INTRODUCTION
The Nydam peat bog in South Jutland, Denmark (see Figure 1), became internationally famous in the mid-19th century when two 20–25-m-long wooden boats from the early 4th century AD were discovered, i.e. the famous Nydam oak ship (Nydam B), which is still preserved and on display in Schleswig, and a fragmentary preserved pine boat (Nydam C). Together with these ships, masses of iron artifacts such as weapons (swords, lances, axes etc.) and personal items (buckles, fibulae, tweezers, etc.), attributed to the late Roman and Migration periods, were found (Bemmann and Bemmann Reference Bemmann and Bemmann1998a, Reference Bemmann and Bemmann1998b). Detailed archaeological analysis of the weapons indicates that sacrificial deposits of weapons were made at Nydam in several distinct phases, between AD 250 and AD 475 (Rau Reference Rau2010).

Figure 1 Location of the Nydam peat bog and the location of the Nydam B oak boat in particular (x).
Carbon is introduced into the iron lattice in variable quantities during the time of production (e.g. Buchwald Reference Buchwald2005), and, provided that the carbon source used for smelting the iron ore is contemporaneous, iron objects can therefore be radiocarbon (14C) dated, as has been successfully demonstrated (e.g. Van der Merwe and Stuiver Reference Van der Merwe and Stuiver1968; Van der Merwe Reference Van der Merwe1969; Cresswell Reference Cresswell1992; Nakamura Reference Nakamura1995; Possnert and Wetterholm Reference Possnert and Wetterholm1995; Cook et al. Reference Cook, Wadsworth and Southon2001; Hüls et al. Reference Hüls, Grootes, Nadeau, Bruhn, Hasselberg and Erlenkeuser2004; Scharf et al. Reference Scharf, Kretschmer, Morgenroth, Uhl, Kritzler, Hunger and Pernicka2004; Oinonen et al. Reference Oinonen, Haggren, Kaskela, Lavento, Palonen and Tikkanen2009). Here we report on the 14C dating of two swords, one ax head, an anchor, and several iron rivets from the famous Nydam oak boat (Nydam B). All these objects had been treated with a waxy conservation agent, and the unique depositional environment at Nydam led to the formation of a siderite corrosion layer on the iron artifacts (Matthiesen et al. Reference Matthiesen, Hilbert and Gregory2003).
To evaluate possible effects of sample handling before and during carbon extraction, modern control samples, i.e. iron with a known, modern 14C content, were produced (Hüls et al. Reference Hüls, Grootes and Nadeau2011) and prepared for 14C measurements by the same methods as the archaeological samples.
MATERIALS AND METHODS
Samples
Ten archaeological iron objects and two modern iron standards were dated (see Table 1 and Figure 2). Both sword fragments represent so-called pattern-welded blades, i.e. different iron alloys with alternating high phosphorus content and medium carbon content (0.2–0.5 wt%C) (Thomsen Reference Thomsen1992; Buchwald Reference Buchwald2005) are forged together. Based on typological features they are thought to have been deposited during the sacrifice 1 (FS4156, AD 250–AD 300) and sacrifice 3 (FS5409, AD 360–AD 400) (Rau Reference Rau2010). The swords were conserved by a waxy coating (∼1 mm thick).
Table 1 Iron samples for AMS-14C measurements.

Table 2 Modern iron standards: carbon content and measured mean carbon isotope composition (charcoal F14C: 1.1667 ± 0.0017; δ13CAMS: –26.4 ± 0.17 ‰VBDB). C-uptake efficiency is calculated by comparison of measured C-content with expected C-content, assuming a charcoal carbon yield of 80wt%.

1 Sample names refer to the production batch, e.g. ST-I-4-1: Standard I, 4th batch, 1st sample.
2 Carbon estimated from CO2-pressure in our graphitization system.

Figure 2 Iron samples from the Nydam peat bog. A: sword fragment FS4156, B: sword fragment FS5409, C: ax Kat.Nr.1865 (© Museum für Archäologie Schloss Gottorf, Landesmuseen Schleswig-Holstein), D: iron bar (find situation, upper scale is 40 cm) and metallographic object carrier of this sample used for C measurements, and E: one example of Nydam rivets (Nydam-13944; upper figure: X-ray image [© Roland Aniol, Museum für Archäologie Schloss Gottorf, Landesmuseen Schleswig-Holstein], lower figure: situation during sample preparation).
