INTRODUCTION
The EC Carbon-14 Source Term (CAST) project aimed to develop understanding of the potential release mechanisms of 14C (carbon-14, radiocarbon) containing waste under conditions relevant for waste packaging and disposal to underground geological disposal facilities. The project focused on the release from neutron irradiated materials—steel, Zircaloy, graphite—and spent ion exchange resins (Williams Reference Williams and Scourse2015). The irradiated metals and spent ion exchange resins are frequently processed with cementitious materials and the 14C release from these four waste forms has been investigated at chemical representative conditions in CAST. These materials and resin may contain 14C within a radionuclide hazardous period. In this paper, this period is determined by the activity concentration at start of disposal, the half-life and exempt level. The 14C exempt level in radioactive waste is 1 Bq/g solid matter (EU 2013) and the 14C half-life is 5700 years. In CAST, the largest measured 14C activity concentrations have been found in neutron irradiated steel from the core of a nuclear plant and neutron irradiated graphite (Neeft Reference Neeft2018). These are 1.08–1.35×105 Bq/g solid matter for neutron irradiated graphite from Vandellós I, a graphite gas cooled reactor in Spain (Toulhoat Reference Toulhoat, Moncoffre, Narkunas, Poskas, Bucur, Ichim, Petit, Schumacher, Catherin, Capone, Shcherbina, Bukaemskiy, Alcalá, Magro, Márquez, Piña, Fachinger, Fugaru, Norris and Zlobenko2018) and a value of 2.7×105 Bq/g solid matter for a stainless steel plenum spring, neutron irradiated in the Gösgen Pressurized Water Reactor (PWR) in Switzerland (Herm Reference Herm, González-Robles, Böttle, Müller, Bohnert, Dagan, Caruso, Kienzler and Metz2017; Mibus Reference Mibus, Diomidis, Wieland and Swanton2018). The 14C activity concentration in waste has decayed to exempt levels within a period of 17–18 14C half-lives, about 100,000 years. There is an engineered containment period up to several hundred thousands of years foreseen in many disposal concepts for high level waste (HLW). Investigation of the 14C source term is therefore of more interest for the low and intermediate level waste (LILW) than HLW for many countries. Processes at ambient deep disposal temperatures are therefore assumed in this paper as there is negligible heat production for the types of waste investigated in CAST.
The biosphere acts as a receptor for any 14C release from the geological disposal system. Processes that control how people might be exposed to any radioactivity from the waste are modeled in a safety assessment and compared with a dose constraint. Another yardstick besides dose constraint needs to be used when a single radionuclide in waste is assessed. In this paper, the natural 14C flux into our living environment is used as a yardstick to compare the calculated potential 14C flux from disposal of radioactive waste. The flux of CO2 emanating from the soil is one of the parameters to determine 14C exposure and a daily flux between 2 to 13 gram CO2 per m2 per day has been found (ISRN 2010). A fairly uniform flux of 2 g CO2 m−2day−1 has been measured in row-crop agroecosystems (Paul Reference Paul, Harris, Collins, Schulthess and Robertson1999). In forests and pastures, higher fluxes have been measured e.g. in temperate climates 2–8 g CO2 m−2day−1 (Kishimoto-Mo Reference Kishimoto-Mo, Uchida, Kondo, Murayama and Koizumi2015) and up to 30 g CO2 m−2day−1 in the tropics (Schwendenmann et al. Reference Schwendenmann, Pendall and Potvin2007). Cosmogenic generated 14C is present as an impurity with a concentration of about 1–1.5 out of 1012 non-radioactive carbon atoms. The natural 14C flux from soil into air is in the order of 109 14CO2 molecules per cm2 per year using a daily flux of 2 g per m2. The paper starts with a brief description of the cosmic generated 14C in order to show that the same processes can be used to determine the 14C activity concentrations in neutron irradiated metals except that some parameter values are different.
MAIN ORIGIN OF 14C
Generation
Natural 14C is only generated by neutron activation. The environmental neutron fluxes originate from collisions with highly energetic protons from the Sun and other stars. The energy of neutrons can be 1 GeV (ICRP 2016). These neutrons lose their energy by collisions with other atoms. Hydrogen atoms are most effective in reducing this energy until a neutron is thermalized. The neutron flux at altitudes typical for intercontinental flights is about 10 neutrons cm−2s−1 (Zanini Reference Zanini, Ongaro, Durisi, Visca, DeAgostini, Fasolo, Pelliccioni and Saavedra2003). At the Earth’s surface, there is more shielding against cosmic radiation and the environmental neutron flux is therefore smaller i.e. about 10−3 cm−2s−1 (Komura Reference Komura, Ahmed, El-Kamel and Yousef2008). The cross sections of the precursors of 14C, their chemical content, and temperature and energy dependent neutron flux are needed as input to determine the 14C generation rate in air. Figure 1 shows the three main precursors. The reaction to 14C neutron cross sections of 15N and 18O are negligible compared to these three precursors and therefore neglected (IAEA 2004).
