to technology’.Footnote 79 With regard to the involvement of the Indian vaccine company Serum Institute of India (SII) in a public-private partnership for the development of a new meningitis vaccine, scholars have found that access to a new technology for vaccine manufacture, namely conjugation technology, was was an important consideration for SII's decision to manufacture the vaccine at the low price of US $0.5 per dose.Footnote 80
Collaboration with global health partnerships as a means to access new technologies is a strategy that has been observed also in a study on Chengdu Biological Products, a large public-sector vaccine manufacturer in China. The authors find that collaboration with PATH ‘provided Chengdu with much vaccine production hardware and software that they would not ordinarily have, and thus gives them an advantage on the international market, even if they cannot control the sales price’.Footnote 81 Scholars researching the growing market for generic ARVs for HIV/AIDS in the early 2000s found that the creation of new markets by humanitarian groups, such as Médecins Sans Frontières, and international financing organisations, such as GFATM, was an important factor in the decisions of Indian companies to supply the drugs at low prices because it increased volumes and improved the predictability of the market.Footnote 82
Finally, research suggests that differences in ownership structure between companies from high-income countries and companies from emerging markets may influence investment decisions. It has been argued that the prevalence of state- and family-ownership among emerging markets companies may make decisions in favour of long-term and less secure investments more likely than the shareholder model of corporate governance and financing that is prevalent among most big pharmaceutical companies from high-income countries. In their study of the Indian biopharmaceutical sector, Wilson and Rao report that ‘[s]everal firms told us that they could consider products that had only modest markets as long as they thought they would be able to at least cover costs; this attitude may reflect the freedom conferred by being privately held (as opposed to publically traded) or … state-owned [companies]’.Footnote 83
The increasing involvement of emerging market companies as suppliers and R&D partners of global health partnerships may therefore be explained by a combination of a policy shift in global health governance towards greater pharmaceuticalisation and a set of internal and external factors that can create a more compelling business case for emerging market companies to produce at low-profit margins than for big pharma firms from Western Europe and the US. In light of the significant role that pharmaceutical companies from emerging markets play as suppliers and R&D partners, their weak representation on the governing boards and among the funders of global health partnerships is puzzling, however.
An explanation may be found in the history of global health partnerships. The ideas and values underlying the rise of public-private partnerships as instruments of governance are based on neoliberalist notions of the role of the state as facilitator, rather than provider, of social policies,Footnote 84 and changing conceptions of the relations between public and private,Footnote 85 both of which have their roots in Anglo-Saxon schools of thought.Footnote 86 Representatives of high-income countries tend to be significantly more numerous on the governing boards and among the funders of global health partnerships than representatives from low- and middle-income countries, not only with regard to representatives from the business sector. Among the global health partnerships analysed here, only the governing board of GAVI shows an equal balance of representatives from high-income countries and low- and middle-income countries. At GFATM and DNDi, approximately one third of board members are from low- and middle-income countries. On the governing boards of the remaining global health partnerships, representatives from low- and middle-income countries make up between 0 per cent and 18 per cent.
Kent Buse and Andrew Harmer trace the under-representation of low- and middle-income countries in global health partnerships back to the early days of their creation. They find that the partnership model was promoted by a small group of individuals and organisations ‘from wealthy, middle-class socio-economic groupings; none were African, indeed all but one were “white”, and only four were female’.Footnote 87 The politics and power dynamics inherent in global health partnerships are evident also in the tension between the terminology of ‘global’ health partnerships and the actual direction of most of their efforts towards low- and middle-income countries. Similarly, there is a mismatch between the absence of emerging market companies on the governing boards of global health partnerships and the language used by many of these organisations, which describes these companies as ‘partners’.
Despite the emergence of pharmaceutical companies from low- and middle-income countries as key suppliers and R&D partners, global health partnerships seem to still be embedded in the power relations of an aid-based model of global health governance.Footnote 88 The question why these dynamics persist goes beyond the scope of this article, but it is an important one. While board representation and provision of financial resources do not automatically guarantee influence,Footnote 89 they certainly can open doors and generate access to information that is important to influence policy.
Conclusion: Implications for global health governance
What are the implications of this analysis for global health governance? The concluding section argues that the increasing involvement of pharmaceutical companies from emerging markets in global health governance can be interpreted as a double-edged sword. On the one hand, their increasing involvement as suppliers of low-cost pharmaceuticals and technologies has enabled governments and global health partnerships to implement a key goal of global health policy: widening access to pharmaceutical treatment and prevention. One the other hand, the analysis points to a fundamental tension between this policy goal and the political economy of how medicines and vaccines are developed and produced.
