Introduction
Depressive disorders have been identified as predictors of premature cardiovascular disease, independent of other recognized psychosocial or ‘lifestyle’ factors (Bunker et al. Reference Bunker, Colquhoun, Esler, Hickie, Hunt, Jelinek, Oldenburg, Peach, Ruth, Tennant and Tonkin2003). Specifically, these disorders are estimated to result in an 80–90% increased risk of cardiovascular disease (CVD) onset (Nicholson et al. Reference Nicholson, Kuper and Hemingway2006) and to exacerbate the progression and prognosis of CVD (Doyle et al. Reference Doyle, McGee, Conroy, Conradi, Meijer, Steeds, Sato, Stewart, Parakh, Carney, Freedland, Anselmino, Pelletier, Bos and de Jonge2015), contributing significantly to the mortality gap (Goldstein et al. Reference Goldstein, Carnethon, Matthews, McIntyre, Miller, Raghuveer, Stoney, Wasiak and McCrindle2015). Potential independent mechanisms by which depressive disorders may directly increase CVD have been hypothesized to include metabolic, immune and sympathetic nervous system dysregulation (Pan et al. Reference Pan, Keum, Okereke, Sun, Kivimaki, Rubin and Hu2012; Penninx et al. Reference Penninx, Milaneschi, Lamers and Vogelzangs2013).
Most commonly, slowly accumulating weight gain due to adverse medication effects or related factors, such as poor diet and low exercise levels, are thought to drive metabolic dysfunction (Chokka et al. Reference Chokka, Tancer and Yeragani2006; McIntyre et al. Reference McIntyre, Park, Law, Sultan, Adams, Lourenco, Lo, Soczynska, Woldeyohannes, Alsuwaidan, Yoon and Kennedy2010). Yet, in young people with emerging mood disorders, we have observed heightened metabolic risk factors before longer-term weight gain or medication exposure (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019). A range of other studies also suggests that perturbed metabolic, inflammatory, autonomic and hypothalamic-pituitary-adrenal axis functions, as well as circadian rhythm disturbances, may play pivotal roles in the observed relationship between depression and metabolic syndrome (Penninx Reference Penninx2017; Penninx et al. Reference Penninx, Milaneschi, Lamers and Vogelzangs2013; Pitsillou et al. Reference Pitsillou, Liang, Hung and Karagiannis2021).
Our prior work highlighted the limitation of relying on standard fasting blood glucose (FBG) for detecting metabolic dysfunction in young people with mood or psychotic disorders (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019; Scott et al. Reference Scott, Hermens, White, Naismith, GeHue, Whitwell, Glozier and Hickie2015). Many people in the early stages of these disorders have elevated fasting insulin or increased insulin resistance (as indexed by the Homeostatic Model Assessment for Insulin Resistance [HOMA2-IR]), even in the absence of abnormal FBG (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019; Scott et al. Reference Scott, Hermens, White, Naismith, GeHue, Whitwell, Glozier and Hickie2015). Therefore, establishing the utility of a more sensitive marker of metabolic dysregulation in young populations has the capacity to guide earlier intervention.
Low-grade inflammation is commonly observed in major mental disorders (Osimo et al. Reference Osimo, Baxter, Lewis, Jones and Khandaker2019; Osimo et al. Reference Osimo, Perry, Cardinal, Lynall, Lewis, Kudchadkar, Murray, Perez, Jones and Khandaker2021), with a notable association between inflammatory or immune-mediated factors (e.g., C-reactive protein [CRP]), and other cardiometabolic factors (e.g., insulin resistance, body mass index [BMI]) (Tickell et al. Reference Tickell, Rohleder, Ho, McHugh, Jones, Song, Hickie and Scott2022). The pathophysiological relationships between these systems, and the extent to which they may simply be markers of illness duration or increasing body weight, are unclear. Further, these markers may not simply be indicators of severity or chronicity but rather they may be associated with specific depressive illness subtypes. In adults, those with atypical symptom profiles have shown a higher propensity for such metabolic and immune disturbances (Lamers et al. Reference Lamers, Milaneschi, Vinkers, Schoevers, Giltay and Penninx2020; Milaneschi et al. Reference Milaneschi, Lamers, Berk and Penninx2020). However, understanding of these potential shared pathophysiological mechanisms remains limited (Scott et al. Reference Scott, Hickie, Crouse and Shin2024).
Consequently, this ongoing longitudinal study examines sensitive metabolic and inflammatory markers in young individuals accessing early mental health intervention services. It addresses three critical research questions with the following exploratory hypotheses:
-
1. What are the trajectories, and demographic and clinical correlates, of sensitive metabolic and inflammatory markers early in the course of major mood disorders?
-
We hypothesize that individuals with major mood disorders exhibit worsening trajectories of HOMA2-IR and CRP early in the course of the disorder. Additionally, we expect that these trajectories will be significantly associated with demographic variables (such as sex and BMI) and clinical characteristics (such as illness subtypes) over time.
-
-
2. Are metabolic and immune/inflammatory markers associated with specific illness subtypes?
-
We hypothesize that the “circadian–bipolar spectrum” is associated with a higher risk of metabolic and inflammatory dysfunctions, while the “neurodevelopmental psychosis” is associated with a higher risk of inflammatory dysfunction.
-
-
3. Is HOMA2-IR a more appropriate measure of early metabolic dysfunction than FBG in young people with emerging mood disorders?
-
We hypothesize that HOMA2-IR will be a more appropriate measure of early metabolic dysfunction than FBG in young people with emerging mood disorders.