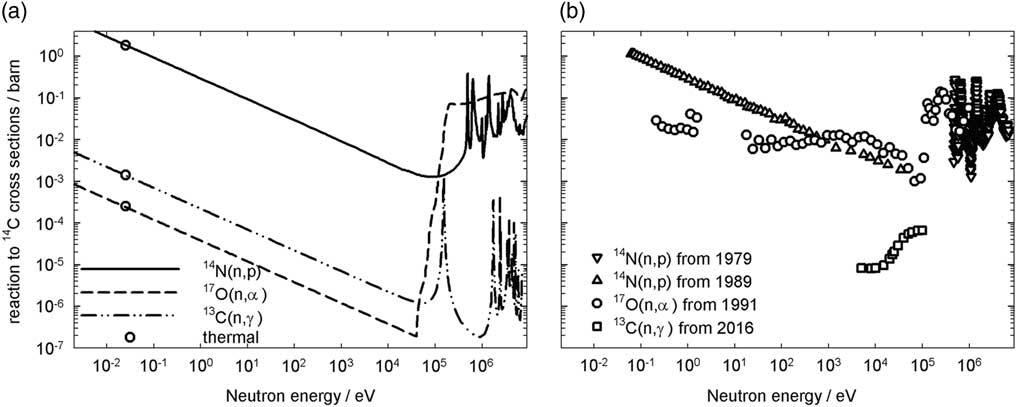
Figure 1 Neutron reaction to 14C cross sections at about 300 K from JEFF 3-2 (a) and EXFOR (b) (NEA 2017).
The natural abundances of 14N, 13C, and 17O are 99.64%, 1.07%, and 0.038% respectively. For scoping calculations, the thermal cross section is frequently used. 14N has the largest thermal neutron cross section as shown in Figure 1a. A combination of the natural abundance and neutron reaction cross sections indicates that the chemical contents of carbon and oxygen need to be respectively five and seven orders of magnitude larger than that of nitrogen in order to contribute the same 14C flux using the cross sections in JEFF-3.2. 14C is therefore mainly generated when nitrogen captures a neutron, because air is made up of 80% nitrogen. The reaction with 17O has been calculated to contribute 0.001% to the natural 14C generation rate using EXFOR database (Kovaltsov Reference Kovaltsov, Mishev and Usoskin2012). 13C has not been included in this calculation. Figure 1b shows the cross sections from EXFOR with more than one or two experimental measurements. At time of these calculations, 13C(n,γ)14C reaction cross section was not available in EXFOR and therefore probably excluded. The thermal cross section for 17O in EXFOR is about two orders of magnitude larger than JEFF 3-2 by which the contribution could be calculated as 0.001% instead of 0.00001%. Despite this difference, the 17O contribution remains negligible.
Neutron activation is also the only relevant process to determine the generated 14C in waste i.e. ternary fission products and decay of actinides such as radium and actinium provide negligible contributions to the 14C content in waste. The origin of neutrons between natural and artificial 14C is different; neutrons are generated by fission of actinides in a nuclear power plant. The energy of generated neutrons in a power plant is about 1 MeV. The operation of a power plant is focused on achieving a large thermal neutron flux, as the cross sections for fission of fissionable isotopes such as 235U are largest for thermal neutrons. Water has a high concentration of hydrogen atoms, and can therefore effectively reduce the energy of neutrons and is consequently frequently used as a moderator in these plants. The neutron flux in the core of a power plant is almost 1014 neutrons cm−2s−1 (Buckau Reference Buckau and Neeft2018) i.e. several orders of magnitude larger than the environmental neutron flux of about 10−3 cm−2s−1 at Earth’s surface. This difference in thermal neutron flux causes 14C to be present in waste at hazardous concentrations, even though nitrogen may only be present at impurity levels in materials used in a nuclear reactor. The nitrogen content in metals, neutron irradiated in a plant, is frequently not specified or measured but the potential 14C inventory in metals can be bounded. Examples of measured nitrogen contents in CAST are 17 ppm and 25 ppm in PWR Zircaloy-4 specimens from two Belgian nuclear reactors Tihange and Doel (Necib Reference Necib, Ambard, Bucur, Caes, Cochin, Fulger, Gras, Herm, Kasprzak, Legand, Metz, Perrin, Sakuragi and Suzuki-Muresan2018) and 0.04% for stainless steel from a Finnish surveillance capsule (Mibus Reference Mibus, Swanton, Suzuki-Muresan, Rodríguez Alcalá, Leganés Nieto, Bottomley, Herm, de Visser-Týnová, Cvetković, Sakuragi, Druyts and Heikola2017). Values twice of three times as large are reported within the CAST project e.g. by waste management organizations (WMOs) but the nitrogen content remains within one order of magnitude from these values.
