INTRODUCTION
In Romania, two CANDU 600 (CANada Deuterium Uranium) reactors are currently in operation at the Cernavoda site and two more CANDU units are foreseen to be commenced in the future. The Romanian strategy for spent fuel and radioactive waste management foresees that the CANDU spent fuel (SF) will be disposed of together with other long-lived radioactive waste (such as non-fuel contact spent ion exchange resins, spent filters, pressure tubes and calandria tubes) in a geological disposal, foreseen to be operational in 2055.
CANDU fuel assembly contains 37 elements made of sintered UO2 pellets (natural uranium) in Zy-4 tubes. The 37 elements are circularly arranged on three rings of 18, 12, and 6 elements respectively, around a central element. CANDU units are designed such as to load and unload their fuel continuously at full power by disconnecting individual pressure tubes. In CANDU nuclear fuel, Zy-4 is used as fuel cladding, and also for end-caps, end support plate, inter element spacers, and pads.
Due to its long half-life (T1/2 = 5730 yr), high mobility in groundwater system and easy incorporation into man via the food-chain, 14C is a radionuclide of concern from a safety point of view. In a nuclear reactor, 14C is generated mainly by neutron activation of stable nitrogen (14N), oxygen (17O) and carbon (13C) isotopes that are present in the fuel, core structural materials, and in reactor coolant and are activated by the neutrons generated from nuclear fission reactions (Yim and Caron Reference Yim and Caron2006).
A particularity of CANDU fuel is the colloidal graphite (CANLUB) deposed on the inside surface of the Zy-4 rods (the average thickness of the graphite layer is 5 μm with a minimum of 3 μm). Therefore, the 14C in CANDU SF claddings is a combination of the 14C formed or attached on the oxide layer, the 14C formed in the Zy-4 metal (mainly from activation of nitrogen impurities) but also small amount of 14C formed in the graphite layer.
MATERIALS AND METHODS
The irradiated Zy-4 samples were obtained from a CANDU spent fuel bundle (GK964F1) irradiated for 1 year in the Cernavoda Unit 2. After a cooling period of around 4 years in the spent fuel cooling pool at the Cernavoda site, the fuel bundle was transferred to RATEN ICN and some fuel elements were extracted for different investigations inside the ICN hot cells. A tube of 10 cm length from one spent fuel element was cut in 6 pieces of around 15 mm long each. After the spent fuel was mechanically removed from these 6 irradiated Zy-4 samples, the Zy-4 tubes were washed in 4M nitric acid (3 washing cycles) and cleaned with deionized water. After air drying, the contact dose rate was between 0.45 and 0.60 mSv/h, below the acceptable limit to carry out further experiments in a fume hood outside the hot cells.
To have real measurements on the 14C content in the irradiated Zy-4 samples prepared for leaching/corrosion tests, each of these 6 samples was further cut into two pieces, one of around 2 mm long for 14C measurement and one of around 8 mm long for leaching/corrosion tests (Table 1). Two more small rings originating from the same spent fuel element were also available and used in the leaching/corrosion tests.
Table 1 Mass and length of the irradiated Zy-4 samples.

The non-irradiated Zy-4 samples were cut from as-received Zy-4 tube with external diameter of 13 mm and wall thickness of 0.4 mm. To obtain a similar oxide film as irradiated Zy-4 claddings, the non-irradiated Zy-4 samples were oxidized in static isothermal autoclaves in representative conditions to CANDU primary circuit (LiOH solution, pH = 10.5, T = 310ºC, p = 100 atm). A target value of 2.5 μm was established for the oxide thickness in order to investigate the post-transition regime and complete the current knowledge on the corrosion kinetics at low temperature.
Leaching and Corrosion Tests
To evaluate the 14C release from the irradiated Zy-4 under chemical conditions relevant to cementitious environment, static leaching tests were performed. To assess the behavior of non-irradiated Zy-4 in the same chemical conditions, leaching tests were also carried out on non-irradiated Zy-4 samples. The corrosion rate was measured both for non-irradiated and irradiated Zy-4 samples by electrochemical methods (accelerated corrosion tests).