-
This exploratory study aims to provide insights into these areas and inform future research directions.
Methods
Data for this study come from the ongoing Neurobiology Youth Follow-up study (Nichles et al. Reference Nichles, Zmicerevska, Song, Wilson, McHugh, Hamilton, Crouse, Rohleder, Carpenter, Ho, Hermens, Wray, Scott, Merikangas, Leweke, Koethe, Iorfino, Naismith, Guastella, Scott and Hickie2021). This study involves longitudinal clinical, biological and pathology assessments of a clinical cohort of young people seeking help from early intervention mental health services in Sydney, Australia, including headspace Camperdown (Scott et al. Reference Scott, Hermens, Glozier, Naismith, Guastella and Hickie2012) and Mind Plasticity (a specialist multidisciplinary clinic). The cohort study targets individuals who are primarily aged 12–30 years who are seeking or have sought help for mental health problems at these early intervention services and associated clinics. The cohort study also uses a follow-back design to re-recruit individuals who participated in our previous studies; for this restricted sub-group, the eligible age-range is raised to 40 years. A subset of participants from the Neurobiology Youth Follow-Up Study also consented to share their historical blood data from a prior study (University of Sydney HREC: 2012/1631). We linked these datasets to examine longitudinal trajectories of blood markers. Exclusion criteria include individuals who are non-English speakers unable to provide consent or those with intellectual disabilities. The study encompasses a comprehensive evaluation of clinical, self-report, neurocognitive, sleep-wake and biological measures (e.g., blood sampling) at baseline, with structured re-assessments at 6, 12, 24 and 36 months. Detailed information for each assessment is stated in the published study protocol (Nichles et al. Reference Nichles, Zmicerevska, Song, Wilson, McHugh, Hamilton, Crouse, Rohleder, Carpenter, Ho, Hermens, Wray, Scott, Merikangas, Leweke, Koethe, Iorfino, Naismith, Guastella, Scott and Hickie2021). In this paper, we report all available blood outcomes of HOMA2-IR, CRP and FBG. For interview-based assessments, we only report the most recent evaluations, as these data are not collected for historical blood data. These include Quick Inventory of Depressive Symptomatology (past week), Brief Psychiatric Rating Scale (mix of lifetime, recent and current items), Young Mania Rating Scale (past 48 hours), Social and Occupational Functioning Assessment Scale (past month); and self-report assessments such as the Kessler Psychological Distress Scale (K10) (past month) and Overall Anxiety Severity Impairment (past week). The effect of specific medications is outside the scope of this paper; therefore, we only report the number of medications in Table 1. Ethical approval for the study was obtained from the Human Research Ethics Committee of the Sydney Local Health District (2020/ETH01272). Informed consent was secured from all participants.
Table 1. Demographics and clinical characteristics of participants (n = 155)

BMI, Body mass index, QIDS, Quick Inventory of Depressive Symptomatology-Clinician Rating; BPRS, Brief Psychiatric Rating Scale; YMRS, Young Mania Rating Scale; SOFAS, Social and Occupational Functioning Assessment Scale; K10, Kessler Psychological Distress Scale; OASIS, Overall Anxiety Severity Impairment Scales.
Measures
Blood samples were collected in a fasting state at a pathology laboratory at each assessment visit and CRP and FBG were calculated by the pathology laboratory using standardized protocols. This paper focused on HOMA2-IR, calculated using fasting insulin and FBG results with the HOMA2 software V.2.2.3 (Hill et al. Reference Hill, Levy and Matthews2013), and CRP. Longitudinal changes were assessed with repeated measurements of these markers. Not all markers were consistently assessed at each sampling point, leading to varying sample sizes (as indicated in figure captions and table descriptions). We defined “elevated risk” by HOMA2-IR greater than 1.5, as outlined in our previous report (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019), insulin level greater than 10 mU/L (consistent with HOMA2-IR >= 1.5) (Shin et al. Reference Shin, Crouse, Nichles, Song, Park, Froggatt, Janiszewski, Chan, Koethe, Hamilton, Carpenter, Iorfino, Mckenna, Scott and Hickie2024), FBG level greater than 5.6 mmol/L, and CRP greater than 3mg/L (Osimo et al. Reference Osimo, Baxter, Lewis, Jones and Khandaker2019).
Assessments included demographic information, BMI measurement and evaluations of mental health symptoms assessed through both clinician-rated and self-reported measures (see supplementary materials), conducted at the last available blood assessment. Our team has proposed three common illness subtypes, based on adolescent-onset and pathophysiological trajectories (Carpenter et al. Reference Carpenter, Iorfino, Cross, Davenport, Hermens, Rohleder, Crouse, Leweke, Koethe and Guastella2019; Hickie et al. Reference Hickie, Hermens, Naismith, Guastella, Glozier, Scott and Scott2013; Hickie et al. Reference Hickie, Scott, Cross, Iorfino, Davenport, Guastella, Naismith, Carpenter, Rohleder and Crouse2019): Participants with primary psychotic disorders or a history of childhood-onset developmental difficulties (such as autism spectrum disorders or learning disabilities) are allocated to the Neurodevelopmental-Psychosis subtype. Those with sleep-wake dysregulation (e.g., high variability or delayed timing of sleep) or significant manic-like symptoms or atypical features (e.g., reduced activation and energy or prolonged fatigue) are assigned to the Circadian–Bipolar Spectrum subtype, regardless of current or past psychotic phenomena. Participants exhibiting anxiety symptoms and evolving depressive symptoms, without clear evidence of the other two subtypes, are categorized into the Hyperarousal-Anxious Depression subtype. This last group serves as the default for individuals with common anxiety and depressive symptoms who do not fit the defining features of the other two subtypes. Each participant was assigned to one of these three subtypes, using our standard algorithm by trained clinical research staff who received specific training and are familiar with the pathophysiological mechanisms model, based on their clinical presentations at each assessment visit.
