INTRODUCTION
Iron Mask Swords are somewhat mysterious iron objects. Recovered mostly from illegal diggings in the 1920s in the province of Luristan, Iran, the about 90 known objects (Muscarella Reference Muscarella, De Meyer and Haerinck1989) are distributed in private and museum collections. Just recently one sword was recovered during a rescue excavation in Nurabad, Luristan, Iran (Hasanpur et al. Reference Hasanpur, Hashemi and Overlaet2015), with an inferred age (datum post quem) around 9th century BC.
All of the known swords are characterized by a disk-shaped pommel on the top of the handle with two mounted bearded heads on two sides, a rectangular shaped handle with two clamps and two kneeling animal figures on the lower part (see Figure 1). The blade is turned 90° with respect to the handle. The overall size of these objects is about 30–60 cm. They may well be the oldest complex iron objects known at present, but without knowing their age, this remains speculative.
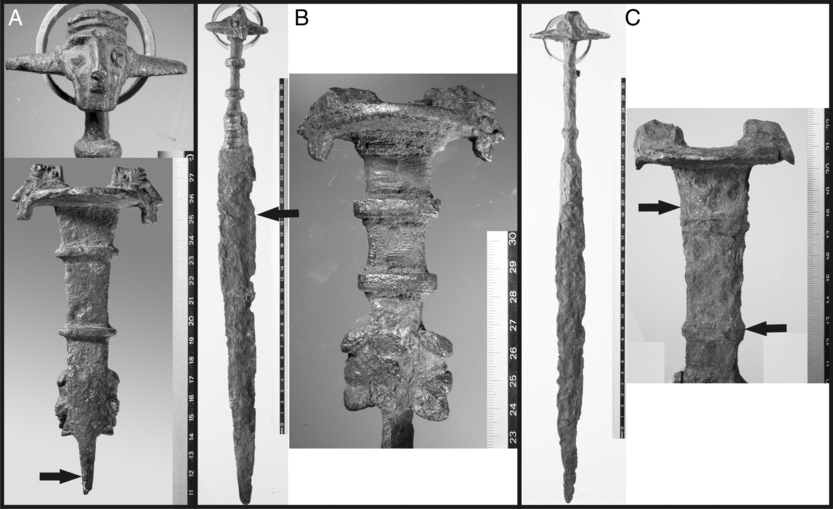
Figure 1 Iron Mask Swords for metallurgical analysis and 14C measurement (A: IR-3743; B: IR-3745; C: IR-3746). Arrows mark the position for sampling for metallurgical and radiometric analyses. Photographs by Agnes Heitmann, Institute for Pre- and Protohistoric Archaeology, Christian-Albrechts-University Kiel, Germany.
Numerous attempts have been made to date these swords by indirect means (see e.g. Muscarella Reference Muscarella, De Meyer and Haerinck1989), but lacking contextual finds, all these efforts are little more than educated guesses. Suggested ages range from 1100 BC–600 BC. A first experimental assessment of the age of these objects came from radiocarbon measurements on two Luristan swords from the Royal Ontario Museum and the Massachusetts Institute of Technology collection (2880 ± 60 BP and 2940 ± 60 BP, respectively; Cresswell Reference Cresswell1992), placing them in the early part of the Iron Age. With respect to their homogeneity in style and structure, an overall short production period was assumed (Moorey Reference Moorey1991).
In this study we present the results of metallurgical analysis and 14C measurements for three Luristan swords, which were acquired recently by one of the authors (HF). After finishing our investigations, the swords were donated to the Royal Museums of Art & History, Brussels.
MATERIALS AND METHODS
Material
All three swords lack an archaeological context, which, in the beginning of our investigation, left open the possibility of a forgery. IR-3743 is a fragment of sword with a moderately well preserved hilt and the upper part of the blade, covered by corrosion with visible solid iron at the broken blade (Figure 1). The sword is sampled below the hilt on one side of the blade. IR-3745 and IR-3746 are complete swords, corroded with visible damages at the blades. The decoration-heads on the pommel are more corroded than on IR-3743. IR-3745 was sampled in the upper half of the blade, IR-3746 was sampled at the handle (two times from the opposite side). All samples are taken by using a cutting disk (∼20–30 × 5 × 5 mm).