Static Leaching Tests
Leaching tests were carried out in static conditions (individual samples prepared for each time established to measure the 14C released in liquid and gas phase: 18 days, 6, 8, 10, 12, and 18 months). Sodium hydroxide (NaOH) 0.01 M was chosen as leachant solution (pH = 12, total dissolved salts of 1100 mg/L, and conductivity of 2.20 mS/cm) relevant for cementitious environment. Even cement pore water contains important amount of dissolved Ca, calcium hydroxide was not chosen as leachant solution to avoid 14C precipitation as carbonate.
Seven glass tubes with silicon lids were adapted to allow N2 purging in the space above the liquid level and after the irradiated Zy-4 samples and leachant solution were introduced in each tube the lids were connected to N2 gas for 48 hr to ensure anoxic conditions. For adequate shielding all the glass tubes with irradiated Zy-4 were placed inside a lead castle during the leaching tests.
At the time interval established to measure the 14C release, N2 gas was purged for 2 hr through selected leaching tube and outgases were washed through gas washing bottles for 14C absorption. After gas collection, the lid was opened, and solution was sampled for 14C measurement.
Method Used for 14C Measurement
As 14C is a pure β-emitter with a fairly low energy compared with the β energy emissions of the other radionuclides it may be difficult to quantify. For 14C measurement by liquid scintillation counting (LSC) it has to be separated and purified from other potential interfering radionuclides (such as 3H, 129I, 137Cs etc.) to get accurate results.
The analytical method used for inorganic and organic 14C measurement in irradiated Zy-4 is based on the acid dissolution and wet oxidation, adapted after the method developed by Magnusson et al. for 14C measurement in spent ion exchange resins and process waters (Magnusson et al. Reference Magnusson, Stenstrom and Aronsson2008).
The experimental setup (Figure 1) consists in a reaction vessel, a dropping funnel, a nitrogen supply and a vacuum pump, two gas washing lines with a catalytic furnace between them. An Erlenmayer flask (300 mL) with a three-hole rubber stopper (two for gas and separatory funnel inlets and one for gas outlet) was used as reaction vessel and a tap-water cooling loop, made of copper tubing that fit the outer side of the Erlenmeyer flask ensured the vapor condensation. The reaction vessel was placed on a heater with magnetic stirring.
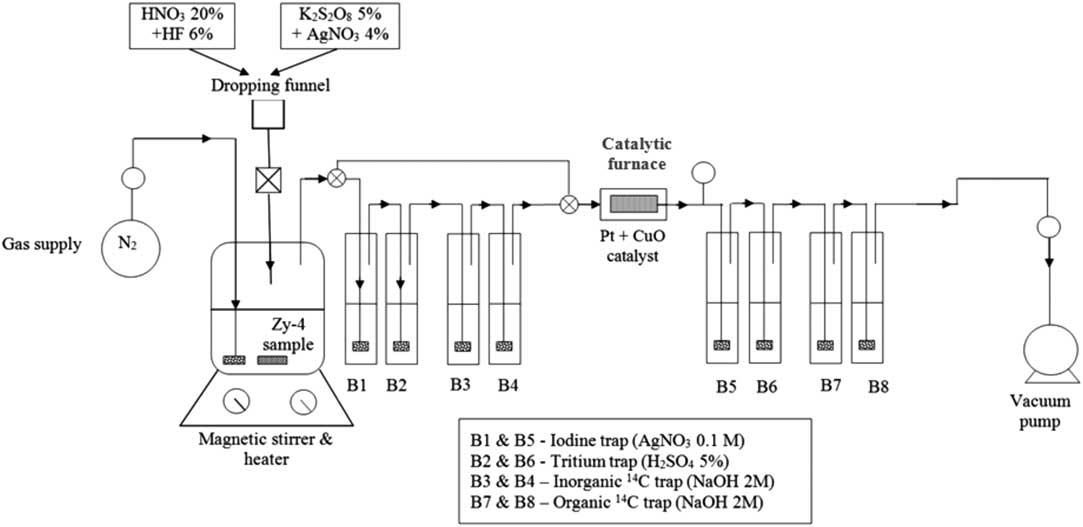
Figure 1 Experimental setup for separation of inorganic and organic 14C.
