INTRODUCTION
Lead white, a basic lead carbonate, has been the most extensively used white pigment by artists since Antiquity up to the 20th century, when it was gradually replaced with less toxic white pigments. Beside the basic lead carbonate “hydrocerussite” Pb3(CO3)2(OH)2, also “Cerussite” Pb[CO3] as well as “plumbonacrite” Pb5(CO3)3O(OH)2 can be found in paintings. This man-made white pigment may be used in the ground or paint layer, pure or admixed with other pigments and additives. In addition, it possesses the advantage of accelerating the oil drying process. Up until the 19th century lead white was the most important white pigment in the art market. In the 18th century, zinc oxide was discovered as an alternative white pigment, however this one only became a real competitor to lead white in the 19th century (Roy Reference Roy1993). Lead white can therefore still be found in early 20th century paintings, when its use declined as its manufacture and sale was first restricted, and then forbidden due to its toxicity. The introduction of titanium white during the 20th century meant that lead white was no longer as central to the artist’s palette (Eastaugh Reference Eastaugh2008). Because of its widespread use throughout history, lead white pigments has been of limited use for the study and dating of works of art. In the course of authentication research, lead white had its moment of glory as its isotopic signature supported the attribution of a Vermeer painting (Ragai Reference Ragai2015). Indeed isotopic analyses of lead white can be used as a fingerprint to trace the origin of the pigment and thereby provide compelling evidence regarding the whereabouts of a painting’s creation (Fortunato et al. Reference Fortunato, Ritter and Fabian2005). In the field of authentication, lead isotopes abundance ratios can thus be used to identify the origin, but no information can be gained regarding the age of the pigment and therefore the object.
Recent developments in accelerator mass spectrometry (AMS), in particular, with respect to sample size downscaling, have allowed the application scale of radiocarbon dating to be widened, in particular to cultural heritage studies (Fedi et al. Reference Fedi, Caforio, Mando, Petrucci and Taccetti2013; Caforio et al. Reference Caforio, Fedi, Mando, Minarelli, Peccenini, Pellicori, Petrucci, Schwartzbaum and Taccetti2014; Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Kuffner, Scherrer and Ferreira2016; Petrucci et al. Reference Petrucci, Caforio, Fedi, Mando, Peccenini, Pellicori, Rylands, Schwartzbaum and Taccetti2016). In most cases, only the support material is sampled, yet the respective radiocarbon results may be misleading, as the support may be older than the actual painting itself, e.g. in cases where old supports have been reused by artists themselves or by forgers. In the search for other candidates suitable for 14C analysis, the natural organic oil binder was found to be ideal (Hendriks et al. Reference Hendriks, Hajdas, Ferreira, Scherrer, Zumbühl, Küffner, Wacker, Synal and Günther2018). Indeed, the 14C clock of natural drying oils initiates with the harvesting of the seeds from which the oil is extracted, hence dating of the oil is very likely to be representative of the time of creation of the paint. However, an offset in the age may also be observed, since artists prized aged oil, which could be kept for long periods before being used to make paint (Carlyle Reference Carlyle2001b).
With the help of radiocarbon analysis, similarly to in-situ formed calcium carbonate in the dating of lime mortars (Ringbom et al. Reference Ringbom, Lindroos, Heinemeier and Sonck-Koota2014 and references therein), lead white can be used as proxy for the time of creation of an object. Indeed, the 14C signature of the carbonate anion is related to the pigment’s production process and its respective date of production. The idea of using lead white as a time proxy was demonstrated by Van Strydonck and co-workers in a recent work regarding the dating of a corpse found in a lead coffin (Van Strydonck et al. Reference Van Strydonck, Boudin, Van den Brande, Saverwyns, Van Acker, Lehouck and Vanclooster2016). In their study, the lead carbonate crust that formed on the bottom inside the lead coffin was dated and allowed to establish the time of death of the corpse in the coffin. Their approach was based on the assumption that the lead carbonate was formed by the reaction of CO2 emitted from the putrefying corpse. Hence the 14C/12C ratio of the carbonate reflected the 14C/12C of the body inside the coffin and henceforth the respective time of death.
This particular study was driven by the omnipresence of lead white in oil paintings by Franz Rederer (1899–1965) and the need to eliminate it. By treating the sample with acid and converting the lead carbonate to a carbon free inorganic fraction by release of CO2 the 14C dating of the organic binder was made possible (Hendriks et al. Reference Hendriks, Hajdas, Ferreira, Scherrer, Zumbühl, Küffner, Wacker, Synal and Günther2018). The work presented herein introduces the possibility of using 14C analysis for the dating of the lead white pigment itself. Due to the many different recipes, the sources of carbon dioxide have evolved and the respective 14C signature will reflect either atmospheric 14C level or fossil derived sources. One of the most critical issues in the 14C dating of artworks is the sampling step. The invasiveness of the method limits its application and hence we demonstrate the additional possibility to date the oil remaining in the sample after the carbonate decomposition. By demonstrating the feasibility of radiocarbon dating the lead white pigment and, additionally, the organic binder from the same sample in a two-step preparation process, the potential of the 14C analysis comes into balance with the sacrifice of taking a sample. We hereby propose new candidates for 14C dating of a painting, as alternatives or complementary to the support material.