Data and statistical analysis
All statistical analyses were conducted using R software (version 4.2.3). Participants were categorized into subgroups based on their baseline risk status (low vs. elevated risk) for each marker at the first blood assessment and into illness subtypes based on the latest clinical presentation.
For question 1, linear mixed-effects models, using the lmer function from the lme4 R package (version 1.1.31), were employed to examine longitudinal trajectories of HOMA2-IR and CRP as dependent variables. Both models included fixed effects for the baseline HOMA2-IR or CRP value, assessment duration, BMI (at the last assessment), sex and illness subtypes, with the participant as a random factor. To account for the limited number of blood assessments at later time points, further analyses were narrowed to changes observed within the first year of the study after excluding outliers (HOMA2-IR >4 or CRP >20 mg/L). Demographic and clinical variables among subgroups were compared using analysis of variance (ANOVA) or Kruskal-Wallis tests.
For question 2, a multinomial logistic regression model was fitted to investigate whether HOMA2-IR and CRP (as independent variables) are indicative of current illness subtypes (as the dependent variable). Age was selected as a covariate in this model due to differing durations for each HOMA2-IR and CRP assessment. The model included fixed effects for longitudinal HOMA2-IR, longitudinal CRP, BMI (at the last assessment), age (at each blood test) and sex. Participants were included as random intercepts to account for individual variability in the probabilities of illness subtypes. This analysis was conducted using the multinom function from the nnet package (version 7.3.18) in R. Adjusted p-values were computed using the false discovery rate (FDR) method, and odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were examined for interpretation.
Additionally, to determine the overall within-individual relationship among paired measures assessed on two or more occasions, we used the ‘rmcorr’ function to examine the repeated correlations between 1) HOMA2-IR and CRP; 2) Age and HOMA2-IR; and 3) Age and CRP.
Trajectories of fasting insulin and FBG were visually examined for question 3.
Results
The sample included 155 participants (100 females (65%), current mean age of 26.9 ± 5.6 years, age at the first blood assessment of 21.0 ± 6.1 years, Table 1) who provided blood samples as part of the Neurobiology Youth Follow-Up Study. 98 of these participants also had historical blood outcomes from a prior study (54 females (66%), current mean age of 28.5 ± 4.8 years). Participants’ ages at each HOMA2-IR assessment ranged from 13-41 years (median age = 25.0 years, mean age = 25.1 ± 5.7 years), and at each CRP assessment from 8 to 41 years (median age = 24.0 years, mean age = 24.1 ± 5.8 years). To examine changes in these markers over time, we included participants who provided at least two blood outcomes (for HOMA2-IR or CRP). The median number of follow-up assessments for HOMA2-IR was three, with a mean and standard deviation of 3.0 ± 1.2 assessments (range: 2 to 9). Participants provided CRP data with a median number of follow-up assessments of four, with a mean and standard deviation of 4.1 ± 2.0 assessments (range: 2 to 16).
Repeated measures correlation analyses applied to data from multiple timepoints revealed a significant positive correlation between HOMA2-IR and CRP levels (r = 0.27, p = 0.005, see Supplementary Materials Figure S1). While age was correlated with HOMA2-IR (r = −0.15, p = 0.026, see Supplementary Materials Figure S1), BMI at each assessment was not available, preventing the calculation of repeated measures correlations between BMI and CRP or HOMA2-IR.
Research Question 1
Longitudinal changes in insulin resistance measured by HOMA2-IR
Follow-up HOMA2-IR data were available for 110 participants, with a median duration of 13.1 months and mean duration of 36.2 ± 40.7 months (range: 3.7–126.2 months). Trajectories for HOMA2-IR over the entire study period and during the initial 24 months were examined, as depicted in Figures 1A and 1B, respectively. Figures 1C and 1D illustrate the HOMA2-IR trajectories between risk-status groups and between illness subtypes during the initial 24 months. Characteristics differences between risk-status groups and between illness subtypes are presented in Table S1 and S2 of the Supplementary Material, respectively.

Figure 1. Changes in Homeostatic Model Assessment for Insulin Resistance (HOMA2-IR) trajectories. Panel A: Overall changes in HOMA2-IR over time (n = 110). Panel B: Changes in HOMA2-IR during the initial 24 months of follow-up (n = 85). Panel C: Trajectories of HOMA2-IR among baseline risk-status groups during the initial 24 months (n = 65 low-baseline-risk (HOMA2-IR<1.5); n = 20 elevated-baseline-risk (HOMA2-IR ≥ 1.5)). Panel D: Trajectories of HOMA2-IR between current illness subtypes during the initial 24 months (n = 59 hyperarousal-anxious subtype; n = 9 circadian-bipolar spectrum; n = 17 neurodevelopmental psychosis).