To ensure that no gases escaped from the system, all dissolution and wet oxidation experiments were carried out under vacuum (0.2 bar below atmospheric pressure) and the carrier gas (N2) was introduced into the system with a flow rate between 60 and 80 mL/min (controlled by a flow meter).
The Zy-4 dissolution was achieved by adding nitric acid (20% HNO3) and diluted fluoric acid (6% HF). Since the inorganic 14C compounds (i.e. carbonates and bicarbonates) are easily decomposed by weak acids to carbon dioxide, the inorganic 14C was released during this acid dissolution mainly as 14CO2 and carried out by the N2 gas and absorbed in the first alkaline gas washing bottles (B3 and eventually B4).The14C released as carbon oxide (14CO) or other organic molecules during acid dissolution, passes through the scrubbing bottles of the first gas washing line and it is oxidized to 14CO2 in the catalytic furnace and subsequently absorbed in the scrubbing bottles of the second gas washing line. After the acid dissolution step is accomplished the first gas washing line is isolated from the system by means of three-way valves placed before the first scrubbing bottle and the fourth one. The 14C released during wet oxidation step (as CO or CH4) are reduced to CO2 in the catalytic furnace and subsequently absorbed in the scrubbing bottles of the second gas washing line.
Three wet oxidation steps were carried out in order to ensure the complete decomposition of the organic 14C-labeled compounds, and the carrier gas was purged into the system for 1 hr in each wet oxidation step. This method distinguishes between 14CO2 released during acid dissolution and 14CO2 released by wet oxidation of hydrocarbons (alcohols, carboxylic acids…) allowing determination of the inorganic and organic fractions of 14C in irradiated Zy-4.
The 14C activity in the alkaline traps was measured by LSC using Hionic Fluor liquid scintillation cocktail with a ratio sample to scintillation cocktail of 1 to 10 mL. All samples were kept in darkness over night before their counting to allow for the chemiluminescence decay.
Aliquots from the reaction vessel solution, as well as from all scrubbing bottles were sampled for gamma measurements using an HPGe ORTEC detector and digiDart analyzer for spectra acquisition and Gamma Vision software for radionuclides quantification.
A similar procedure as the one described above was used for inorganic and organic 14C measurements in the alkaline solutions sampled from the leaching tests, except the acid used for inorganic 14C release. In the solution case, the mixture of nitric and fluoric acid used for Zy-4 dissolution and inorganic 14C release was replaced by concentrated sulfuric acid (H2SO4 6M).
Accelerated Corrosion Tests
The linear polarization resistance (LPR) method was selected to determine the uniform corrosion rate of the spent fuel claddings. This technique is fast and enables to detect the instantaneous corrosion rate and it was applied on the irradiated Zy-4 samples extracted from the leaching tests after sampling the gas and liquid phases for 14C measurements as well as on non-irradiated Zy-4 samples (with or without oxide layer on their surface).
A potentiostat/galvanostat AUTOLAB 302, with NOVA 1.11 corrosion software, was used for the electrochemical tests. The corrosion cell consists of a glass cell (90 mL), equipped with a five-holed lid: four holes for electrodes (Ag/AgCl reference electrode, two Pt counter electrodes, and the working electrode) and a hole for the bubbling tube. After the electrodes mounting, the vessel lid has been tight by adding resin or glue around them (see Jobbágy et al. Reference Jobbágy, Druyts, Sakuragi, Fulger, Bucur and Bottomley2015 for more details on experimental setup).
All electrodes and the bubbling tube were immersed into 30 mL NaOH 0.01M solution (pH = 12 and conductivity of 2.1 mS/cm). Before starting each experiment, the device was calibrated using an AUTOLAB Dummy Cell 2 and nitrogen gas was bubbled into solution for 2 hr at the beginning of each electrochemical test and also during the test. The electrochemical assembly was mounted into a Faraday cage during all electrochemical tests to protect the experimental setup against electromagnetic interferences from external sources.