The ax head, from sacrifice 4 (AD 380–AD 420) (Rau Reference Rau2010), was sampled by staff of the Archäologische Landesmuseum at Schloss Gottorf, Schleswig, Germany. A triangular section was cut from one face of the ax head, leaving a black, waxy surface coating on three sides of the sample and exposing fresh iron surfaces on the other sides.
The six iron rivets/nails were collected during the 1989–1997 excavation at Nydam by the National Museum of Denmark and are thought to be associated with the Nydam B oak boat, whose construction was dated dendrochronologically to AD 310–AD 320 (Bonde Reference Bonde1990). All rivets are heavily corroded and apparently soaked in wax.
Sample Nydam-3421 originates from a triangular metallurgical sample, set in a resin sample holder and polished from one side. This sample was taken from a large iron bar, a possible anchor shaft, collected during the Nydam excavation in 1993 (see Figure 2), and thought to be associated with either the Nydam (B) oak ship or the Nydam (C) pine boat (early 4th century AD; Rieck Reference Rieck and Rau2013). Metallographic examination show a ferritic/phophorferritic composition with a chemical signature of slag inclusions in accordance with an origin of iron made in western Jutland/Denmark at that time (Buchwald Reference Buchwald2005). In contrast to the rivets and the sword samples, this sample appeared almost pure on the polished upper surface and was contaminated with resin on the sides and below.
Two iron samples with a modern 14C signature were produced using ultra pure iron powder (99.998%Fe) and powdered modern charcoal (F14C: 1.1667 ± 0.0017; δ13CAMS: –26.4 ± 0.17‰VBDB) (Hüls et al. Reference Hüls, Grootes and Nadeau2011), heated up to ∼1600°C in an argon-flushed muffle oven. Sampling, preparation and 14C measurement were done in parallel to the archaeological samples to check for effects occurring during the sample preparation.
Sample Preparation
All iron samples were reduced in size by cutting and surface grinding prior to thermal carbon extraction in order to minimize possible contamination from modern tools used as was observed previously when using coring or milling (Scharf et al. Reference Scharf, Kretschmer, Morgenroth, Uhl, Kritzler, Hunger and Pernicka2004; Hüls et al. Reference Hüls, Grootes and Nadeau2011).
Swords, the ax head, and modern standards: cutting into sections using a cutting disk and subsequent thorough abrasion with a corundum grinding tool to remove a significant portion of the sample surfaces.
Iron rivets: surface cleaning with an iron wire metal brush until solid iron was exposed.
Cleaned samples were cut into 3–4-mm sized pieces with a metal shear followed by grinding of cut edges with a corundum grinding tool.
Sample Nydam-3421 (triangular shaped ∼ 17 × 13 mm, 1.5 mm thick), after removal from the metallographic sample holder, was just abraded with a corundum grinding tool and further cut into smaller pieces.
In order to remove wax and/or unknown organic substances, either from conservation agents on the samples or from sample handling, the samples were then subjected to a Soxhlet type serial extraction with solvents. In sequence, they were extracted three times each with boiling tetrahydrofurane (THF), chloroform, petroleum-ether, acetone, and methanol and water, followed by drying ∼60°C in an oven.
Carbon Extraction Method A
Between 600 mg and 1600 mg of prepared sample material (see also Table 3) was sealed in prebaked quartz tubes together with CuO (5 times amount of sample material; Hüls et al. Reference Hüls, Grootes, Nadeau, Bruhn, Hasselberg and Erlenkeuser2004) and combusted at 1000°C for 24 hr.
Table 3 Radiocarbon measurements on Nydam iron objects.

Carbon Extraction Method B
The severe contamination of Nydam rivets (corrosion with siderite, i.e. FeCO3 [Matthiesen et al. Reference Matthiesen, Hilbert and Gregory2003], soaking in PE wax as indicated by FTIR-ATR measurements) required additional cleaning efforts. To remove both contaminants, a step-combustion procedure with a low-temperature (LT, ∼570–600°C) step, followed by a high-temperature (HT, >970°C) step, was applied (due to crystal structure properties, carbon [dissolved in the iron lattice] diffusion is lower at temperatures <700°C in comparison to higher temperatures). For this purpose, precleaned sample material as outlined above was combusted
(B1) in a closed quartz tube together with CuO (500 mg) at 600°C for 4 hr (low-temperature CO2 fraction LT); after collecting the LT-CO2 fraction, the residue was combusted in closed quartz tubes together with CuO (5 times amount of sample material) at 1000°C for 24 hr (high-temperature CO2 fraction HT).