LEAD WHITE PRODUCTION
Lead white may be produced following many different processes. The traditional stack (or Dutch) process involves the exposure of strips of metallic lead to acetic acid and fermenting organic material, the latter of which provides both heat and a higher concentration of carbon dioxide. Over several weeks the combined action of air, acetic acid, and carbon dioxide successively converts the metallic lead to lead hydroxide, lead acetate basic lead acetate, and finally to lead carbonate hydroxide (see Equations 1–4)(Sharma Reference Sharma1991), commonly known in artist jargon as basic lead white. Many patents and variations of the process have been published, the most popular being the stack or Dutch process, where metallic lead is placed in ceramic pots and the floor of the chamber is covered with manure. This process knew very little alteration in the 18th century and was the most common way of producing lead white pigment until the end of the 19th century. However, more efficient means were explored. In 1749 James Creed patented the so-called chamber method, where the innovation was to supply heated gas to the chamber (Eastaugh Reference Eastaugh2008). The patent describes how the use of organic fermenting material could be negated by using the heat of fire. Whether Creed fully understood the chemical process taking place is unclear, as the existence of carbon dioxide was only discovered in the late 18th century and was referred to as “fixed air”. Some years later in Germany, a new turn in the production of lead white occurred. Near Klagenfurt, the family factory of Herbert was renowned for producing lead white of excellent quality (Sedlacek Reference Sedlacek1938). Herbert implemented the Dutch process to his advantage, using fruit must vinegar from his own orchard as raw starting material, supplying the reaction with the necessary gases and vapors. His success gave way to the construction of a second factory in 1792, which was the first to implement the so-called German process. This practice was first described by Gustav Dietel in 1839 in Eisenach, where a charcoal-heated furnace was coupled to the chambers to provide more heat and a constant carbon dioxide stream. This process was continuously adapted and by the beginning of the 19th century could be found in most of Europe under different names, such as the French process in France or the Rowe process in the United Kingdom. In the 19th century the number of new processes flourished thanks to the development of industrialization and modern chemistry. The metallic lead could be exposed in a dry environment or immersed in water and the final product was achieved by precipitation or electrolysis. A short review for the different preparation processes described above can be found in Pérez-Villares and Bailón-Moreno (Reference Pérez-Villares and Bailón-Moreno2017), while an extensive review was written by Stols-Witlox (Stols-Witlox et al. Reference Stols-Witlox, Megens and Carlyle2012).
In many cases, natural and industrially synthesized pigments can be differentiated based on the presence/absence of byproducts/contaminants. For example, the pigment ultramarine blue is nowadays artificially synthesized but it used to be based on the mineral lazurite extracted from Lapis Lazuli. There are several ways of distinguishing lazurite from synthetic ultramarine, for example based on their elemental composition and the identification of specific mineral phases (Favaro et al. Reference Favaro, Guastoni, Marini, Bianchin and Gambirasi2012). In the case of lead white, although particle morphology analysis by scanning electron microscopy does point to a clear difference between the traditional method versus the modern, no dating information can be gained. Radiocarbon, however, has the power not only to discriminate between production processes but can also give a time range. In order to date lead white pigment, the specific source of carbon dioxide used in the formation of the carbonate anion (see Equation 3) is of interest.
When CO2 is produced from fermenting organic material as described in the stack process, the carbonate will carry the 14C signature of the atmosphere and can be used for dating the time of production. With the advent of the alternative methods, such as the chamber process, the carbon dioxide source varied considerably from coal fires to the addition of calcium carbonate or potassium carbonate to vinegar (Eastaugh Reference Eastaugh2008). The 14C signature of carbon dioxide was thus radically changed as in such material the 14C content is very low and resulting 14C ages are more than thousands of years old. As a result, lead white issued from industrial production cannot per se be dated but can be used as a marker for identifying lead white made with alternative methods to the traditional stack process.
MATERIALS
Reference Material
Lead White Pigment and Oil Paint Samples
Two different sources of lead white pigment, both presumably prepared using modern methods, were provided from the MOLART Fellowship 1999 reference collectionFootnote 1 (Carlyle Reference Carlyle2001a; Carlyle Reference Carlyle2005): one which had been prepared commercially in the 20th century by the Schoonhoven CompanyFootnote 2 , and the second, which had been purchased from Kremer Pigmente. Lead white pigments made following the Dutch/Stack Process had been prepared in separate batches between 2002 and 2005 for the HART project1. Samples with creation dates were provided from the HART project reference collection (Table 2) and consisted of both washed and unwashed lead white pigment (washing was carried out with Millipore water, see Carlyle (Reference Carlyle2005)). Oil paint samples were also provided from the MOLART and HART reference collections. In both cases the oil had been extracted from linseeds purchased for the projects (Electra linseed seeds from Flevo Vlas Loonwerk B.V obtained in 1999 and 2005).
Paint Reconstructions
In order to assess if the method developed for measuring 14C ages of the lead carbonate within the paint matrix successfully eliminated the interference of the binder, a series of lead white oil paint reconstructions of different origin and date were tested (Table 2).
In a second step, the possibility of dating the oil binder residue after the carbonate decomposition was pursued. Various organic solvents (see Table 1) were tested to optimize the extraction of the organic phase. Preliminary studies were conducted on paint of known composition, namely a mixture of umber pigment and chalk (CaCO3). Additionally, from the SIK-ISEA collection, lead white paint prepared in 1981 was tested for sequential carbonate and oil dating. Paint reconstructions from both the MOLART and HART projects were also used (Table 2).
Table 1 Solvents tested for the organic phase extraction.

Table 2 Overview of the reference sample material used in this study. The extraction date of the oil from the seeds is irrelevant for 14C analysis, as the process will not impact the 14C signature, therefore only the purchase date of the seed is listed.

a) Kremer Pigmente GmbH & Co, Aichstetten, Germany.
b) Supplier and purchase date unknown.
Case Studies
Among the objects made available for the study was an oil on canvas painting by Franz Rederer (1899–1965) entitled Bildnis Margrit mit roter Jacke und Konzertkleid, 1962, 140×100 cm, SIK-ISEA, Zurich (Figure 1). The results of the radiocarbon dating of the canvas and oil binder from this panting has been previously reported (Hendriks et al. Reference Hendriks, Hajdas, Ferreira, Scherrer, Zumbühl, Küffner, Wacker, Synal and Günther2018). For this study, a sample of green paint from the reverse of the canvas was collected. Being unvarnished and never having been restored, the material from this painting presents ideal case study material as no new sources of carbon have been added. The green paint was known to bear some lead white pigment and provided therefore fitting material for the subsequent dating of the oil after the carbonate decomposition.

Figure 1 (Left) Bildnis Margrit mit roter Jacke und Konzertkleid, by Franz Rederer, 1962, oil on canvas, 140× 100 cm, SIK-ISEA (archive Nr. 98 511). Middle: reverse side of the painting showing the sampled area in red. Photograph: SIK-ISEA (Philipp Hitz). (Right) green paint sampling on the reverse of the painting, where the scale used is 5 mm. (Please see electronic version for colors.)