Discharge
Not all generated 14C is present in the waste. The part that is discharged might change the main origin of 14C in waste. 14C generated by neutron activation of nitrogen is loosely bound for neutron irradiated graphite. This type of 14C might be released during reactor operations and therefore not be present in irradiated graphite destined for disposal as explained by the Spanish WMO for the Vandellós I, a graphite gas cooled reactor. Only 14C from neutron activation of 13C needs to be assumed to determine the 14C inventory (Buckau Reference Buckau and Neeft2018). For steel and Zircaloy, the diffusion of carbon within these metals at neutron irradiation temperatures in a nuclear plant is so small that it can be assumed that there is no redistribution of 14C in these metals i.e. the 14C activity concentration determined by neutron activation resembles the 14C activity concentration in waste. The only discharge from these irradiated metals is spalling of corrosion products in cooling and moderating water. Ion exchange resins are used to reduce the volume of waste by concentrating radionuclides from water. Not all 14C generated during reactor operations remains in the coolant, 14C discharge during reactor operations can be more than 90% (Capouet Reference Capouet, Boulanger, Vandoorne, Gaggiano, Norris, Williams, Schumacher, Rübel, Nummi, Poskas, Narkuniene, Grigaliuniene, Vokál, Mibus, Rosca-Bocancea, Hart, Ferrucci, Levizzari, Luce, Diaconu, Cuñado Peralta, Owada, Kienzler, Wieland, Van Loon and Walke2017). 14C can also be discharged from spent resins at specific storage conditions. These conditions are explained in the next section.
DISPOSAL OF 14 C CONTAINING WASTE
Carbon Speciation
The mitigation mechanisms within engineered and natural barriers to limit the exposure of 14C released from the waste depends on the carbon speciation. This speciation may strongly depend on the pH of water, redox potential and radiation.
pH
The pH value of pore waters in engineered barriers such as concrete and bentonite and the host rocks considered for geological disposal (clay, rock salt and granite) is at least 7. There are many carbon compounds but only a number of them have been measured in CAST. Table 1 shows the acidity constants of carbon compounds measured in CAST that have a typical pH dependent speciation.
Table 1 Acidity constants of carbon compounds measured in CAST (CRC 2015; McMurry Reference McMurry1992).

The acidity constants of carboxylic acids are usually less than 5. Consequently, carboxylic acids can only be present as their conjugate bases in barriers and at least one of the following mitigation mechanisms that involve charged species would act in barriers: reduced diffusion accessible porosity, sorption, ion-exchange and precipitation.
Some carbon compounds are unstable and dissociate into H2O and a carbon-containing gas e.g. carbonic acid and formic acid. For dissociated carbonic acid, CO2 is the predominant compound at a pH below 6.35. Air bubbling is used to homogenize spent resin slurry stored in tanks. The pH of fluids with spent ion exchange resins was measured to vary between 5 and 8 and 14CO2 was measured to be released at these storage conditions (Arensson Reference Aronsson, Lillfors-Pintér and Henning2016). The equilibrium concentration of carbon dioxide is 1.35×10−9 mmol/L at natural atmosphere pressure and 25ºC i.e. highly insoluble by which the inorganic 14C content in these resins can be significantly reduced by air bubbling. Also for formic acid, a fast dissociation into CO and H2O is proposed (McCollum Reference McCollom and Seewald2007) but a reduction in this organic carbon content requires a pH below 3.75 and therefore not expected at storage.