Sample Preparation for Metallurgical Analysis
Iron samples IR-3743, IR-3745, and IR-3746 were embedded in PolyFast™ (Struers) with a SimpliMet™ XPS1 press (Buehler), grinded, polished, and etched with 3% Nital solution. After microstructure analysis of Nital etched cross sections, samples were polished again, etched with an Oberhoffer solution to reveal possible phosphorous (P) contents, followed by a second microstructure analysis of Oberhoffer etched cross sections. Microstructure analysis were done with reflecting light microscopy (MeF3A from the company Reichert-Jung). The carbon content was estimated by comparison with microstructures of known composition (Wever and Rose Reference Wever and Rose1954; Rose and Hougardy Reference Rose and Hougardy1972; Orlich and Rose Reference Orlich and Rose1973; Orlich and Pietrzeniuk Reference Orlich and Pietrzeniuk1976). Following microstructure analysis, mechanical properties such as micro-hardness were determined (Micro-hardness Micro-Duromat 4000E from the company Reichert-Jung with a force of 100P [20 s] and an increase of 40P/s). Finally, the samples were inspected with SEM/EDX (Zeiss Gemini Ultra55plus, EDX-detector: Oxford X-act SD-detector. EHT: 5 kV, measuring time: 600,000 counts, calibration: standardless) for point analysis of the chemical composition of the metallurgic samples.
Sample Preparation for 14C Measurement
All surface areas from the different sword samples are thoroughly grinded using a corundum grinding tool, thereby removing the corrosion layer at the outside and possible surface-contaminated iron from the cutting process. Subsequently, smaller pieces are cut (length/width/thickness ∼5/5/3 mm) with a metal cutter, again followed by a thorough grinding of shear surface areas. Finally, pieces are solvent cleaned using hot tetrahydrofuran, chloroform, ligroin, acetone, methanol, and water, using a computer-controlled “Soxhlet”-type series extractor (Bruhn et al. Reference Bruhn, Duhr, Grootes, Mintrop and Nadeau2001). Carbon dioxide is released by closed-quartz-tube combustion with CuO (∼5–6 times amount of sample weight) at 1000°C/24 hr (Cook et al. Reference Cook, Wadsworth and Southon2001; Hüls et al. Reference Hüls, Grootes, Nadeau, Bruhn, Hasselberg and Erlenkeuser2004, Reference Hüls, Grootes and Nadeau2011) and subsequent cryogenic purification of CO2 (–70°C by dry-ice/ethanol slurry for removing H2O, –130°C [frozen n-pentane] cool-trap to withhold SO2 [Kusakabe Reference Kusakabe2005]).
AMS Measurements
The sample CO2 was graphitized by the Bosch reaction with an iron catalyst (Vogel et al. Reference Vogel, Southon, Nelson and Brown1984; Nadeau et al. Reference Nadeau, Grootes, Schleicher, Hasselberg, Rieck and Bitterling1998). The resulting mixture of graphite and iron powder was pressed into aluminum target holders for AMS 14C measurements with a 3MV HVEE Tandetron AMS system. 14C measurements are normalized to modern Oxalic Acid II standard (NBS SRM 4990C) and corrected for isotopic fractionation and background effects (Nadeau and Grootes Reference Nadeau and Grootes2013). 14C ages are translated to calendar ages using the software package OxCal4 (Ramsey and Lee Reference Ramsey and Lee2013) and the IntCal13 dataset (Reimer et al. Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey, Buck, Cheng, Edwards, Friedrich, Grootes, Guilderson, Haflidason, Hajdas, Hatté, Heaton, Hoffmann, Hogg, Hughen, Kaiser, Kromer, Manning, Niu, Reimer, Richards, Scott, Southon, Staff, Turney and van der Plicht2013).
RESULTS AND DISCUSSION
Metallurgy
Only a brief summary of the metallographic analyses is given here to provide the necessary details to evaluate and support the reliability of the 14C dates. A more comprehensive overview and discussion of the metallurgy will be given in a separate publication (Petri et al. in prep.).
The microscopic analysis of samples IR-3743, IR-3745, and IR3746 (see Figure 2) reveal in general two distinctive zonal structures with gradual transitions:
• IR-3743 (sampled on the upper part of the blade): Zone 1 contains a fine-grained ferritic structure with strings of cementite along grain boundaries (only detected in SEM/EDX), the estimated C-content is about ∼0.02 wt%. Zone 1 contains small slag inclusions (Fayalite + Wüstite + Glass; more slag inclusions are observed in zone 1a). Zone 2 consists of a very fine-grained structure with mainly Ferrite, globular Pearlite, and Cementite along grain boundaries. This zone contains more slag inclusions. The C-content is estimated to between 0.1 and 0.5 wt%. The hardness ranges from about 100 mHV to about 200 mHV, whereas the hardness of zone 2 was higher than that of zone 1.