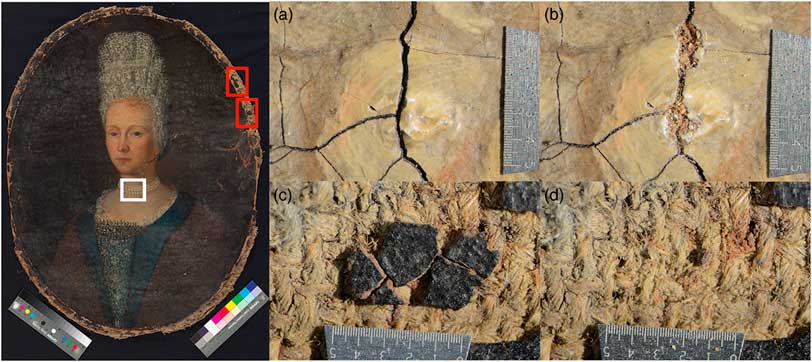
An additional oil on canvas painting unattributed and with no signed date, shown in Figure 2, also belonging to the SIK-ISEA collection, was sampled for lead white containing paint and analyzed. Based on stylistic assessment the painting of the half-length female portrait is estimated to be around the 17th century. Thanks to fashion details a time of origin can be assessed, indeed the high headdress, also known as Fontange, was very popular in the period 1685–1715Footnote 3 . The canvas and brown paint were also sampled, to compare the lead white radiocarbon age with the ages of the support and organic binder. A more detailed overview of the sampling location is given in Figure 1 and Figure 2.

Figure 2 (Left) Untitled half-length portrait of a young woman wearing a lace bonnet, pearl necklace and fur-trimmed cape, unsigned, ca. 17th century, 57×78 cm, SIK-ISEA (Archive Nr. 171108 0002). Photograph: SIK-ISEA (Philipp Hitz). The lead white sampling location is marked by the white box, while the sampled brown paint and canvas are marked in red. (Right) The four details indicate the sampling location of lead white containing paint prior sampling (a) and after (b) and similarly for the brown paint prior sampling (c) and after (d). The scale used represents 5 mm. (Please see electronic version for colors.)
METHOD
Analytical Techniques for Paint Characterization of the Two Case Studies
Suitable paint locations bearing lead white were identified and characterized by combining X-ray fluorescence (XRF), Fourier-transform infrared (FTIR), and Raman spectroscopy. The XRF study was conducted with a Bruker AXS ARTAX 800 system equipped with a Rh target (Bruker, Karlsruhe, Germany). The XRF spectra were acquired using the following parameters: excitation spot <100 µm, generator voltage 50 kV, current 600 µA, helium atmosphere, and acquisition time 100 s. Paint locations, which showed intense Pb signals, were sampled using a 250 µm steel micro chisel (Electron Microscopy Sciences, USA) for subsequent FTIR and 14C analysis. FTIR spectra of the samples were collected in transmission mode on a Perkin Elmer System 2000 (Perkin Elmer, Massachusetts, USA) using a diamond cell. Acquisition parameters covered a spectral range from 4000–580 cm–1 with a resolution of 4 cm–1 and 16 scans. Complementary Raman analyses were acquired on a Renishaw in Via system with a diode type 785 laser source. The focus on the sample was achieved with a Leica DM microscope, using objectives of 50× and 100× magnification. The spectra were recorded using a laser power of 0.01–1 mW on sample and a measurement time between 30 and 200 s. The collected data was interpreted using known reference databases (Burgio and Clark Reference Burgio and Clark2001; Scherrer et al. Reference Scherrer, Zumbuehl, Delavy, Fritsch and Kuehnen2009).
Carbonate Preparation for AMS 14C Analysis
In the context of artwork dating, sampling size is a crucial issue. In this study when working with reference material, sufficient material was available for graphitization of the carbonate (equivalent of 0.5–1 mg C), while paint samples collected from oil paintings (see Case Studies) were too small and were therefore measured directly on the AMS as carbon dioxide released from the carbonate decomposition.
In order to yield 1 mg of carbon required for graphitization, ca. 20 mg of pure lead white pigment was necessary. Indeed, due to the heavy lead cation, lead white contains only 5% in weight of carbon when pure or even less when mixed with binder and/or other pigments as is often the case in real paint samples. Consequently, if the pigment to binder ratio is 1:1, twice as much paint is required to provide 1 mg C. Therefore, a mass of ca. 40 mg for paint reconstructions was used. The desired amount of material was weighed into 12 mL Exetainer® vials which were closed with septum screw caps (Labco, Lampeter, UK). The vials were flushed with He (70 mL/min) for 10 min in the carbonate handling system (CHS) (Wacker et al. Reference Wacker, Fulop, Hajdas, Molnar and Rethemeyer2013a). Similarly to the processing of calcium carbonate (see Equation 5 and 6), lead carbonates samples were further dissolved by the addition of 0.5 mL analytical grade phosphoric acid 85% (Merck, Darmstadt, Germany) using a gastight syringe to avoid the contamination of the vial headspace with atmospheric CO2 (Hamilton Company, Nevada, USA) and left to react overnight at 75°C.
The coupling of the system to a commercially available auto-sampler (PAL-GC, CTC, Zwingen, Switzerland) allowed the direct transfer of the liberated CO2 by the carbonate decomposition to the automated graphitization equipment (AGE) (Wacker et al. Reference Wacker, Nemec and Bourquin2010c). The amount of produced CO2 was manometrically quantified in a calibrated volume and further converted to C.
In the case of the samples from the paintings, significantly smaller amounts of material were available, usually less than 1 mg total, which includes the binding media, other pigments and lead white. In such small samples, the actual carbonate content is in the order of micrograms. The measurement was performed using the gas ion source, where the carbonate graphitization step was bypassed and the CO2 produced by the carbonate decomposition was directly fed into the MICADAS through the coupling of CHS to the gas ion source (GIS) interface (Fahrni et al. Reference Fahrni, Wacker, Synal and Szidat2013; Wacker et al. Reference Wacker, Lippold, Molnar and Schulz2013b). The samples were therefore weighed into 4 mL Exetainer® vials closed with septum, flushed with Helium and acidified with 0.5 mL H3P04.