Entire study duration: A linear mixed model predicting HOMA2-IR over the entire study period revealed significant main effects for the baseline HOMA2-IR level (β = 0.83, p < 0.001) and study duration (β = 0.01, p < 0.001), indicating that higher HOMA2-IR values and longer study duration were associated with higher subsequent HOMA2-IR levels (Table 2). There was a significant interaction between the baseline HOMA2-IR and study duration (β = −0.59, p < 0.001), suggesting a differential rate of change in HOMA2-IR over time according to baseline HOMA2-IR levels, with smaller changes in participants with higher baseline levels. Additionally, BMI showed a positive association with HOMA2-IR, with a one-unit increase in BMI corresponding to a 0.03 increase in HOMA2-IR (p < 0.001). Illness subtypes and sex were not significantly associated with HOMA2-IR.
Table 2. Longitudinal changes in insulin resistance measured by Homeostatic Model Assessment for Insulin Resistance (HOMA2-IR)

Two separate models were run to predict HOMA2-IR: 1) over the entire study duration and 2) for the initial 24-month period. Fixed effects included baseline HOMA2-IR, duration, BMI, sex and illness subtypes, with interaction terms for HOMA2-IR and duration. #At baseline; †‘hyperarousal-anxious depression’ was the reference group.
Initial 24 months: Further analysis was conducted to predict changes in HOMA2-IR during the initial 24 months using the same predictors. The results were similar to the models for the entire study period (Table 2). Baseline HOMA2-IR and duration were again positively associated with longitudinal HOMA2-IR (β = 0.84, p < 0.001; β = 0.04, p < 0.001, respectively). Additionally, a significant interaction was detected between the baseline HOMA2-IR and assessment duration (β = −0.03, p < 0.001), again indicating a lower rate of change in HOMA2-IR over time in participants with higher baseline levels. BMI was also positively associated with HOMA2-IR, with a one-unit increase in BMI corresponding to a 0.02 increase in HOMA2-IR (p < 0.001). In this model, the “Circadian-bipolar spectrum” subtype was associated with higher levels of HOMA2-IR compared to the “Hyperarousal-anxious” subtype (β = 0.24, p = 0.004). Model outcomes are presented in Table 2.
Longitudinal changes in inflammatory marker (CRP)
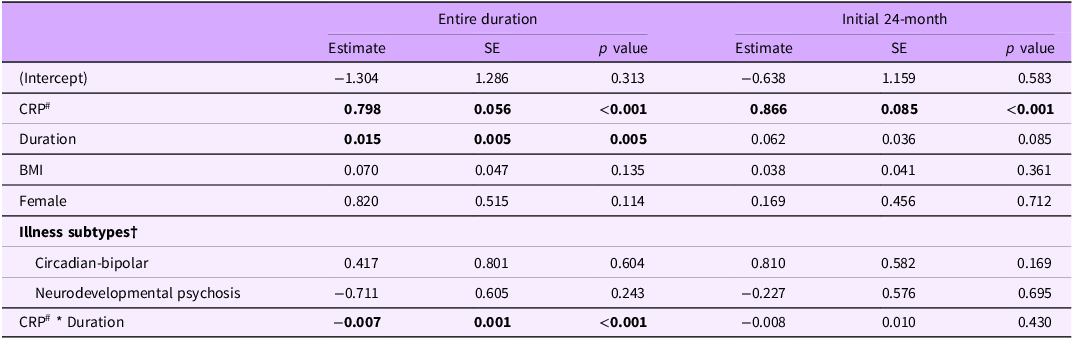
Longitudinal CRP data were available for 111 participants, with a median duration of 29.6 months and mean duration of 48.0 ± 44.2 months (range: 1.3–196.4 months). Changes in CRP over the entire study period and during the initial 24 months were examined (Figures 2A and 2B, respectively). Figures 2C and 2D present CRP trajectories between risk-status groups and between illness subtypes during the initial 24 months. Characteristic differences between risk-status groups and between illness subtypes are presented in Table S1 and S2 of the Supplementary material, respectively.
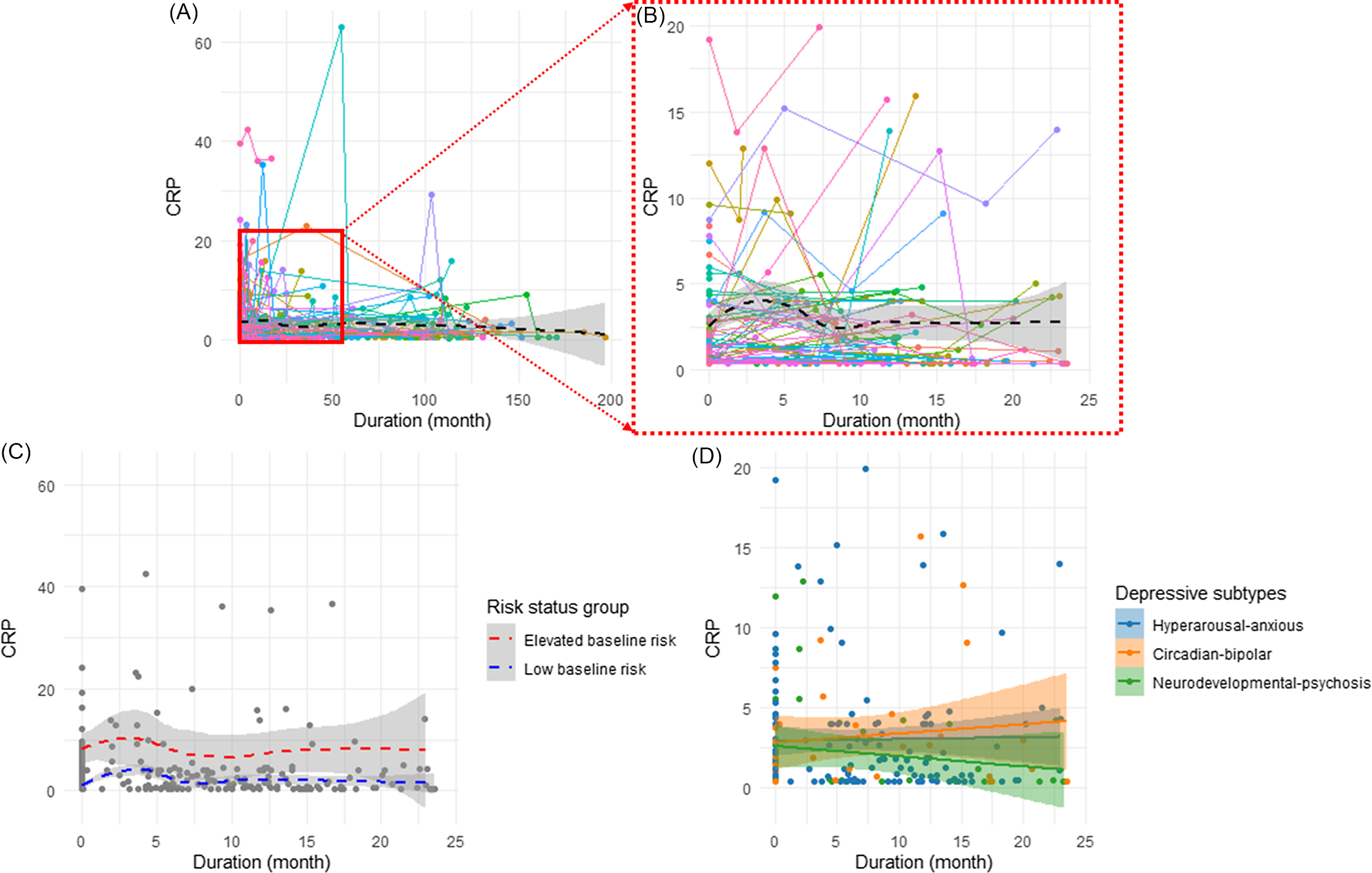