• IR-3745 (sampled at the blade): This sample shows a high content of very large slags (Fayalite + Wüstite + Glass). Within general ferritic grains of zone 1, Cementite needles are observed, particularly well developed in zone 1a. Overall, the C-content is low (∼<0.02 wt%). Zone 2 consists of Ferrite and Pearlite, the Pearlite being partially globular. The carbon content varies between 0.02 and 0.1 wt%. The hardness ranges from about 100 mHV to about 185 mHV, while the hardness of zone 2 tends to be higher than that of zone 1. The inner part of the blade is softer than the outer part, but the highest hardness values are not measured at the cutting edges.
• IR-3746 (sampled at the handle): The sample shows two structural zones with no clear borders. Zone 1 contains a fine-grained ferritic-paerlitic Widmanstätten structure, the Pearlite being mostly globular. The slag content is relatively low, the slags are mostly fayalitic. The C-content raises continuously from about 0.1 wt% to the carbon content of zone 2. Zone 2 contains a fine-grained pearlitic structure, the Pearlite being mostly globular. Zone 2 shows some slag inclusions (Fayalite). The estimated C-content is about 0.7–0.8 wt%. The two metallurgical samples of this sword are comparably similar, even though being from the opposite ends of the handle. This indicates the manufacture of the handle from a rather homogenous single iron bloom. The hardness ranges from about 115 mHV to about 230 mHV, whereat the hardness of zone 2 was higher than that of zone 1.

Figure 2 Microstructures of samples IR-3743 (A), IR-3745 (B), and IR-3746 (C1, C2) (Nital etched; left side pictures show whole metallographic sample, right side gives close-up). Numbers give structural zones (see description in text). Photographs by I. Petri, Institute for Pre- and Protohistoric Archaeology, Christian-Albrechts-University Kiel, Germany.
For all samples no clear borders or linear structures such as bands of slag inclusions, oxidation horizons, or phosphorous enriched bands (“white lines”), characteristic for welding of different pieces, are observed. The zonal structures also do not show clear borders but gradual transitions into the next structure. As such, the structural zones appear original from an inhomogeneous iron bloom and are not caused by forge-welding of pieces of a different carbon content or by subsequent carburization of the finished object. The amount of slag inclusions and their composition indicates their origin from the bloomery process. Their elongated appearance and their orientation within the samples indicates subsequent hammering into form. The globular Pearlites show that the material has been intensively annealed over several hours. This is supported by the hardness measurements, even the areas with a C-content of about 0.7–0.8 wt% do not exceed a hardness of 230 mHV. As such, the intensive annealing was probably done to soften the material for subsequent fixing of the assembled different parts of the swords. For example, the outer part of the sample from sword IR-3743 shows traces of intensive cold working. Here the metal has been driven cold against the side of the animal figure that sits directly over the sample to clamp it into place.
The composition of the slag inclusions for the three sword samples by SEM-EDX analysis (see Table 1) give further insight into the production process. For example, the so-called G-value (Buchwald Reference Buchwald2005), which is the ratio of (non-reduced) oxides coming from charcoal ashes, impurities of the used ore, and furnace construction material, divided by the oxides from the iron ore, gives information about the reduction grade of the ore during the iron smelting process. For the arbitrarily selected Luristan sword samples of this study, a gradual increase in G-values are observed, starting with a low value for IR-3745 (G = 19), an intermediate value for IR-3743 (G = 52) to a higher value for IR-3746 (G = 76). Much higher values (>400) are observed for iron which is produced with the more advanced and efficient blast furnace technique (Buchwald Reference Buchwald2005), developed during the late medieval period. The estimated low G-values between 19–76 are thus further proof for a production via the bloomery process, not to mention that the swords are genuine and not fakes. Admittedly, further and more detailed analysis with more samples would be needed for confirmation.
Table 1 SEM-EDX measurements of slag inclusions (calculated averages from N single point measurements; n.d.: not detected).