Oil Extraction for AMS 14C Analysis
Lead white oil paint, which is a mixture of lead hydroxycarbonate and an organic binder, contains carbon from two different sources, which can be independently dated. The possibility of radiocarbon dating the oil remaining in the sample after the carbonate decomposition was investigated following two strategies (see also under Results and Discussion):
1. Organic phase extraction. The treatment of the carbonate bearing paint with phosphoric acid results in the formation of lead phosphate embedded within the binding media. This viscous solution, when given a few mL milli-Q® water (Merck Millipore, Darmstadt, Germany) forms a suspension. The oil molecules can be separated from the aqueous matrix based on a liquid-liquid extraction. For this purpose various solvents were tested as displayed in Table 1. The separation of the aqueous and organic phase was achieved by removing the aqueous phase with a syringe (1 mL, Codan medical Ag, Baar, Switzerland) and needle (0.8×80 mm, B. Braun, Melsungen, Germany) and were further deposited into tarred cylindrical tin cups (0.5 mL, Elementar Analysensysteme GmbH, Langenselbold, Germany). The organic solvent associated with the organic phase was then left to evaporate. The process was repeated several times, until 2–3 milligrams of oil were collected. The vessels and respective content weere then graphitized in the AGE system (Wacker et al. Reference Wacker, Nemec and Bourquin2010c).
2. Modification of the protocol for carbonate decomposition. The use of hydrochloric acid for the removal of lead carbonates prior to dating of the organic binder was successfully demonstrated in a previous study (Hendriks et al. 2017). Hence, beginning with another paint sample the dissolution of the carbonates was pursued using hydrochloric acid (Merck Millipore, Darmstadt, Germany) instead of phosphoric acid. After the carbonate graphitization, the remaining material was given an additional 5 mL HCl 1M and left to react for several hours at 80ºC in the shaker. The remaining material was then washed with milli-Q® water, dried and graphitized.
Radiocarbon Analysis
In addition to the carbonate and oil analysis, the support material of the painting used as case study was also dated. Samples were first cleaned by Soxhlet extraction (Bruhn et al. Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001) followed by standard ABA procedure (Hajdas Reference Hajdas2008). The cleaned canvas samples were then graphitized.
All 14C AMS measurements were carried out on the Mini Carbon Dating System MICADAS at the Physics Department of ETH (Synal et al. Reference Synal, Stocker and Suter2007; Synal Reference Synal2013), which allows for the analysis of both graphite and gaseous samples (Ruff et al. Reference Ruff, Szidat, Gaggeler, Suter, Synal and Wacker2010; Wacker et al. Reference Wacker, Bonani, Friedrich, Hajdas, Kromer, Nemec, Ruff, Suter, Synal and Vockenhuber2010a).
Data evaluation was conducted using the data reduction program BATS (Wacker et al. Reference Wacker, Christl and Synal2010b). Radiocarbon ages were converted to calendar ages using the OxCal v.4.3.2 software (Ramsey Reference Bronk Ramsey2008; Ramsey Reference Bronk Ramsey2009) with either the IntCal13 atmospheric calibration curve or the post-bomb atmospheric NH1 calibration curve for samples dated post-1950 (Hua et al. Reference Hua, Barbetti and Rakowski2013; Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013). Data extending into 2000 and after were calibrated with the CALIBomb software (Stuiver and Reimer Reference Stuiver and Reimer1993; Stuiver et al. Reference Stuiver, Reimer and Reimer2018) and the post-bomb calibration data set from Levin et al. (Reference Levin, Kromer and Hammer2013).
RESULTS AND DISCUSSION
The radiocarbon dating results for the lead white are divided into sub-sections. Firstly, the feasibility of lead white pigment dating by radiocarbon analysis is evaluated and discussed using pigment samples from the MOLART and HART project. Secondly, the results regarding the subsequent dating of the organic binder, after the carbonate 14C dating on paints made with documented pigments and oil of known date, are presented. Finally, results from the two case studies (SIK-ISEA collection), including the spectroscopic characterization of the paint samples, are given followed by a discussion of the radiocarbon ages of the differently dated materials of these artworks.
Feasibility of Lead White 14C Dating
The lead white pigment samples from both the MOLART and HART project offer an ideal opportunity to compare the traditional stack process and modern manufacture. The respective 14C signatures displayed in Table 3 demonstrate that stack produced lead white bears atmospheric 14C levels while the commercially manufactured modern lead white samples studied are devoid of 14C.
Table 3 Radiocarbon ages of the MOLART and HART lead white pigments and oil paint samples measured on ca. 1 mg C. The exact amount of C was derived from the manometrical quantification of the carbon dioxide in a calibrated volume prior to the graphitization. The preparation date refers to the production year of the lead white pigment. All dates were calibrated in OxCal 4.3.2 using the post-bomb atmospheric NH1 calibration curve. The first time interval hitting the curve as it rises between 1956 and 1957 can be discarded as the MOLART and HART project were initiated after 1999 and 2002, respectively, only the second time window is of relevance (highlighted in bold).
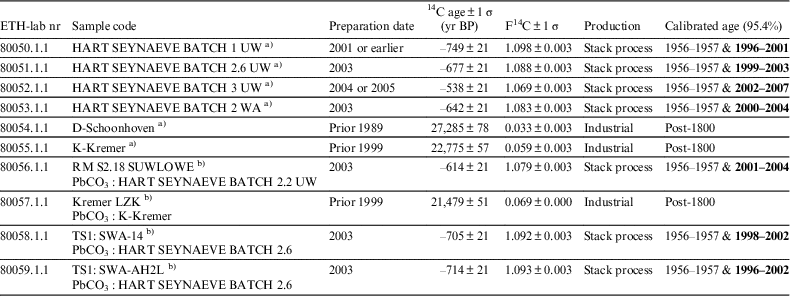
a) Pigment powder UW=unwashed, WA=washed.
b) oil paint reconstruction.