Many of the achievements of universal access policies would not have been possible without emerging market companies supplying low-cost, high-quality medicines and vaccines. For example, the rapid scale up of HIV/ AIDS treatment in low- and middle-income countries from a few hundred thousand people in the early 2000s to almost ten millionFootnote 90 today has been built largely on ARVs produced by pharmaceutical companies from emerging markets.Footnote 91 Also, the significant reduction of meningitis cases in Africa, which in 2013 was the lowest in ten years, has been attributed to the introduction of a vaccine produced by an Indian company in collaboration with a public-private partnership.Footnote 92
Moreover, the rapid growth of biopharmaceutical innovation in emerging markets provides potentially great opportunities for global health governance because the types of innovation produced by emerging market companies may be well aligned with the needs of patients in low- and middle-income countries. The International Business literature has found that the low average income in the domestic markets of emerging market companies has ‘spurred innovations to serve people at the middle or bottom of the economic pyramid’.Footnote 93 Many emerging market companies seem to have developed particular strengths in developing or adapting products so as to lower production costs without necessarily affecting quality.Footnote 94 In addition, many companies from these countries were found to have the skills and customer knowledge required to make products easier to use in the more difficult conditions of low- and middle-income countries.Footnote 95
Several examples of such innovations by pharmaceutical companies from emerging markets exist that have played a significant role in global health governance. In 2001, the Indian company Cipla introduced the first internationally recommended 3-in-1 fixed dose combination of three key ARVs (Stavudine + Lamivudine + Nevirapine) to treat HIV/AIDS. The combination of the three ingredients in one fixed-dose treatment made it much easier for patients in low- and middle-income countries to adhere to the complex drug regimen required for successful treatment. More recently, the Brazilian Butantan Institute developed an innovative process to produce a cellular pertussis vaccine at a cost of only US $0.12–0.15 per dose compared with its counterpart costing US $8 per dose.Footnote 96 The involvement of emerging market companies as R&D partners, therefore, may provide an opportunity to access the specific skills required for the development of products that are more affordable and adapted to the needs of users in low- and middle-income countries.
The growing involvement of emerging market companies in global health governance therefore provides great opportunities for the implementation of global access policies. Yet, the analysis in the previous section of why emerging market companies have become involved in global health governance points to the fragility of this contribution. It shows that the pharmaceuticalisation of global health governance has created great pressure on governments and global health partnerships to find ever-cheaper sources of medicines and vaccines. It is this price pressure that has made them turn to suppliers from emerging markets. Yet, while emerging market companies may be able to produce at lower profit margins than their counterparts in Western Europe and the US, they too are profit-oriented organisations. The partnership language used to describe many of the new organisations in global health can obscure the fact that companies – including emerging market firms – engage in global health partnerships for commercial reasons. They are not predominantly partners in global health but first and foremost business partners in the legitimate pursuit of profit for their owners. It is therefore not surprising that a growing number of pharmaceutical companies from emerging markets are responding to the continuous price pressure from global health partnerships with concern.Footnote 97
The tension between global health policy goals that require access to pharmaceuticals with extremely small profit margins and the organisation of pharmaceutical supply by for-profit organisations is familiar to most scholars and practitioners in global health governance. In fact, the creation of public-private-partnerships to widen pharmaceutical access and the use of partnership language are manifestations of this tension. While the increasing involvement of emerging market companies in global health partnerships seems to have mitigated this tension at first glance, a closer look reveals that it has also brought it further to the fore.
The tension between political demand and market demand for pharmaceuticals affects not only the global politics of HIV/AIDSFootnote 98 but also the global response to a wide – and growing – range of other diseases, including malaria and tuberculosis, neglected tropical diseases, pandemic influenza, bioterrorism and, most recently, antibiotics. In light of the threat that some of these diseases pose to public health it seems risky to base policy responses on the quest for ever cheaper sources of commercial supply. Pharmaceutical companies can play a role in the development and production of medicines and vaccines for which there is effective market demand, and some companies are willing to produce at lower profit margins than others. Yet, for-profit operating companies will not take on the costs and risks of producing goods for which the market is very small or unpredictable. If global health governance is to pursue the goal of providing access to medicines and vaccines globally and sustain the successes already achieved, greater investment from the public in the development, production, and procurement of medicines and vaccines is required.