Figure 2. Changes in C-reactive protein (CRP) trajectories. Panel A. Overall changes in CRP over the time (n = 111). Panel B. Changes in CRP during the initial 24 months (n = 68). Panel C. Trajectory of CRP between baseline risk-status groups during the initial 24 months (n = 51 low-baseline-risk (CRP < 3); n = 17 elevated-baseline-risk (CRP ≥ 3)). Panel D. Trajectory of CRP between illness subtypes during the initial 24 months (n = 45 hyperarousal-anxious subtype; n = 11 circadian-bipolar spectrum; n = 12 neurodevelopmental psychosis).
Entire duration: The model predicting CRP over the entire study period revealed a significant main effect of baseline CRP (β = 0.80, p < 0.001), indicating an association between higher baseline CRP and increased subsequent CRP levels. There was also a main effect of study duration (β = 0.02, p = 0.005), suggesting increased CRP levels over the study duration. The interaction between the baseline CRP and duration was also negatively associated with longitudinal CRP (β = −0.01, p < 0.001), suggesting a lower rate of change in CRP over time in young people with higher baseline CRP. However, there was no significant association between longitudinal CRP levels and different illness subtypes, sex, or BMI (p > 0.05).
Initial 24 months: The association between CRP during the initial 24 months and the same predictors were further assessed. During the first 24 months, only baseline CRP was positively associated with longitudinal CRP (β = 0.87, p < 0.001) with no other significant predictors identified. Both outcomes of linear mixed models are presented in Table 3.
Table 3. Longitudinal changes in inflammatory marker (C-reactive protein [CRP])
Two separate models were run to predict CRP: 1) over the entire study duration and 2) for the initial 24-month period. Fixed effects included baseline CRP, duration, BMI, sex and illness subtypes, with interaction terms for CRP and duration. #At baseline; †‘hyperarousal-anxious depression’ was the reference group.
Research Question 2
Based on our hypothesis for Question 1, which suggested that illness subtypes would differentially predict the trajectories of metabolic markers, we further explored these associations. Question 2 aims to delve deeper into the specific relationships between metabolic and inflammatory markers and distinct illness subtypes. In particular, we hypothesized that the ‘circadian-bipolar spectrum’ subtype would be significantly linked to metabolic and inflammatory dysfunctions. To test this hypothesis, we employed a multinomial logistic regression model was used to explore the relationships between current depressive illness subtypes (the outcome variable) and longitudinal metabolic, inflammatory markers and other predictors. HOMA2-IR emerged as the only significant predictor (OR: 2.32, 95% CI: 1.24–4.33, p = 0.008, FDR-p = 0.033) for the ‘circadian-bipolar spectrum’ subtype. This finding suggests that a one-unit increase in HOMA2-IR level was associated with a 2.3-fold increase in the risk of belonging to the circadian-bipolar subtype, compared to the ‘hyperarousal-anxious’ subtype (Table 4).
Table 4. Multinomial logistic regression comparing predictors of illness subtypes to the reference group ‘Hyperarousal-anxious’ subtype
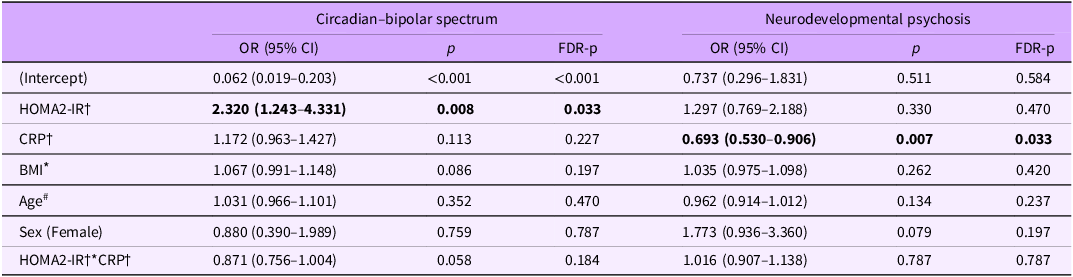
† Longitudinal levels.