14C Measurements
The three Luristan swords IR-3743, -3745, and -3746, give 14C ages between 2800 BP and 3360 BP (see Table 2), calibrated to 1745 BC–900 BC (Figure 3). The oldest measured sword is IR-3745 with a low carbon content (<0.1 wt%C) and a low G-value. Samples IR-3743 and IR-3746 contain a higher carbon content (0.4 and 0.6 wt% C, resp.) and give 14C ages slightly older and younger, resp., than previously measured Luristan swords (Cresswell Reference Cresswell1992). For both samples, the higher C-content allowed a duplicate carbon extraction and subsequent 14C measurements, which gave reproducible results. To test for possible fractionation effects occurring during graphitization and AMS-measurement of the small sample of IR-3745 (220 µg C measured), a comparable amount of CO2 of sample IR-3746 (260 µg C) was graphitized and measured (see Table 2). The result is in agreement with the measurements done on normal sized samples (∼1 mg C), indirectly supporting the 14C measurement of IR-3745.

Figure 3 Calibrated sample ages of IR-3743, IR-3745, and IR-3746. For comparison, previously dated Luristan swords (Cresswell Reference Cresswell1992) are shown in pale gray.
Table 2 Carbon yields and radiocarbon ages of samples IR-3743 (KIA51495), IR-3745 (KIA52135), and IR-3746 (KIA 52134).

1 Part of available sample CO2 of IR-3746 has been reduced using a comparable small amount (∼0.26 mg C) as was available for graphitization and AMS 14C measurement of sample IR 3745 to test possible fractionation effects in analyzing small samples.
Regarding the overall similarity of about 90 known Luristan swords (Muscarella Reference Muscarella, De Meyer and Haerinck1989), a rather limited production period, between the 10th to 8th century BC, was suggested (e.g. Moorey Reference Moorey1991; Overlaet Reference Overlaet, Stöllner, Slotta and Vatandoust2004), which is in contrast to the longer period suggested by our 14C dates. This raise the question to what extent 14C ages measured from extracted carbon from iron is reliable.
14C dating of iron in principle measures the age of dissolved carbon within the iron lattice, incorporated during the production of the iron. The main carbon source is coming from the fuel used for smelting the iron ore and for subsequent forging. A reasonable assumption for early metal production is that charcoal, made from almost contemporaneous trees, was used, which should give an age effect of only a few decades (Rehder Reference Rehder1991). For a local iron production site in Northern Germany from the 3rd century AD, anthracological analysis have indicated a wood age range up to a few decades (Dörfler and Wiethold Reference Dörfler, Wiethold, Haffner, Jöns and Reichenstein2000), which, although not directly applicable, lends further support for a small wood age effect. And indeed, for a number of studies regarding 14C dating of iron objects, a low wood age effect seems to have been indicated (e.g. Van der Merwe Reference Van der Merwe1969; Cresswell Reference Cresswell1992; Igaki et al. Reference Igaki, Nakamura, Hirasawa, Kato and Sang1994; Nakamura Reference Nakamura1995; Possnert and Wetterholm Reference Possnert and Wetterholm1995; Cook et al. Reference Cook, Wadsworth and Southon2001; Scharf et al. Reference Scharf, Kretschmer, Morgenroth, Uhl, Kritzler, Hunger and Pernicka2004; Park et al. Reference Park, Burr and Jull2010; Hüls et al. Reference Hüls, Grootes and Nadeau2011; Leroy et al. Reference Leroy, L’Héritier, Delqué-Kolic, Dumoulin, Moreau and Dillmann2015).
Further carbon contamination sources could influence the carbon isotope composition during the production process, as was summarized by Craddock et al. (Reference Craddock, Wayman and Jull2002), namely the addition of fossil carbon, either coming from the use of fossil fuel sources (e.g. coke), or the use of calcite or lime as a flux ingredient (to increase the reduction efficiency and Fe yield from the ore), or coming from the geological material within the ore itself. The use of fossil fuel, such as pit-coal or coke, was introduced much later in iron technology, in the 11th century AD in China and the 17th century AD in Europe (Wikipedia 2018). If fossil fuel would have been used here at any stage of the production process (smelting, smithing), the resulting 14C age would become much older (>>10,000 years) as, for example, was shown by Park et al. (Reference Park, Chunag and Gelegdorj2008) and Craddock et al. (Reference Craddock, Wayman and Jull2002).