In the particular case of the HART lead white produced following the stack process by Jeff Seynaeve, radiocarbon analysis can target the year of production. Indeed, upon calibration of the four radiocarbon ages of the samples he prepared (rows 1–4 in Table 3) two calendar ages are obtained due to the particular feature of the calibration curve, also known as the Bomb-peak. The first point occurs between 1954 and 1960 as the bomb peak rises and the second point occurs as it decreases. Both time windows are displayed in Table 3. Since the MOLART and HART projects were initiated in 1999 and 2002 respectively, the first time window can be dismissed as only the second time interval post-1960s is of relevance (highlighted in bold in the table). For each sample of the stack process lead white pigment, the second calibrated time interval correlates with the production year of the respective batch. Lead white prepared following modern processes cannot be dated “per se” but can be used as a marker for identifying lead white produced with alternative industrial methods to the traditional stack process. It is unfeasible to date lead white pigments prepared with radiocarbon-dead material. The chamber process patented by James Creed in the mid-18th century was the first to describe the use of fires, however when the wood was replaced by coal is unknownFootnote 4 . Gustav Dietel was the first to write a clear protocol describing the use of charcoal in 1838. In this work, for simplification, any radiocarbon dated lead white affording ages of more than a thousand years is designated as having been produced using 14C devoid material, which arose with the beginning of industrialization, hence post-1800 was chosen as terminus post quem.
In oil paints, pigments and other additives are dispersed in a carbon rich organic binding medium. The influence of this additional carbon source was studied to determine if the presence of an oil binder would interfere with the measured carbonate 14C ages. From the last four samples ETH-80056, ETH-80057, ETH-80058, and ETH-80059 in Table 3, it can be seen that the binder does not affect the dating of the carbonate from the lead pigment. The reaction specifically targets the carbonate and its decomposition to CO2. These results thus demonstrate the potential of applying this technique to real case studies. Additionally, in contrast to the dating of the organic binder (Hendriks et al. 2017), the method here proposed is unaffected by the presence of varnish layers or restoration materials. Indeed, newly added varnish layers as well as consolidants, which are all carbon-bearing materials, will not react with the acid used to decompose the lead white and therefore will not interfere with the 14C dating of the carbonate. Moreover, the presence of organic pigments, carbon black or carbon containing impurities in the original paint (except CaCO3) will not disturb the result. This means that the method proposed has the advantage of not requiring any sample preparation in order to remove possible contaminations.
Comparison of Different Preparations for the Two-Step Radiocarbon Dating of Lead White Oil Paint
This work was undertaken to test the feasibility of successive radiocarbon dating of the lead white pigment and the organic binder from one paint sample. Two approaches to recover the oil fraction after the carbonate decomposition were tested and results were compared (Figure 4).
Organic Phase Extraction after Carbonate Decomposition
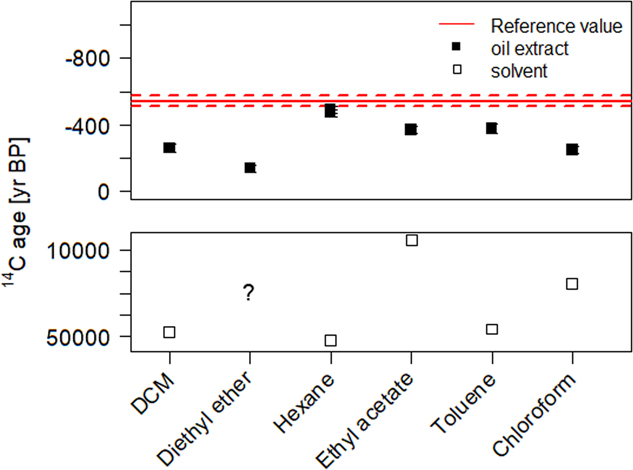
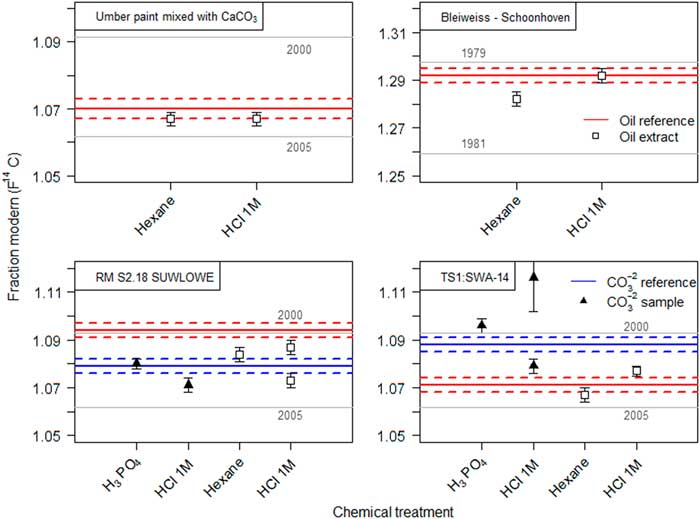
In the standard carbonate dating protocol (Wacker et al. Reference Wacker, Fulop, Hajdas, Molnar and Rethemeyer2013a), carbonates are decomposed by the reaction with phosphoric acid. Lead white reacts similarly to calcium carbonate and forms the corresponding lead phosphate salt. Upon addition of milli-Q® water to the remaining material after the carbonate decomposition a white suspension is formed. By adding an organic solvent, the hydrophobic oil fraction is extracted from the aqueous phase. Generally, for 14C analysis the use of organic solvents should be avoided, as they potentially introduce additional C contamination. Highly volatile solvents were therefore preferred. All solvents tested in this study bear ages from 5000 to 50,000 yr BP (see bottom plot of Figure 3). Hence in the case that the solvent did not fully evaporate, this would translate to an older age than expected. The measured 14C concentration of the different oil extracts are displayed in Figure 3. After the organic phase extraction and solvent evaporation, the radiocarbon dated extracts were expected to match the reference value of the binder from sample made with umber pigment (14C age=–544±20, F14C=1.070±0.003) (Hendriks et al. Reference Hendriks, Hajdas, McIntyre, Kuffner, Scherrer and Ferreira2016). Most extracts were, however, much older than anticipated, thus indicating residual solvent contamination. However, in the case of hexane, results demonstrate that this effect could be reduced to its minimum and the corresponding extract afforded matching results with the reference value.