* BMI at the last assessment.
# Age at each blood assessment.
By contrast, for the ‘neurodevelopmental psychosis’ subtype, longitudinal CRP showed a negative association (OR: 0.69, CI:0.53–0.91, p = 0.007, FDR-p = 0.033), suggesting that a one-unit increase in CRP level was associated with a 31% reduced likelihood of belonging to the ‘neurodevelopmental psychosis’ subtype, compared to the ‘hyperarousal-anxious’ subtype. Other examined factors did not predict illness subtypes.
Research Question 3
We examined the changes in fasting insulin and FBG levels over time, in addition to HOMA2-IR. Visual inspection revealed distinct trajectories for the two markers, with noticeable changes in insulin levels compared with a relatively flat trajectory for FBG levels (Figure 3). Furthermore, while 27% (n = 30 of 110) of participants had a HOMA2-IR score greater than 1.5, and 41% (n = 46 of 111) had insulin levels higher than 10, only 4.6% (n = 6 of 131) had an abnormally high FBG (5.6 mmol/L).
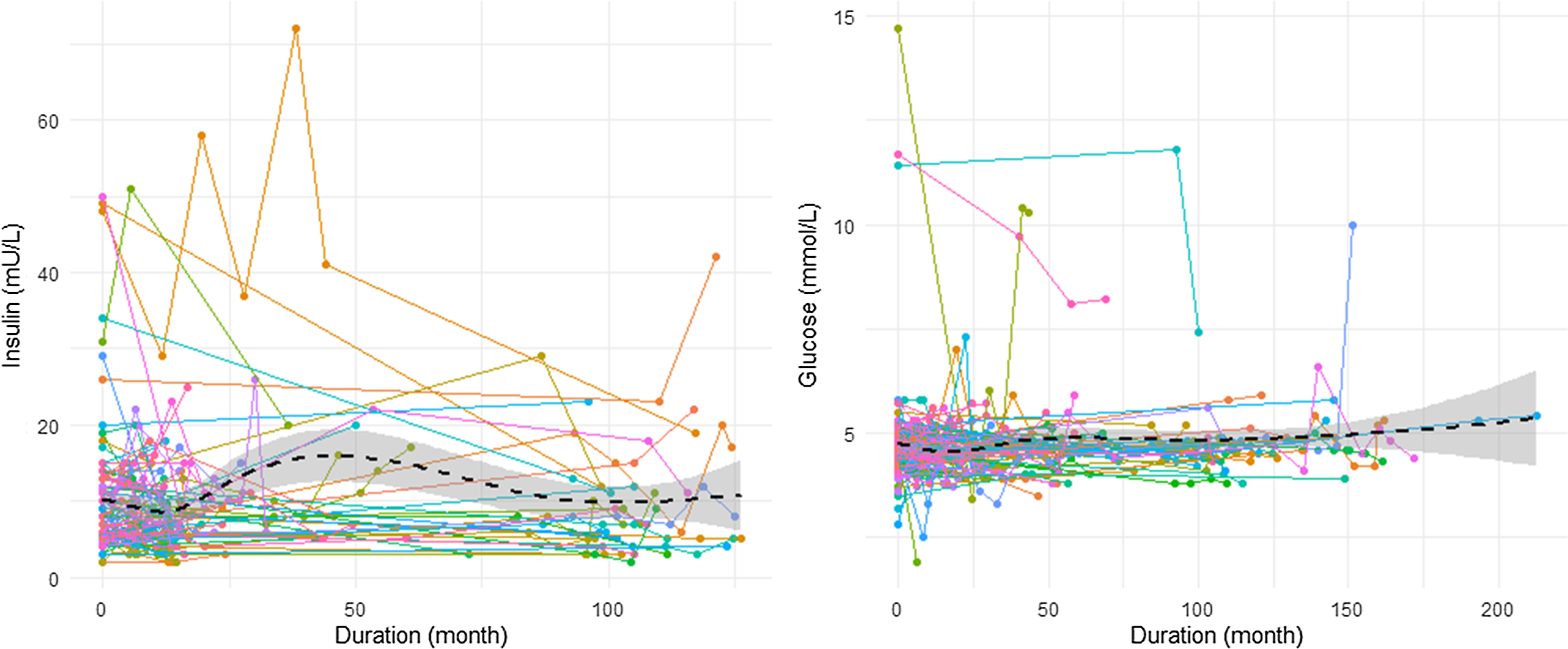
Figure 3. Changes in fasting insulin and fasting blood glucose levels over time (n = 111 for insulin, n = 131 for glucose).
Conclusion
This preliminary analysis of an ongoing study of the longitudinal trajectories of metabolic and inflammatory markers in young people with emerging mood disorders, examined predictors of longer-term changes in HOMA2-IR and CRP and their relationships with specific illness subtypes.