Electrochemical tests were carried out both on non-irradiated and irradiated Zy-4 samples by scanning through a potential range around the corrosion potential (Ecorr). At the beginning of the test, around 2.5 cm2 from the Zy-4 sample was immersed in solution and kept for 10 min without electrical connection to stabilize the Ecorr.
The applied potentials were ± 10 mV and ± 25 mV vs. Ecorr using a scan rate of 0.16 mV/sec. The polarizing potentials are sufficiently small to avoid disruption of the oxide layer. Following polarization, the Tafel plots were generated using NOVA 1.11 software and corrosion rates were computed (ASTM G 102, 2004).
The Faraday’s law was used to calculate the corrosion rate (CR) and mass loss rate (MR), as expressed in Equations (1) and (2)
where: CR is the corrosion rate, mm/yr; MR is the mass loss rate, mg/dm2d (mdd); ρ is the Zy density, g/cm3; icorr is the corrosion current, μA/cm2; EW is the equivalent weight (dimensionless; for Zy-4 EW = 23); K1 and K2 are constants (K1 = 3.27E-03 mm × g/μA/cm/yr and K2 = 0.0895 mg × cm2/μA/dm2/d).
RESULTS AND DISCUSSION
14C Measurements in Irradiated Zy-4 Samples
From preliminary tests carried out to estimate the volumes of acids used in the dissolution step, it was determined that 20 mL of 20% HNO3 and 20 mL of 6% HF were enough to completely dissolve the Zy-4 matrix.
To optimize the experimental conditions and chemicals used for inorganic and organic 14C measurements, a bunch of acid dissolution/wet oxidation tests were carried out in controlled conditions, using non-irradiated Zy-4 samples immersed in solutions with known activities for the radionuclides of interest (14C in inorganic form as sodium carbonate/bicarbonate and organic forms as sodium acetate and lauric acid, 3H, 129I, 60Co, 137Cs, 241Am and 152Eu). With these tests, the 14C recovery, the reproducibility of the results and efficiency of acid dissolution/wet oxidation method to distinguish between inorganic and organic 14C species were evaluated. The results show that with this analytical method, good 14C recovery and efficient purification are obtained:
inorganic 14C recovery: between 96% and 99%, with an average value of 98% and standard deviation of 1.78
organic 14C recovery: between 94% and 98%, with an average value of 96% and standard deviation of 1.55
no gamma emitters were identified in the solutions sampled from alkaline gas washing bottles, and 14C specters were free of interferences.
An average memory effect of less than 1% was observed (no washing step was performed between the recovery step and the memory one).
The standard deviation of the results of six similar tests carried out in controlled conditions was less than 15%, proving the good reproducibility of the results.
Seven irradiated Zy-4 samples were used to evaluate the 14C content and its inorganic and organic ratio. For this, each irradiated Zy-4 tube was introduced in the reaction vessel together with a solution of 2 M NaOH (10 mL) and the analytical procedure described above was applied. The experimental results obtained for these samples (reported in Table 2) show that the irradiated Zy-4 used for leaching/corrosion tests contains around 2E + 04 Bq of 14C per gram of Zy-4, mainly as organic compounds.
Table 2 14C content in the irradiated Zy-4 samples used in leaching/corrosion tests.
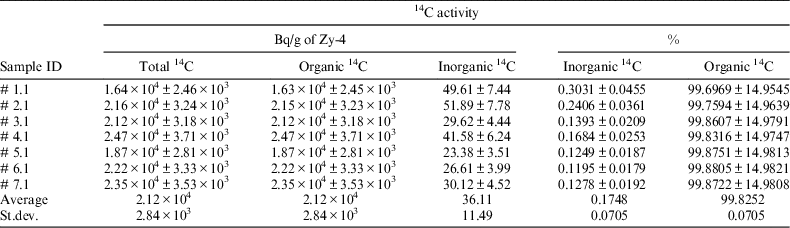
Total 14C content measured with this analytical method is in the same order of magnitude as the value estimated by modeling for an average burn-up of 7 MWd/kg U (1.78E + 04 Bq/g of Zy-4). Details on the modeling of the 14C accumulation during CANDU fuel irradiation are given in CAST D3.13 report (Necib et al. Reference Necib, Bottomley, Ali Bahri, Broudic, Bucur, Caron, Cochin, Druyts, Fulger, Herm, Jobbagy, Kasprzak, Legand, Iorgulis, Metz, Perrin, Sakuragi, Suzuki-Muresan and Tanabe2016).