Redox Potential
Reduced 14C species are expected at alkaline pH and reducing conditions from thermodynamics. The identified carbon species released from steel are low molecular weight organics up to C5 (Wieland Reference Wieland and Hummel2015). At alkaline reducing conditions, dissolved organic carbon compounds have been measured to be the major 14C species released from neutron irradiated steel, except for specimens with the highest calculated 14C activity concentrations (Mibus Reference Mibus, Diomidis, Wieland and Swanton2018), neutron irradiated Zircaloy (Necib Reference Necib, Ambard, Bucur, Caes, Cochin, Fulger, Gras, Herm, Kasprzak, Legand, Metz, Perrin, Sakuragi and Suzuki-Muresan2018) and neutron irradiated graphite (Toulhoat Reference Toulhoat, Moncoffre, Narkunas, Poskas, Bucur, Ichim, Petit, Schumacher, Catherin, Capone, Shcherbina, Bukaemskiy, Alcalá, Magro, Márquez, Piña, Fachinger, Fugaru, Norris and Zlobenko2018). A quantification of released organic carbon into carboxylic acids, alcohols and aldehydes has not been completed within CAST but this quantification could reduce the conservatism in assessments since organic carbon species are assumed to be non-retarded species in safety assessments (Capouet Reference Capouet, Boulanger, Vandoorne, Gaggiano, Norris, Williams, Schumacher, Rübel, Nummi, Poskas, Narkuniene, Grigaliuniene, Vokál, Mibus, Rosca-Bocancea, Hart, Ferrucci, Levizzari, Luce, Diaconu, Cuñado Peralta, Owada, Kienzler, Wieland, Van Loon and Walke2017) but conjugate bases of carboxylic acids are charged species in which at least one of the four just described mitigation mechanisms within the barriers could act.
Radiation
Other radionuclides present in waste can contribute to radiolysis of pore water. During dissociation of water, hydrogen ions and hydroxyl radicals are generated (Dzaugis Reference Dzaugis, Spivack and D’Hondt2015). Radiolysis of water can have an impact on the 14C speciation since the OH radicals can decompose organic compounds. This decomposition is an oxidation by which CO2 and CO can be formed from organic carbon species. OH radicals are produced by chemicals such as potassium persulphate (K2S2O8) for the experimental determination of the organic 14C content in samples such as leachate solutions of irradiated Zircaloy corrosion as proposed by Magnusson (e.g. Bucur Reference Bucur, Fulger, Florea, Dobrin, Diaconescu, Tudose and Uta2017 and Sakuragi Reference Sakuragi2017), leachate solutions of irradiated graphite (e.g. Toulhoat Reference Toulhoat, Moncoffre, Narkunas, Poskas, Bucur, Ichim, Petit, Schumacher, Catherin, Capone, Shcherbina, Bukaemskiy, Alcalá, Magro, Márquez, Piña, Fachinger, Fugaru, Norris and Zlobenko2018), acidic solutions with spent ion exchange resins (e.g. Rizzato Reference Rizzato, Rizzo, Heisbourg, Večernik, Bucur, Comte, Lebeau and Reiller2015) and acidic solutions with dissolved irradiated steel and Zircaloy (Mibus Reference Mibus, Diomidis, Wieland and Swanton2018). The main dissolved carbon phase was inorganic carbon for the leachates in which neutron irradiated steel samples with the highest calculated 14C activity concentrations between 1.55–2.87×105 Bg/g solid matter were exposed to alkaline, reducing conditions (Visser Reference de Visser-Týnová, Stijkel, Swanton, Otlet and Walker2018). At the alkaline conditions representative for cementitious materials, there may be competition between CO2 gaseous release and deprotonation into its thermodynamically stable form CO3 2– by which this inorganic carbon could be oxidized organic carbon that was released during steel corrosion at reducing conditions. Oxidation by OH radicals of dissolved organic carbon species may also have occurred during the experimental investigations in CAST in which neutron irradiated steel and neutron irradiated Zircaloy were exposed portlandite solutions. Gases released from these solutions were measured to be organic gaseous carbon as well as carbon dioxide (Druyts Reference Druyts and Caes2018). Radiolysis may be the reason why both reduced and oxidized hydrocarbons can exist simultaneously in solutions in contact with corroding irradiated metals.
Processes Determining Speciation and 14 C Exposure
The types of waste investigated in CAST have a negligible heat production and processes at ambient temperature are therefore assumed to take place in an engineered barrier made of cementitious materials, natural barriers and biosphere. These processes can limit the 14C flux from the waste into the biosphere and can change the carbon speciation.
Biosphere and Natural Barriers
As described in the introduction, the minimum in natural 14C emanation rate from soil into air is in the order of 109 14CO2 molecules cm−2yr−1. Organic carbon species are however frequently measured to be released from waste at alkaline, reducing conditions and not 14CO2. Organic carbon species such as carboxylic acids, alcohols, aldehydes and gaseous methane are food sources for microbes; organic carbon is converted to inorganic carbon by microbes in the root zone and below. The University of Nottingham has investigated the extent of this conversion for the most fast expected migrating organic carbon species: methane; most radioactive methane migrating from a disposal facility is likely to be converted to 14CO2 in the soil (Lever Reference Lever and Vines2015). Consequently, any 14C species released from the waste and potentially reaching the biosphere can be assumed to enter air as 14CO2.