The use of calcite as a flux ingredient is more difficult to disprove. Using modern charcoal as fuel, roasted iron ore, and calcite as ingredients for a smelting experiment, Oinonen et al. (Reference Oinonen, Haggren, Kaskela, Lavento, Palonen and Tikkanen2009) studied the effect of the addition of calcite with respect to the 14C concentrations of the resulting iron bloom. Measured Ca and 14C concentration within the iron bloom confirm the incorporation and apparent mixture of modern (from the charcoal) and fossil carbon (from the calcite), with an inferred contamination of more than 20% (> 2000 years). Unfortunately, their data (e.g. measured calcium concentrations) cannot be compared directly with our data as they have made the measurements on bulk iron bloom samples.
CaO concentrations measured in the slag inclusions of our Luristan sword up to 11% (IR-3743) may also come from the ore used to make the iron. For example, the Apatite-Magnetite Deposit in central Iran (Esmailiy et al. Reference Esmailiy, Zakizadeh, Sepidbar and Kanaanian2016), would provide elements such as Al, Ca, K, and P, which become enriched during the smelting process within the slag inclusions. Depending on the pre-conditioning of the iron ore before smelting, e.g. roasting of the ore (Buchwald Reference Buchwald2005), a significant portion of fossil carbon will get eliminated before the ore enters the smelting ovens. Indirect support for the reliability of 14C dating of iron comes from the 14C dating of some iron objects from the war booty of Nydam, Denmark, which are in good agreement with archaeological age estimates (Hüls et al. this issue), although elevated CaO concentrations up to 11 wt% were observed in some slag inclusions (Buchwald Reference Buchwald2005).
Lacking hard evidence of a possible contribution from fossil carbon sources during the iron production of the three Luristan swords, we are inclined to accept our 14C age estimates at this stage with, however, a larger uncertainty.
As was indicated by Scharf et al. (Reference Scharf, Kretschmer, Morgenroth, Uhl, Kritzler, Hunger and Pernicka2004) and Hüls et al. (Reference Hüls, Grootes and Nadeau2011), the sampling of iron objects for 14C measurements could potentially contaminate the overall extracted carbon due to the addition of fossil carbon from the modern steel tools used for cutting, which, however could be minimized by using larger iron pieces with a thorough surface cleaning. Recent 14C measurements on experimentally produced modern iron (Hüls et al. Reference Hüls, Grootes and Nadeau2011, Reference Hüls, Meadows and Rau2019) and known archaeological iron from the early 1st millennium AD, seem to indicate that, instead of a previously assumed contamination by modern cutting tools, an intrinsic age offset, which is probably related to effects during the carbon uptake during the production process itself, may be possible, with a magnitude up to 100 years.
When applying an age offset of approximately 100 years to the measured 14C ages of swords IR-3743, IR-3745, and IR-3746, the calibrated age range shifts to an indicated production period between 1650 BC and 800 BC, which is still a few centuries older than discussed by Moorey (Reference Moorey1991) and Overlaet (Reference Overlaet, Stöllner, Slotta and Vatandoust2004).
CONCLUSIONS
For an arbitrary selection of three Luristan iron mask swords, metallurgical analysis and 14C measurements have been performed. Earlier studies of these swords have indicated a mixture of a rather primitive iron production process and a sophisticated craftsmanship in the design of the decoration with a timing in the early Iron Age, around 1000 BC.
The microstructural analysis of the sword iron samples shows in general a heterogeneous mixture of low to intermediate carburized iron with a considerable slag content, indicative of a production via the bloomery process. Different to later iron objects, no clear signs of an intense forging process are observed, illustrating the early stage in iron making. The chemical composition of the slag inclusions seems to indicate an increase in the efficiency with respect to iron yield during the smelting process. Our 14C measurements indicate a comparatively long production period between 1650 BC and 800 BC, in contrast to earlier studies suggesting a short production period around 1000 BC. Further studies would be needed to gain more and detailed insight into the evolution of the early iron making during the transition of Bronze Age to the Iron Age.
ACKNOWLEDGMENTS
The authors are indebted to the Prof. Dr. Werner Petersen-Stiftung of Kiel, Germany, who financed part of this research. Thanks are also due to Profs. L. Kienle, R. Adelung, and the team of the Institute of Materials Science, CAU, Kiel, for letting us use their facilities. The competent help of Mrs. K Brandenburg, Mr. M.-D. Gerngroß, Mrs. Heitmann, Mr. K. Rath, Mrs. M. Schwitzke, and Mrs. Ch. Szillus is gratefully acknowledged. We appreciate the constructive comments given by two anonymous reviewers helping to shape the manuscript.