Conclusion for research Question 1
We confirmed our hypothesis for Research Question 1, observing a significant increase in insulin resistance (indicated by HOMA2-IR) and low-grade inflammation (by CRP) over time in our cohort of young people with mood or psychotic disorders. This indicates an increasing risk for some young people, despite access to clinical services. Additionally, metabolic trajectories were associated with BMI and illness subtypes. At baseline, one-third of the participants exhibited emerging insulin resistance (HOMA2-IR>=1.5 – 27%) or low-grade inflammation (CRP>=3mg/L – 27%) at their initial blood test. This is consistent with our previous study, which reported that 22% of young people presenting to primary mental health services showed an elevated risk of insulin resistance (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019). Similar prevalence rates of low-grade inflammation have been reported in individuals with lifetime depression (20–25%) (Osimo et al. Reference Osimo, Perry, Cardinal, Lynall, Lewis, Kudchadkar, Murray, Perez, Jones and Khandaker2021; Pitharouli et al. Reference Pitharouli, Hagenaars, Glanville, Coleman, Hotopf, Lewis and Pariante2021) and acutely ill depressed patients (25–30%) (S. R. Chamberlain et al. Reference Chamberlain, Cavanagh, de Boer, Mondelli, Jones, Drevets, Cowen, Harrison, Pointon, Pariante and Bullmore2019). People with higher baseline HOMA2-IR or CRP levels were likely to experience continuing increases over time, emphasizing the importance of early detection and intervention. Interestingly, the rate of increase in HOMA2-IR or CRP over time appears to diminish in people with higher baseline level in these markers, possibly reflecting an impact of medications or interventions received during the course of illness. However, these factors were not examined in this analysis. Another possibility is that baseline levels are already near an upper threshold, making further marked increases physiologically less likely within a certain period. Consistent with our previous work, BMI – a major modifiable risk factor – also played a role in increasing the risk of metabolic dysregulation (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019). Although our study of an inpatient cohort (Tickell et al. Reference Tickell, Rohleder, Ho, McHugh, Jones, Song, Hickie and Scott2022) found that higher BMI and being female were associated with increases in CRP – suggesting a potential sex difference in inflammatory markers among young people with mood disorders – the current study did not find BMI or sex effects on longitudinal CRP levels. This discrepancy implies that other factors, such as circadian rhythm disruptions, genetic predispositions, or chronic fatigue, might play a more substantial role in influencing CRP levels. For instance, elevated CRP levels persist in major depressive disorder even when corrected for BMI, particularly in treatment-resistant cases (Samuel R. Chamberlain et al. Reference Chamberlain, Cavanagh, de Boer, Mondelli, Jones, Drevets, Cowen, Harrison, Pointon, Pariante and Bullmore2019), indicating that inflammation in mood disorders may be driven by factors beyond BMI. Future research should explore these alternative factors – such as circadian disruptions, genetic predispositions, or chronic fatigue – and their roles in inflammatory and metabolic dysfunctions. Additionally, examining the effects of interventions and medications on these trajectories could provide further insights into managing and mitigating these risks in young people with mood disorders.
Conclusion for research Question 2
We hypothesized that the “circadian–bipolar spectrum” is associated with a higher risk of metabolic and inflammatory dysfunctions, while the “neurodevelopmental psychosis” is associated with a higher risk of inflammatory dysfunction. Our findings partly support this hypothesis. Specifically, higher HOMA2-IR levels had a 2.3-fold increased likelihood of belonging to the “circadian-bipolar spectrum” subtype (characterized by atypical features, manic-like symptoms, or circadian disturbance) compared to the hyperarousal-anxious subtype. This is consistent with recent studies that have identified metabolic or inflammatory alteration in atypical subtypes (Fernandes et al. Reference Fernandes, Salagre, Enduru, Grande, Vieta and Zhao2022; Lamers et al. Reference Lamers, Milaneschi, Vinkers, Schoevers, Giltay and Penninx2020; Milaneschi et al. Reference Milaneschi, Lamers, Berk and Penninx2020). Given that these subtypes may exhibit circadian disruption – and considering the well-documented relationship between disrupted circadian systems and metabolic disruption (Stenvers et al. Reference Stenvers, Scheer, Schrauwen, la Fleur and Kalsbeek2019; Zimmet et al. Reference Zimmet, Alberti, Stern, Bilu, El-Osta, Einat and Kronfeld-Schor2019) – our findings suggest that circadian dysfunction may be a key mediator of metabolic dysregulation in cohorts such as this.
Contrary to other studies reported higher peripheral inflammation or increased CRP levels in people with psychotic disorders (Jacomb et al. Reference Jacomb, Stanton, Vasudevan, Powell, O’Donnell, Lenroot, Bruggemann, Balzan, Galletly, Liu, Weickert and Weickert2018; North et al. Reference North, Bruggemann, Cropley, Swaminathan, Sundram, Lenroot, Pereira, Zalesky, Bousman, Pantelis, Weickert and Shannon Weickert2021; Zhang et al. Reference Zhang, Lizano, Guo, Xu, Rubin, Hill, Alliey-Rodriguez, Lee, Wu, Keedy, Tamminga, Pearlson, Clementz, Keshavan, Gershon, Sweeney and Bishop2022), we did not observe a significant increase in CRP levels in the “neurodevelopmental psychosis” subtype. Instead, we found a negative association between higher levels of CRP and membership in the “neurodevelopmental psychosis” subtype. From our results, as indicated in Figure 2 and Table S1, CRP levels in the elevated range were observed less often (only 10% (n = 3)) and the CRP levels decreased over time in this subtype, contributing to the observed negative association. This decrease over time might be related to specific treatments received by this subtype, suggesting that high longitudinal CRP could potentially reflect less effective anti-inflammatory treatment effects. In future studies, it will be important to further investigate the impact of these treatments on CRP levels and their effectiveness in managing inflammation in this subtype.