The natural barriers in a geological disposal system are the host rock and surrounding rock formations. There can be two mitigating mechanisms within the surrounding rock formations to limit the 14C flux into the biosphere: dilution and travel time. A common dilution factor is 104 for deep geological disposal of waste (IAEA 2003). Travel time in the surrounding rock formations is too specific for the geological setting and therefore no common values are available but it has been calculated for the clay host rock within CAST. Diffusion can assumed to be the main migrating process for 14C species since the pore water in natural barriers such as clay and salt—but also engineered barriers such as bentonite and concrete—is stagnant. Typical diffusion values for a non retarded tracer such as tritiated water (HTO) in clay perpendicular to the bedding plane are 0.6–1.5×10−11 m2s−1 for Opalinus Clay and less than 10−10 m2s−1 for Boom Clay (Mazurek Reference Mazurek, Gautschi, Marschall, Vigneron and Lebon2008). The 14C flux is reduced by more than 103 for a conservative tracer within a clay formation with a diffusion value of 10−10 m2s−1 (Capouet Reference Capouet, Necib, Schumacher, Mibus, Neeft, Norris, Nummi and Rübel2018). Dispersion in the host rock further reduces the 14C flux from waste into air but the factor depends on the disposal concept and a common value can therefore not be given. Consequently, the reduction factor in 14C flux by the natural barriers into the biosphere can be at least 107. In clays, the negatively charged clay minerals overlap other charges and conjugate bases of carboxylic acids have therefore smaller values for diffusion than similar sized neutral organic carbon species such as alcohols and aldehydes due to anion exclusion by which the anion accessible porosity is smaller than the accessible porosity for neutral species.
The 14C speciation within clay, salt and engineered barriers is not expected to be changed due to lack of microbial activity by space restriction. The viable microbial size is 0.2 μm and the connecting pore throat of clay host rocks such as Boom Clay is between 10 to 50 nm (Wouters et al. Reference Wouters, Janssen, Moors and Ley2016) and in well hydrated Portland based cement also a pore size between 10 and 50 nm has been found (Smart et al in NEA 2012). Microbes may stay in a dormant phase in clay and salt host rocks and engineered barriers.
Cementitious Materials
The initial redox potential in concrete depends strongly on the type of cement used for the production of engineered barriers. For ordinary portland cement (OPC), CaO is made by baking carbonate in limestone without specific control of the heating environment. Blast furnace slag (BFS) cement is made in reducing environments as a by-product of steel. OPC concrete lacks electroactive species and is therefore largely unbuffered, being slightly oxidizing after fabrication. Ingress of oxygen can be too slow to prevent a local reduction at the interface between corroding metal and concrete (Wang Reference Wang2013). Above all, the amount of oxygen trapped during fabrication of cementitious materials can oxidize only a negligible fraction of metals. BFS concrete contains small amounts of FeS2 and has therefore a reducing environment after fabrication. An oxygen penetration front into the concrete is observed as a loss of the blueish color in above ground civil infrastructure made with this BFS concrete. The oxygen exposing levels in underground facilities may be too small to reach the concrete-waste interface and reducing environments may be assumed at start of the disposal. In Finland and the Netherlands, BFS cement is used for waste processing (Buckau Reference Buckau, Bottomley and Neeft2016).
Typical values for diffusion in OPC paste for neutral tracers such as HTO are at room temperature 1.1–7.7×10−11 m2s−1 (Takiya Reference Takiya, Watanabe, Kozaki and Sato2015). These HTO diffusion values are maximum values, for blended cements the permeability is smaller than Portland based concrete due to the more refined pore structure (Jackson Reference Jackson, Mulcahy, Chen, Li, Li, Cappelletti and Wenk2017). With superplasticizers, concrete with a smaller water-cement ratio can be made resulting in a smaller permeability of concrete (Gascoyne Reference Gascoyne2002). The waste package will always be in an environment that changes eventually the chemical and physical properties e.g. the pH of concrete will reduce until the pH of the surrounding environment and the diffusion value may change. The evidence of the chemical resistance of engineered concrete on long timescales is available for example the Roman marine concrete structures that have either fully immersed in seawater or partially immersed in shoreline environments have remained intact for the last 2000 years (Jackson Reference Jackson, Mulcahy, Chen, Li, Li, Cappelletti and Wenk2017). The changing rate of chemical and physical properties of cementitious waste packages embedded in host rocks such clay and salt are expected to take place at a smaller rate because the pore water is stagnant. Figure 2 shows potential processes by cementitious materials that can reduce the 14C flux from waste into the surrounding environment.