(B2) with high-purity O2 (∼50 mL/min) in an electric resistivity oven, attached to a gas manifold for subsequent cryogenic CO2 purification, at ∼570°C (LT-CO2) for 4 hr, followed by a ∼980°C combustion (HT-CO2) for 5 hr.
The resulting sample CO2 was cryogenically cleaned using a dry-ice/ethanol and a n-Pentane cooling trap (freezing T ∼130°C) to remove and separate H2O and possible SO2 from sample CO2 (e.g. Kusakabe Reference Kusakabe2005).
AMS Measurements
The sample CO2 was graphitized by the Bosch reaction with an iron catalyst (Vogel et al. Reference Vogel, Southon, Nelson and Brown1984; Nadeau et al. Reference Nadeau, Grootes, Schleicher, Hasselberg, Rieck and Bitterling1998). The resulting mixture of graphite and iron powder was pressed into aluminum target holders for accelerator mass spectrometry (AMS) 14C measurements with a 3MV HVEE Tandetron AMS system. 14C measurements are normalized to modern Oxalic Acid II standard (NBS SRM 4990C) and corrected for isotopic fractionation and background effects (Nadeau and Grootes Reference Nadeau and Grootes2013). 14C ages are converted to calendar ages using the software package OxCal4 (Ramsey and Lee Reference Ramsey and Lee2013) and the IntCal13 dataset (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté?, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013).
RESULTS AND DISCUSSION
Modern Iron Samples
The measurements of the two modern iron standards show depleted 14C concentrations compared to the charcoal and are in good agreement with previous measurements with comparable sample material (Hüls et al. Reference Hüls, Grootes and Nadeau2011; see Table 2). In addition to the earlier study, detailed geometric information and mass-balancing during the grinding cleaning step were documented, revealing an overall material removal of 30–36% and, on average, a removal of ≥ 0.1 mm from the sample surface. With this amount of material removed from the sample surface, the explanation of observed average 14C differences as proposed by Hüls et al. (Reference Hüls, Grootes and Nadeau2011) (i.e. surface contamination by cutting tools) may be questioned. Instead, we speculate that fractionation effects occurred during carbon dissolution into the metal. For example, the measurements of sample ST-I-4-2 (0.1 wt%C) with the lowest amount of charcoal used (see Table 2), show a rather low efficiency in carbon uptake (e.g. overall C-content in metal vs. charcoal carbon available), depleted 14C concentrations (ΔF14C ∼1.9%), and increased δ13C values (–5‰VPDB) with respect to the carbon source. For the remaining 4 standards with a higher carbon incorporation efficiency, one standard (ST-I-4-3, 1.9wt%C) give a 14C concentration in agreement, the other standards give depleted 14C concentrations (ΔF14C ∼1%), compared to the charcoal source. Clearly, more experimental smelting studies would be needed to verify if a fractionation effect could occur during real iron production, e.g. smelting, which, consequently, would be needed to be considered in 14C dating of iron.
Nydam Swords and Ax Head
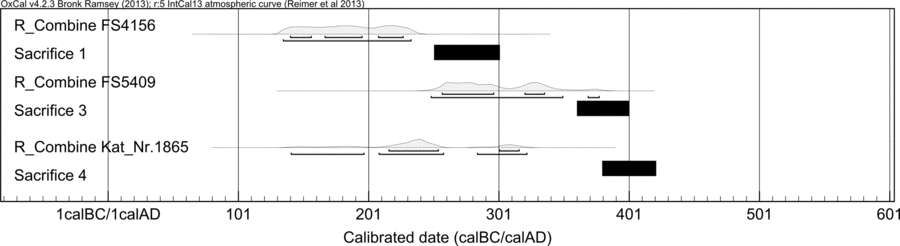
Multiple 14C AMS measurements of the three Nydam weapon samples gave internally consistent and reproducible 14C ages (Table 3, Figure 3). With one exception, all carbon extractions from the iron lattice were done by method A. For one sample of FS4156, carbon extraction was done by a step-combustion with a low and high temperature (570°C and 980°C, respectively, method B2). The measured 14C concentration is consistent to the measurements done with CO2 extractions by method A, which seems to indicate that the mechanical surface and subsequent solvent cleaning is sufficient for this type of sample material.