Conclusion for research Question 3
Regarding our third question, we confirmed that HOMA2-IR is a more appropriate measure of early metabolic dysfunction than FBG. Our study found that standard FBG measures are relatively insensitive for capturing early metabolic dysregulation associated with depression. We have previously reported elevated insulin resistance without concurrent alterations in FBG levels in young people with emerging mental illness (Scott et al. Reference Scott, Carpenter, Iorfino, Cross, Hermens, Gehue, Wilson, White, Naismith and Guastella2019; Scott et al. Reference Scott, Hermens, White, Naismith, GeHue, Whitwell, Glozier and Hickie2015), emphasizing the crucial need to monitor both insulin and glucose for assessing insulin resistance. Additionally, another group found that the effects of a circadian-based therapy (light therapy) differed according to the level of insulin resistance in people with depression and type 2 diabetes, suggesting the potential of insulin resistance to guide personalized treatment (Brouwer et al. Reference Brouwer, van Raalte, Lamers, Rutters, Elders, Van Someren, Snoek, Beekman and Bremmer2021). Future studies should investigate HOMA2-IR as a key variable to explore the pathophysiological mechanisms underlying metabolic dysfunction and evaluate the benefits of metabolic interventions (e.g., metformin) in young people with mood disorders.
Several strengths and limitations are worth noting. A major strength of this study is its longitudinal design with a minimum of 2 to a maximum of 16 repeated blood assessments, which allows for the observation of changes over time in metabolic and inflammatory markers within young individuals with mood disorders. Despite this, there are some limitations. The small sample size, particularly within specific illness subtypes. Hence, further investigations involving larger cohorts are warranted to validate these findings and establish more robust conclusions. Additionally, our longitudinal data assessment times varied, as we capitalized on data from young people who participated in two cohort studies separated by a minimum of 5 years. Longitudinal studies with more frequent assessments are needed to better understand the temporal relationships between metabolic-inflammatory markers and the evolution of specific illness subtypes. Furthermore, due to the lack of BMI data at each assessment, we could only include BMI at the latest assessment in the models.
In conclusion, our study advocates for incorporating sensitive metabolic and inflammatory markers, such as HOMA2-IR, into routine clinical screenings for young people with emerging mood disorders. Standard glucose measures alone are not sensitive to metabolic disturbances, emphasizing the use of more sensitive and frequent metabolic markers, such as fasting insulin resistance. By integrating these markers into clinical practice, clinicians can identify metabolic dysfunctions early, guide personalized treatment plans, and implement targeted interventions. Regular monitoring of these markers will facilitate ongoing adjustment of therapies, improving management and outcomes for individuals with mood disorders. Training clinicians to effectively use these markers will further enhance the integration of this approach into standard care practices, ultimately supporting better long-term health and well-being for this population.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/dep.2024.8.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Acknowledgments
The authors acknowledge the Gadigal people of the Eora nation, upon whose ancestral lands our research was conducted. We pay our respect to elders past and present. We acknowledge all First Nations Australians, in their ongoing struggles for sovereignty and justice. We express our gratitude to all participants for giving their time to contribute to this study. Gratitude is extended to the donors whose support has facilitated the execution of this study.
Author contributions
Conceptualization: MS, SM, JSC, IBH; Data collection & Project administration: EP, NZ, AN, YJCS, CJ, MP, KC, DF, TW; Data analysis & visualization: MS; Supervision: JSC, IBH, EMS; Writing – Original draft: MS; Writing – Review, Editing & Supporting: all authors. Sarah McKenna and Joanne S. Carpenter contributed equally to the article.
Financial support
The Neurobiology Youth Follow-up Study is supported by philanthropic funding, for which donor(s) who are families affected and wish to remain anonymous. MS receives a research fellowship supported by a philanthropic donation from a family affected by mental illness who wishes to remain anonymous. SM is supported by Cottle Family Fellowship. JJC is supported by National Health and Medical Research Council (NHMRC) EL1 Investigator Grant (GNT2008196). ET is supported by a Breakthrough Mental Health Foundation Fellowship. JSC is supported by the Stephen Francis Bequest. IBH is supported by a NHMRC L3 Investigator Grant (GNT2016346).
Competing interests
The authors declare that there are no competing financial interests in relation to the work described. Potential conflicts of interest may arise from the following: The Brain and Mind Centre (BMC) operates early intervention youth services at Camperdown under contract to headspace. Professor Hickie has previously led community-based and pharmaceutical industry-supported (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca, Janssen Cilag) projects focused on the identification and better management of anxiety and depression. He is the Chief Scientific Advisor to, and a 3.2% equity shareholder in, InnoWell Pty Ltd which aims to transform mental health services through the use of innovative technologies. EMS is Principal Research Fellow at the BMC, The University of Sydney. She has received honoraria for educational seminars related to the clinical management of depressive disorders supported by Servier, Janssen and Eli Lilly Pharmaceuticals. She has participated in a national advisory board for the antidepressant compound Pristiq, manufactured by Pfizer. She was the National Coordinator of an antidepressant trial sponsored by Servier.
Ethical standards
The data presented in this study was part of the projects approved by the Human Research Ethics Committee of the Sydney Local Health District (2020/ETH01272) and the University of Sydney (2012/1631).












Comments
No accompanying comment.