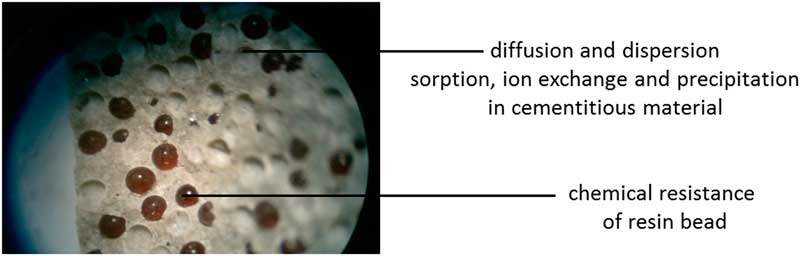
Figure 2 Cleavage fracture of a cementitious matrix with indicated processes for disposal that may reduce the potential 14C exposure. Resin beads are 0.5–2 mm.
The low diffusion values in especially blended cements may reduce the 14C flux from waste into the surrounding environment. The range in diffusion accessible porosity for neutral species is assumed to be the same for anionic species since the anionic species are not repelled by the positively charged cementitious mineral surfaces. The waste form is a fraction of the processed waste volume and dispersion will therefore also reduce the 14C flux.
Inorganic and organic carbon anionic compounds can be very insoluble and may precipitate in cementitious materials due to the high calcium content. Carbonate as well as oxalate will precipitate due to the low solubility products of calcium carbonate of 3.36×10−9 and calcium oxalate hydrate of 2.32×10−9 (CRC 2015). The dissolved calcium content at a pH of 12.5 is 20 mmol/L and at a pH of 10 about 0.5 mmol/L (Vehmas Reference Vehmas2017). This knowledge can be used to bound the potential dissolved amount of radioactive carbon; the maximum in 14CO3 2– is 4×102 Bq/g pore water i.e. 4×108 Bq/m3 in pore water at a pH of 12.5 and this content can be further reduced with knowledge of the non-radioactive released CO3 2–. For example, the non-radioactive carbon content is three orders larger of magnitude than the 14C content in steel (Neeft Reference Neeft2018).
The equilibrium activity concentration of 14C in pore water can be further reduced by sorption. The sorption of dissolved calcium on silanol sites overlaps the negative charge of calcium silicate hydrate surfaces by which uptake of anionic radionuclides can take place. Sorption is expressed as Rd values i.e. a ratio between adsorbed concentration on a solid divided by the dissolved concentration and Rd values for 14CO3 2– of more than 103 mL/g i.e. 1 m3/kg have been measured with batch sorption experiments (Pointeau Reference Pointeau, Coreau and Reiller2008). The conjugate bases of dicarboxylic acids have the same charge and are therefore expected to have the same behavior but experimental investigations have not yet been found. Sorption values for lower charged conjugate bases, formate and acetate, are available; Rd values are significantly smaller than carbonate: 1.1×10−3 m3/kg for formate and 3.3×10−3 m3/kg for acetate as determined with batch-type experiments. A fraction of formate was strongly bound, presumably ion-exchanged with SO4 2– in the ettringite structure. These results suggest that the small formate ion had access to further parts in the pore space which is not available for the larger acetate ion (Wieland Reference Wieland, Jakob, Tits, Lothenbach and Kunz2016). In other literature, Rd values are named Kd values, solid-liquid distribution coefficients and used to determine the retardation factors for diffusion (e.g. EPA 1999). The diffusion value then becomes about 5000 times for 14CO3 2–, 7 times for HCOO− and 3 times for CH3COO− smaller than HTO. Consequently, the majority of released 14CO3 2– from waste is expected to decay within cementitious materials provided that the pH of pore water remains high enough for sorption during the radionuclide hazardous period.
14 C SOURCE TERM
The neutron irradiated metals investigated in CAST have undergone a special chemical treatment in the nuclear plant and this treatment has an impact on the source term at start of disposal. Reducing conditions are preferred to limit the corrosion of metals during reactor operations; the oxygen content can be below 1 ppb in a PWR (Buckau Reference Buckau and Neeft2018). An iron-oxide layer of a few nm is present on the metallic surface after neutron irradiation (Mibus Reference Mibus, Swanton, Suzuki-Muresan, Rodríguez Alcalá, Leganés Nieto, Bottomley, Herm, de Visser-Týnová, Cvetković, Sakuragi, Jobbágy and Lavonen2015) and zirconia is also observed on Zircaloy (Gras Reference Gras2014). The 14C release from irradiated metals including this oxide-layer is being studied, to simulate the waste form conditions for disposal as closely as possible. Zirconia (Gras Reference Gras2014) and iron-oxide such as magnetite (Grenthe Reference Grenthe and Puigdomenech1997) are thermodynamically stable at a pH representative for cementitious conditions. The oxide layer on these metals found after neutron irradiation has a large impact on the corrosion rate since the corrosion process is bounded to equilibrium between diffusion of water through the oxide layer and dissolution at the solid-liquid interface. 14C can be incorporated in the metal-oxide layer and be released when metal-oxide dissolves. Table 2 summarizes the corrosion mechanisms to calculate the 14C release rate at start of disposal of neutron irradiated materials.