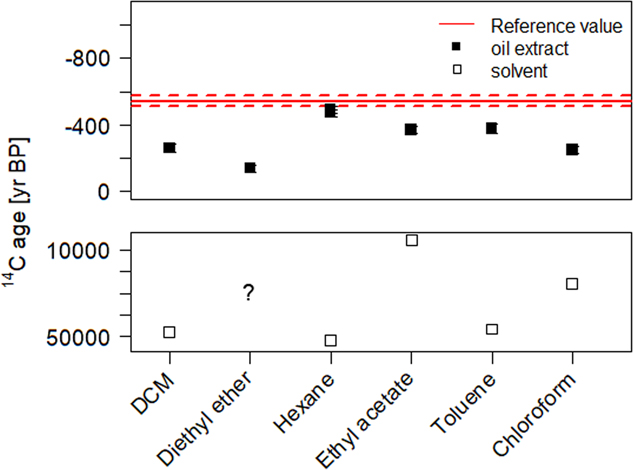
Figure 3 Radiocarbon ages of the extracted oil fraction from the SIK-ISEA umber and calcium carbonate sample after carbonate decomposition with H3PO4 and successive phase extraction using a variety of solvents. The reference value (in red) represents the measured 14C concentration in the umber paint (reference material) and enables an assessment of the efficiency of the oil extraction procedure (filled squares) and potential contamination from solvent residues. For comparison, all the solvents were also dated (empty squares) only diethyl ether could not be dated as it is highly volatile. (Please see electronic version for colors.)
Since hexane showed the most promising results, it was tested on several other paint reconstructions. The corresponding results are summarized in Table 4 and displayed in Figure 3. The Bleiweiss Schoonhoven paint was hereby effectively dated in two steps; the carbonate yielded more than 20,000 yr of age, indicating an non-traditional production while the oil was successfully dated to 1979–1981. Similar results were obtained for paint reconstructions from the MOLART and HART projects, where both the lead white and oil dating afforded corresponding results within the expected dates.
Table 4 Radiocarbon ages of the SIK-ISEA umber and calcium carbonate, SIK-ISEA lead white paint, MOLART and HART lead white samples following the two step dating of the carbonates followed by the oil. The amount of carbon collected at the end of the graphitization process was derived from the manometrical quantification of carbon dioxide in a calibrated volume. The preparation date refers to production year of the lead white pigment. All dates were calibrated in OxCal 4.3.2 using the post-bomb atmospheric NH1 calibration curve. The first time interval, which hits the curve as it rises between 1956 and 1957, can be dismissed and only the second is of relevance (highlighted in bold).

*Directly measured as CO2 using the GIS interface.
Chemical treatment: a) Carbonate decomposition with H3PO4 followed by hexane extraction of the oil fraction, b) HCl 1M.
This extraction procedure has however its downfalls; after the hexane extraction the remaining hydrophobic paint material aggregates and sticks to the vial walls. Thus, this laborious and time-consuming transfer of material to the tin cups results in low yields.
Alternative Carbonate Decomposition by Hydrochloric Acid
Phosphoric acid is the standard acid for the preparation of carbonates for 14C analysis. Its substitution with hydrochloric acid was pursued based on knowledge gained in a prior study (Hendriks et al. 2017), where lead carbonates were successfully removed prior to 14C dating of the organic binder by using hydrochloric acid. This second approach based on the acid substitution has several advantages, namely no additional organic solvent is used and it is less labor intensive as the sample only requires drying. Comparable carbon dioxide yields were achieved using either phosphoric acid or 1M/0.5M HCl, while only half of the amount of carbon was gained using 0.2M HCl. A blank assessment was performed using different HCl molarities (1M, 0.5M, and 0.2M) and no significant difference in the blank level was observed. Thus, the use of 1M HCl was determined to be the most efficient procedure.
The sequential dating of the carbonate followed by the organic binder was also tested using 1M HCl on several paint reconstructions (see Table 4 and Figure 4). The F14C of the oil binder in the paint pigmented with umber and calcium carbonate after 1M HCl treatment, matches the reference date of the oil. The oil binder of the lead white paint prepared in 1981 could be dated to 1979–1980. Similarly to the hexane extraction procedure, both carbonate and oil fraction from two MOLART and HART samples (codes RM S2.18 SUWLOWE and TS1: SWA-14), could be dated following the 1M HCl treatment. However, it should be noted that sample size turned out to be a crucial factor. Large aggregates of sample material tended to hinder the carbonate decomposition reaction, as the diffusion rate of the acid within the core was limited, resulting in an incomplete reaction and biased F14C value of the oil fraction. Nevertheless, in the case of smaller samples the sequential radiocarbon age of the oil fraction agrees with the reference value and hence validates the method. Thus it is important to monitor and ensure complete carbonate decomposition by FTIR analysis.

Figure 4 Results for the two-step dating of lead white oil paint measured on 1 mg of C. Solid lines depict the age of the reference material, dashed lines the respective uncertainty. Dating of the recovered oil fraction (square symbols) by hexane extraction or by using 1M HCl is compared in the top figures. The effect of substituting phosphoric acid with 1M HCl for carbonate decomposition and subsequent dating of the oil fraction within the same sample is compared in the lower figures. (Please see electronic version for colors.)
In comparison to the standard protocol involving H3P04, the use of 1M HCl results in similar carbon dioxide yields after the carbonate decomposition. For the 1M HCl process, much higher yields are observed for the recovered material, an advantage for subsequent oil dating.
CASE STUDIES
Spectroscopic Analysis of the Paintings Investigated
The dating of lead carbonate requires the characterization of paint mixtures in order to identify and select lead carbonate containing paint. The results of the paint characterization combining XRF, FTIR and Raman analysis are displayed in Table 5. The identification of lead as main element in the unsigned baroque painting is not surprising, as up until the 19th century lead white was widely used in oil paint. Additionally, among the elements identified by XRF, varying concentrations of calcium were also found. This finding has important implications for the radiocarbon dating of the lead carbonate, as the presence of calcium carbonate will introduce a source of contamination. In sample P03 pearl, traces of Ca were observed in the XRF but no calcium compound was discernible in the FTIR spectra. On the contrary in the paint sample P04 brown, Ca was identified as major component, which was confirmed by FTIR as being dolomite. For this reason and to avoid contamination sources, only the lead white from P03 pearl was radiocarbon dated and the brown paint was avoided. However, no other C-bearing pigments were found in the P04 brown paint sample and therefore the brown paint was deemed suitable for further 14C analysis of the organic binder.