Figure 3 Calibrated sample ages for swords FS4156, FS5409 and ax Kat_Nr 1865. Black bar below probability curves give the time span of sacrifices 1, 3, and 4, respectively.
Calculated weighted mean 14C ages of swords FS4156, FS5409, and the ax head of 1824 ± 9 BP, 1734 ± 13 BP, and 1791 ± 13 BP, respectively, are in reasonable agreement with the expected deposition time of sacrifices 1, 3, and 4 (Figure 3). Calibrated sample ages are older by about 0–60 years, which is within or slightly more than an expected old-wood effect (e.g., average wood-age used to make charcoal for iron ore smelting) of only a few decades, as indicated in the study of Leroy et al. (Reference Leroy, L’Héritier, Delqué-Kolic, Dumoulin, Moreau and Dillmann2015). For example, variable (average) wood ages up to several decades are estimated by anthracological analysis of sample material from a 3rd–5th century AD iron production site in Joldelund, northern Germany, not far from Nydam (Dörfler and Wiethold Reference Dörfler, Wiethold, Haffner, Jöns and Reichenstein2000). An additional reservoir effect, caused by fractionation during iron production as seen in modern test samples, cannot be rejected nor confirmed.
Nydam Iron Rivets
Carbon extracted by method A gave 14C ages varying from ∼2300 BP to 4300 BP, much older than expected and thus indicating incomplete removal of carbon-containing contaminants (see Table 3). In the course of sample preparation, we recognized two contamination sources. All analyzed iron objects from the Nydam peat bog are significantly corroded, and for the Nydam iron rivets this corrosion consists almost exclusively of iron carbonate (siderite, FeCO3; Matthiesen et al. Reference Matthiesen, Hilbert and Gregory2003). After recovery, these rivets were further contaminated by soaking in liquid wax.
Due to the high thermal stability of the carbon dissolved in the iron, a step-combustion procedure was applied to remove low-temperature volatile carbon sources such as siderite (Gallagher and Warne Reference Gallagher and Warne1981) and wax before the final extraction of carbon from the iron lattice with higher combustion temperatures.
The LT-CO2 fractions from the Nydam rivets give varying apparent 14C ages greater than 4000 BP, giving evidence of a remaining contamination in the precleaned iron sample, originating from a mixture of wax and siderite.
With the exception of Nydam-12444, the HT-CO2 fractions give 14C ages ranging from 1920 to 2020 BP. A double low-temperature combustion and subsequent high-temperature CO2 extraction by method B2 of one subsample of Nydam-13944 gave a 14C age in accordance to the measurement of the other subsample with only one low-temperature combustion, and in agreement to the results obtained for Nydam-13707, -6263, and 6279 (see Table 3). Apparently, the first low-temperature combustion was already sufficient for a removal of low-temperature stable carbon fractions, since the 2nd low-temperature combustion did not produce CO2.
The HT-CO2 fractions of Nydam-12444, extracted by method B1 (using closed-tube combustion), gave 14C ages of 2207 ± 22 BP and 2565 ± 35 BP (Figure 4), significantly older than the HT-CO2 measurements of the other iron rivets. The sample material after surface and solvent cleaning of Nydam-12444 contained visibly more foreign substances in comparison to the other rivets, which, assuming incomplete removal of exogenous carbon by the low-temperature combustion using method B1, could explain the measured 14C differences of the HT-CO2 fraction of this sample. Maybe a longer-lasting low-temperature combustion time (i.e. >4 hr) is needed to completely remove the low-temperature carbon fraction.

Figure 4 Calibrated sample ages Nydam iron rivets (pale gray area). Dendro-age for Nydam B is indicated.
Nevertheless, the overall close agreement between the HT-CO2 14C measurements between the rivets Nydam-13705, -13707, -13944, -6263, and Nydam-6279, and the result of the double precombusted (at 570°C) subsample of Nydam-13944, indicate sufficient removal of siderite and wax contamination for these samples.
Calibrated sample ages of Nydam-13705, -13707, -13944, -6263, and Nydam-6279 lie between to 90 BC–AD130 (see Figure 3), which is at least 200 years older than the dendro-date for the construction of the large Nydam oak boat (i.e. AD 310–AD 320; Bonde Reference Bonde1990).