Table 2 14C release mechanisms and rates at start of disposal.

For steel, the 14C release rate is linearly related to the iron release rate. The iron release rate has not been measured in CAST but the 14C release rate is linearly related to the hydrogen release rate because hydrogen is not picked up by steel. An upper limit for corrosion of stainless steel during anaerobic corrosion at alkaline conditions is 0.01 μm per year (Mibus Reference Mibus, Diomidis, Wieland and Swanton2018). The surface areas of all steel materials are the largest for claddings when it is assumed that both sides of the cladding are exposed to cementitious pore water. It may take 70 half-lives of 14C to have disposed stainless steel claddings with a thickness of 0.45 mm as used in a research reactor (Conrad Reference Conrad1997) to be corroded. This is a larger period than the maximum of the radionuclide hazardous period of 18 half-lives of 14C. Consequently, the main hazardous 14C content decays within the waste form and is not released to the encapsulation, the cementitious material. At start of disposal, a reasonable 14C release rate is in the order of 1011 14C molecules per cm2 per year i.e. two orders of magnitude larger than the 14C emanation rate from soil. After three half-lives of 14C, the 14C release rate has reduced an order of magnitude.
For Zircaloy, the 14C release rate cannot be related to the hydrogen release rate because more than 90% of the hydrogen generated during anaerobic corrosion is picked up by Zircaloy (Sakuragi Reference Sakuragi2017). The corrosion rates from measured adsorbed and released hydrogen are as low as corrosion rates determined from released non-radioactive nickel and chromium: below 1 nm per year. The smaller corrosion rates measured with zirconium are attributed to the small solubility product of zirconia (Necib Reference Necib, Ambard, Bucur, Caes, Cochin, Fulger, Gras, Herm, Kasprzak, Legand, Metz, Perrin, Sakuragi and Suzuki-Muresan2018). Assuming a value of 1 nm per year and a 14C content of 104 Bq/g, a reasonable 14C release rate is in the order of 109 14C molecules per cm2 per year at start of disposal i.e. comparable to the 14C emanation rate from soil.
Graphite is chemically inert and therefore another release mechanism needs to be proposed than chemical corrosion. Common values for radiolytic corrosion for neutron irradiated graphite were 10−5 to 10−7 g per m2 per day (Toulhoat Reference Toulhoat, Narkunas, Zlobenko, Diaconu, Petit, Schumacher, Catherin, Capone, Lensa, Piña, Williams, Fachinger and Norris2015) i.e. 4×10−12 to 4×10−14 m per day using a density of 2250 kg per m3 for graphite. Consequently, the radiolytic corrosion rate ranges between 1.6 nm per year and 0.016 nm per year i.e. similar to or smaller than the corrosion rate of neutron irradiated Zircaloy. At start of disposal, 14C release rates from neutron irradiated graphite are expected to be similar to natural 14C emanation rate from soil but a radiolytic corrosion rate is expected to decrease on the long-term due to decay of radionuclides by which radiolysis of the pore water is reduced. Some “hot spots” (Toulhoat Reference Toulhoat, Moncoffre, Narkunas, Poskas, Bucur, Ichim, Petit, Schumacher, Catherin, Capone, Shcherbina, Bukaemskiy, Alcalá, Magro, Márquez, Piña, Fachinger, Fugaru, Norris and Zlobenko2018) i.e. intergranular pores with 14C containing gas, may cause some high 14C peaks and the presence of these spots depends on the chemical and temperature operated conditions of the neutron irradiated graphite.