Table 5 Combined results from the multi-technique approach to determine the paint composition of selected samples from the paintings under study: the half-length female portrait by an unknown artist and Bildnis Margrit mit roter Jacke und Konzertkleid by Franz Rederer. The XRF data is reported in bold for major elements, while traces are indicated in brackets.

In the case of Margrit’s painting, zinc was found as the main element followed by lead and titanium, hence indicating that the artist probably used a commercially blended white containing lead white because of its drying properties. Although lead white was not found to be the main paint component, its dating was nonetheless pursued.
Radiocarbon Results for the Case Studies
Radiocarbon data of the selected samples from the female portrait are summarized in Table 6, while 14C results for Rederer’s painting are presented in Table 8. The quantities of paint sampled for this study range from approximatively 5 mg to hundreds of micrograms of total material. This includes the binding media, other pigments and lead white. The resulting C content was far below the desired 1 mg for graphitization and consequently the 14C analyses were measured as CO2 directly, resulting in higher measurement uncertainty. From the data in Table 6 the mean values were determined for samples P03 pearl and P04 brown taken from the female portrait. All the 14C ages were calibrated to the corresponding calendar ages in Table 7.
Table 6 Selected samples from the female half-length portrait for 14C analysis including sample description, initial weight, carbon content, and respective radiocarbon age.

Table 7 Painting, sample number, description, measured 14C ages and respective calibrated age range using IntCal13 (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatte, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013).

Half-Length Female Portrait (ca. 17th Cent.)
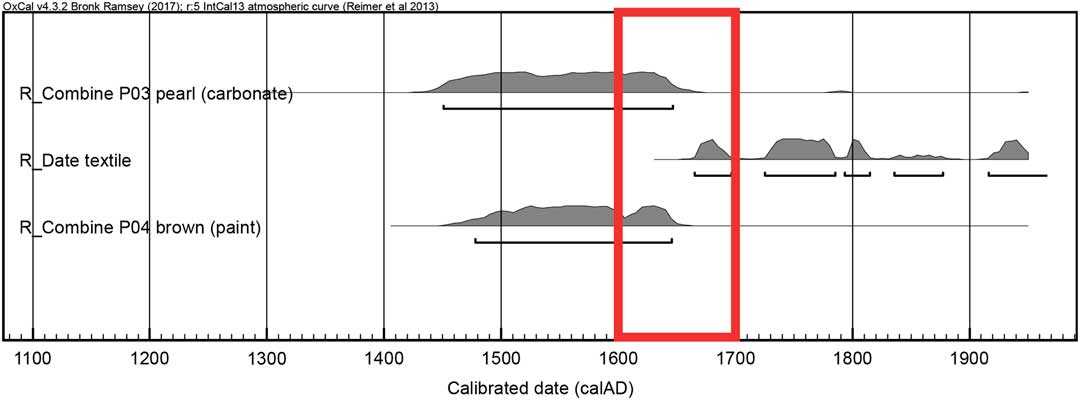
In the study of the half-length female portrait, we sampled not only lead white paint but also canvas threads and the brown paint for radiocarbon dating. Upon calibration the textile’s 14C age hits the curve at several points, yielding multiple time intervals ranging from the mid-17th to the mid-20th century. These results are in accordance with the art historical dating of the artwork, namely the female portrait was painted during the turn of the late 17th and early 18th century. However, it is also worth keeping in mind that dating of an object based on a fashion style gives only the earliest possible date, it could well be a later copy, which cannot be ruled out by the wide range of calibrated radiocarbon age. Thus, the 14C age of the canvas does not provide any decisive argument regarding the age of the painting. Therefore the dating of other materials than the support, such as the organic binder and the lead white pigment, become important. The mean value of the two carbonate measurements is 341±53 BP, which after calibration yields a time range between the mid-15th to the mid-17th century and a matching result is obtained on the organic binder from the brown paint. One can conclude that the additional dating of the lead white and oil helps to narrow down the time window given by the 14C age of the canvas, hereby excluding a possible later creation during the 18th, 19th or 20th century (Figure 5). These results also highlight offsets between the age of materials, which can be due to possible storage times, for instance the pigments and oil may have been stored for the time of one generation in comparison to the canvas. A direct correlation between the age of the pigment and the age of the oil must not always be the case, as either one could be much older than the other. It is known that painters kept materials around in their studios for decades, which were then passed on to other artists after their death.
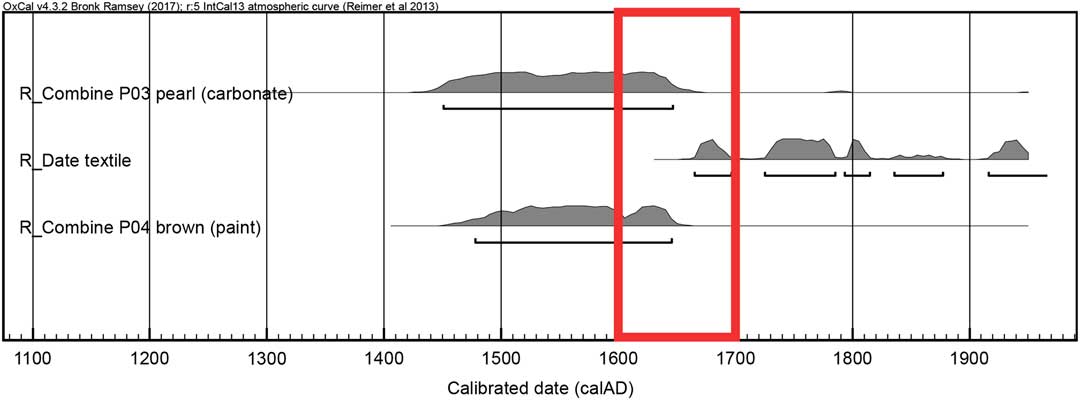
Figure 5 Calibrated ages for the set of samples collected on the female portrait. The red box highlights the time range where the calibrated ages of all three materials (carbonate, canvas, and binder) are in agreement. (Please see electronic version for colors.)