Assuming a complete removal of siderite and wax contamination by combustion below 600°C, additional contaminating carbon sources need to be considered. Possible preaged carbon sources could be the use of older trees for charcoal production, and/or fossil carbon added during the iron making (e.g., carbonates in ore or flux, geogenic material of smelting oven wall lining). None of the mentioned causes can be rejected nor confirmed without further metallographic analysis. Likewise, the possibility of an aging effect, caused during iron making as seen in modern test samples, cannot be excluded.
In contrast to the precleaning of the swords and ax head samples, contamination during the mechanical removal of corrosion and conservation coating of the rivets also remains possible. As outlined above, corrosion and wax was removed using a metal (iron) brush. While the mineral corrosion product and wax seem softer compared to the iron brush, the brush show a considerable wear after usage. A tiny amount of metal from the metal brush, containing fossil carbon as shown in previous studies for modern iron (a.o. Cook et al. Reference Cook, Wadsworth and Southon2001; Hüls et al. Reference Hüls, Grootes, Nadeau, Bruhn, Hasselberg and Erlenkeuser2004; Scharf et al. Reference Scharf, Kretschmer, Morgenroth, Uhl, Kritzler, Hunger and Pernicka2004, etc.), could be responsible for an additional observed age difference, since the irregular shaped sample surface prevent additional cleaning by surface grinding using a corundum grinding tool.
Finally, one need to mention the possibility of a reuse of older iron, as was indicated by Disser et al. (Reference Disser, Dillmann, Leroy, Merluzzo and Leroy2016) in their study of Carolingian architectural iron fastenings.
Anchor Nydam-3421
The metallurgical sample of an iron bar from a ship anchor gave a 14C age 2575 ± 35 BP, which is obviously too old with respect to its expected archaeological age (4th century AD). Although this sample appeared comparably well preserved, similar to the sword and ax head samples, the apparent age difference indicates the abundance of a significant amount of exogenous carbon, which likely comes from the embedding resin.
CONCLUSIONS
In this study we present the results of 14C measurements for 10 archaeological iron objects from the war booty offering site at Nydam, SE Denmark. Additionally, we measured the 14C composition of iron samples made with modern charcoal to evaluate possible contamination effects during handling of iron samples.
Measured 14C concentration of modern iron samples are depleted with respect to the original 14C composition of the charcoal used for the making of the standards. Due to the large amount of surface material removed during sample preparation, the measured isotopic differences may indicate isotope fractionation effects, requiring further studies to verify if this is a common effect occurring during iron making.
Multiple 14C measurements of sample material from two swords and one ax head give consistent age estimates. Corrected and calibrated sample ages are in good agreement with expected ages and indicate small old-wood effects.
A step-combustion carbon extraction procedure was applied for the 14C dating of 6 iron rivets to remove low-temperature volatile contamination before extraction of assumed original carbon dissolved in the iron. Measured 14C ages of HT-CO2 fractions of 5 rivets gave consistent 14C ages of 1920–2020 BP, about 200–300 years older compared to the dendro-age of the Nydam B oak boat (AD 310–AD 320, equivalent to ca. 1760 BP). Single or multiple aging effects such as the use of old wood during iron production and/or contamination with fossil C (during iron production or precleaning) might be responsible for the observed age difference. The reuse of older iron may also be a possible explanation.
The 14C age of an iron anchor fragment, associated to the Nydam ships (early 4th century AD), is significantly older than the inferred archaeological age, probably due to inefficient cleaning of sample material prior carbon extraction for 14C measurements.
ACKNOWLEDGMENTS
For valuable information on conservation methods of the Nydam artifacts, comments and assistance with the choice of potential samples: Jan Bruun Jensen, Curation and Cultural Heritage Conservation, National Museum of Denmark, Eva Salomonsen, Curation and Cultural Heritage Conservation, National Museum of Denmark, Henning Matthiesen, Environmental Archaeology and Materials Science, National Museum of Denmark. For the allowances to sample the Nydam iron material: Michael Gebühr, Archaeological Museum Schleswig-Holstein, Poul Otto Nielsen, Keeper of the Danish Prehistoric Collections and Environmental Archaeology at the National Museum of Denmark. The comments of two reviewers are appreciated.