Processed spent ion exchange resins are already disposed in European countries in near-surface facilities in Sweden, Finland, Hungary, Slovenia, Spain, and France (Buckau Reference Buckau, Bottomley and Neeft2016). The 14C activity concentration measured by the French and Swedish WMOs was in the order of 103 Bq/g wet resin (Capouet Reference Capouet, Boulanger, Vandoorne, Gaggiano, Norris, Williams, Schumacher, Rübel, Nummi, Poskas, Narkuniene, Grigaliuniene, Vokál, Mibus, Rosca-Bocancea, Hart, Ferrucci, Levizzari, Luce, Diaconu, Cuñado Peralta, Owada, Kienzler, Wieland, Van Loon and Walke2017) but orders of magnitude larger values have been measured by the Hungarian WMO (Buckau Reference Buckau, Bottomley and Neeft2016). Chemical reactor operations, for example control of air ingress and pH coolant controller, and storage conditions of spent resins, for example air bubbling and drying, all have an impact have an impact on the 14C content (Neeft Reference Neeft2018). The majority of the 14C activity concentration measured from resins in CAST was from resins that were unconditioned for waste processing (Reiller Reference Reiller2018). Attempts to measure 14C release from spent resins in alkaline media have been made in CAST but 14C has not been measured yet to be released in cementitious pore water. A 14C source term is therefore not given in this paper but chemical and microbial degradation of resins may be excluded as a 14C release mechanism due to the high microbial and chemical resistance. Resins are organic matter and are therefore considered as a potential food source for microbes but the usable energy for microorganisms would barely be sufficient to breakdown ion exchange resins (Abrahamsen Reference Abrahamsen, Arnold, Brinkmann, Leys, Merroun, Mijnendonckx, Moll, Polvika, Ševců, Vikman and Wouters2015). The microbial degradation in intact cementitious materials is also expected to be limited due to space restriction. The chemical resistance of resins is generally larger than inorganic ion exchangers and resins are therefore preferred (IAEA 2004). Chemical degradation of organic materials can be initiated by a nucleophilic attack of OH− ions on a carbon atom with a partial positive charge. Such carbon atoms are generally not present in polystyrene, the basic material for ion exchange resins (Loon Reference Van Loon and Hummel1995; Abrahamsen Reference Abrahamsen, Arnold, Brinkmann, Leys, Merroun, Mijnendonckx, Moll, Polvika, Ševců, Vikman and Wouters2015). A degradation rate of resins representative for the disposal conditions is therefore not available.
The release mechanism of 14C from resins may not be corrosion i.e. mass loss of the waste form as used for the neutron irradiated materials. Only specific carbon species can be concentrated by ion exchange resins namely as anions and anionic compounds. The anionic 14C is fixed to the functional groups and 14C release might require ion exchange. The common functional groups in anion exchangers bear nitrogen for example a tertiary amino group. The affinity typically increases with increasing charge on the exchanging anion and increasing atomic number (decreasing hydrated ionic radii). For anions, a typical series for affinity is (IAEA 2002):
14C as acetate has a lower affinity than oxalate. Note that inorganic carbon is not reported in this series but CO3 2− is expected to have a high affinity due to the large negative charge. Ingress of SO4 2− and Cl− might cause release of 14C containing species. For deep geological disposal and near surface disposal in caverns such as Finland and Sweden, a sufficient ingress of these anions might require several half-lives of 14C for concrete due to low diffusion values, retarded ingress by precipitation, for example Friedel and Kunzel salt (Seetharam Reference Seetharam and Jacques2015) and sorption to positively charged sites (Pointeau Reference Pointeau, Coreau and Reiller2008). Also for sorption on the positively charged sites of cementitious minerals, a typical affinity series can be made: Cl−<I−<CO3 2– i.e. Rd value measured for CO3 2– is larger than Cl−. If 14C release takes place by ion-exchange than the 14C-flux to the surrounding environment may be significantly reduced by sorption within cementitious minerals as an engineered barrier.
CONCLUSION
The maximum in 14C source terms of the neutron irradiated metals Zircaloy and steel at disposal in cementitious environments are, respectively, similar to and 100 times larger than the natural 14C emanation rate from soil into air, using a 14C activity concentration of 105 Bq/g iron and 104 Bq/g Zircaloy. The small 14C source terms are caused by the high chemical resistance of these metals at these chemical disposal conditions: high pH and reducing environment by which corrosion is bounded by diffusion of water through the thermodynamically stable metal-oxide layer and dissolution at the solid-liquid interface. The majority of 14C is not expected to be released but to decay within the waste form. The potential 14C release of neutron irradiated graphite and spent ion exchange resins cannot be determined with a chemical degradation process. The maximum in radiolytic corrosion rates for neutron irradiated graphite found in literature are comparable to Zircaloy in cementitious environments or smaller. Geological disposal of these neutron irradiated materials in clay or salt host rocks further reduces the 14C flux from the waste into air at least 107. Consequently, 14C flux from disposal of this waste into air is radiologically insignificant compared to the natural 14C emanation rate from soil.
ACKNOWLEDGMENTS
The research leading to these results has received funding from the Seventh Framework Programme FP7 (2007–2013) under grant agreement n°604779, the CAST project. The author wishes to thank all the participants of CAST workshops for regulators, waste management organizations and waste generators, the careful review and feedback on the overviews by the CAST WP Leaders and the valuable comments from an anonymous reviewer and Neil Chapman on the manuscript of this paper. CAST reports can be downloaded from http://www/projectcast.eu until March 2023.