What has not been investigated and is worth mentioning is the potential impact of remineralization of the lead carboxylate in heavily saponified lead white oil paints. This form of alteration of lead pigmented oil paint layers has been observed in various paintings spanning the 15th to 20th century (Keune Reference Keune2005). However, much of the research up to now has been descriptive in nature of the phenomenon and the causes of the remineralisation are still presently unexplained (Boon et al. Reference Boon, van der Weerd, Keune, Noble and Wadum2002). Since the mechanism are still uncertain, it is not clear if the remineralization of lead carboxylates to lead carbonates will interfere with the 14C dating of the carbonate. In the female portrait studied, the distinct peak at 1528 cm–1 in the FTIR spectra can be attributed to the COO- group of metal carboxylates, commonly referred to as soaps. This raises the question of possible remineralisation back to lead carbonate or lead hydroxycarbonate, which may introduce some probably very minor but at the moment unknown error in the dating of the pigment. In the current case we surmise that the age of the carbonate produced following the traditional stack process should be in agreement with the one of the binder as demonstrated in Figure 5, and such remineralization process should bear little impact on the measured 14C age of the global lead carbonate fraction.
Bildnis Margrit mit Roter Jacke und Konzertkleid (1962)
In the case of Rederer’s painting, the negative radiocarbon ages indicate that the dated samples are post-1950, hence the respective 14C ages and uncertainty are additionally given as fraction modern F14C (see Table 8).
Table 8 Radiocarbon results given as 14C ages and fraction modern for the samples of Rederer’s painting.

a) Data already published in Hendriks et al. (Reference Hendriks, Hajdas, Ferreira, Scherrer, Zumbühl, Küffner, Wacker, Synal and Günther2018).
b) Extract after carbonate measurement with H3PO4 using Hexane.
The characteristic signature of fossil fuels was detected in the carbonate of P05 green, thus indicating that the lead white pigment was made following a modern industrial process. This result is compatible with the hypothesis that the artist, Franz Rederer, who lived in the 20th century, in his paint used lead white that came from a commercial industrial source. Indeed, the production of lead white following modern industrial methods began in the 19th century and its identification in Rederer’s painting dated to 1962 is fully appropriate.
It is interesting to highlight the sample size, indeed as already mentioned pure lead white pigment contains only 5% C. In paint samples this C content becomes even lower as the lead white is mixed with an organic binder and is applied to the painting. Upon mixing with other pigments, in this case with ultramarine and PG7, this C contribution from the lead white can go down to 1%. Nevertheless, both spectroscopic analysis and AMS measurement enabled its identification.
As already mentioned in a prior study, the Rederer’s painting is an ideal case study in the sense, that it has a legitimate provenance, has undergone no restoration and is not varnished. Thus, the additional extraction of the binder and subsequent dating after carbonate measurement could proceed without additional cleaning steps. Phosphoric acid was used to decompose the lead white and liberate carbon dioxide, then the oil was extracted from the remaining viscous material by an organic phase separation involving hexane. The results are straightforward; the oil dates to –597±22 yr BP, which upon calibration using the post-bomb atmospheric NH1 curve (Hua et al. Reference Hua, Barbetti and Rakowski2013) affords two time windows, namely 1957 and 2001–2005. The later can be excluded as it postdates the artist’s death in 1965. The radiocarbon dating of the remaining organic binder affords a growing season year for the oil seeds in 1957, which is in line with the signed date of 1962. Hence from one unique sample weighing 15 mg, followed by a two-step preparation, both the lead white and organic binder were successfully dated and results correlate with the time frame of the artist’s activity.
CONCLUSION
This work demonstrates the potential of using radiocarbon analysis for dating other materials than an artwork’s support. The procedure presented for the dating of the lead white pigment has the advantage of being material specific, since only the inorganic carbonate will be released as carbon dioxide independent of any other carbon containing material (organic pigment, varnish, ...). A distinction between manufacturing processes with different CO2 sources can be achieved based on the different 14C signature. The results show that the radiocarbon ages for lead white pigments produced following the traditional stack process match their production years. Lead white pigments from other industrial methods cannot provide the age as such but can be used as a marker to indicate manufacturing processes which do not follow the traditional stack method. Lead carbonate can therefore be used as a proxy for the time of creation of the painting (by providing a creation date no earlier than the time of the pigment manufacture where the stack method was used), and for identifying later interventions or detecting pigment anachronisms.
Furthermore, this work is not only a feasibility study, as the method developed for dating lead carbonate was successfully applied to the analysis of two real case oil paintings. The measured 14C ages of the lead white pigments are chronologically compatible with the period to which the paintings were attributed.
The two-step method of sample preparation introduced here allows the sequential dating of the carbonate, followed by the dating of the organic binder. This ability to derive two-fold information from a single sample is important since it reduces the sampling required and hereby increases the value of the 14C analysis. It is important to note that while the lead white pigment dating is material specific, for analysis of the organic component (the binder), no additional materials, such as varnish, retouching or consolidants can be present in the sample since they will influence the 14C results.
To conclude, radiocarbon dating has great potential for application to studies of works of art, however one must always bear in mind that it is the time of formation of carbon-bearing material, which is radiocarbon dated and not the time of painting, thus an offset between radiocarbon age and artwork creation must always to be considered.
Acknowledgments
This study was funded by an ETH-grant (ETH-21 15-1). The authors gratefully acknowledge the contribution of both the MOLART and HART projects which allowed preliminary feasibility studies. We wish to thank the Swiss Institute for Art Research (SIK-ISEA) for their collaboration in the study and for the access given to their painting collection. In particular we wish to acknowledge Jens Stenger for the introduction to handling of the XRF instrument, as well as Lukas Wacker and Negar Haghipour for their help running the CHS-AMS measurement. Also to be thanked are the reviewers who helped to improve this manuscript.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/RDC.2018.101