Since this method also allows measurement of tritium content and of other gamma emitters, the concentration activity of these radionuclides in irradiated Zy-4 samples was measured (see Table 3) in solutions sampled from the acidic scrubbing bottles (for tritium) and from the reaction vessel (for gamma emitters).
Table 3 Radionuclide content in the irradiated Zy-4 samples used in leaching/corrosion tests.
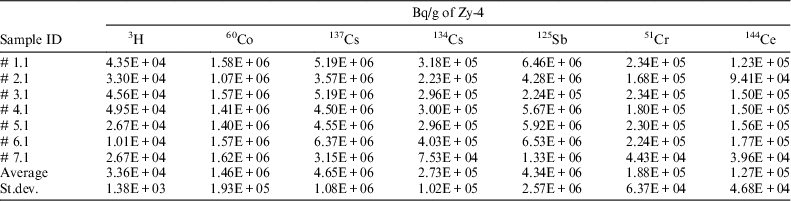
The experimental results for gamma emitters measured in the solution obtained after the irradiated Zy-4 dissolution are similar with those measured directly on the small ring cut from the spent fuel tube. The preliminary acid dissolution/wet oxidation tests carried out in controlled conditions as well as the results obtained for gamma emitters show that the analytical method used for inorganic and organic 14C allow a very good recovery of the radionuclides contained by the analyzed sample.
14C Release under Alkaline Conditions
Static leaching tests were carried out over a period of 18 months, starting from April 2016. Individual irradiated Zy-4 samples were prepared for each sampling interval (7 samples in total). Before opening the leaching vessels to sample the liquid phase for 14C measurement, N2 gas was purged in the space above the liquid level and the outgases were washed through gas washing bottles similar with those comprising the gas washing lines in the acid dissolution/wet oxidation method. By this means, the 14C released in gaseous phase was quantified.
The amount of 14C released in liquid phase was measured after 18 days, 6 months, 8 months, 12 months, and 18 months of immersion in alkaline solution, applying the analytical procedure described above in this paper. The measured 14C activities (both as total 14C as well as inorganic and organic fractions) in liquid phase are reported in Table 4.
Table 4 The amount of 14C released in liquid phase, Bq/g of Zy-4.
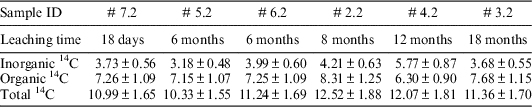
Beside the 14C released as soluble species during leaching tests, an amount of 4.99 Bq/g of Zy-4 was measured in gaseous phase, as inorganic 14C species (no organic 14C in gas phase was measured).
As it can be observed from the above reported data, similar amount of 14C released in liquid phase was measured after 18 days and 6, 8, 12, and 18 months (an average of 11.42 Bq of 14C /g of Zy-4). These data suggest that very small fraction of 14C is available as instant release, this fraction representing only 0.077% (considering both soluble and gaseous 14C species) from the initial 14C content in irradiated Zy-4. Since the irradiated Zy-4 samples used for leaching tests were pre-washed in 4M nitric acid before their use in the leaching tests, the 14C that was absorbed from the moderator on the oxide layer of CANDU fuel during its irradiation was most likely released during these pre-washed cycles. Consequently, the amount of 14C released during the leaching tests could come from the fresh Zy-4 metal from the edges of the samples (created by sample cutting) exposed directly to the leaching solution.
By acid stripping/wet oxidation tests carried out on the leachant solutions it was determined that the 14C released in liquid phase is preponderant as organic species (around 64% of the 14C released in solution was in the organic form).
The scanning electron microscopy (SEM) investigations carried out on the irradiated Zy-4 sample after its immersion for 6 months in NaOH solution (Figure 2), evidence large cracks on the oxide surface of irradiated Zy-4 generated probably by the machining of the irradiated Zy-4 samples from the tube. Therefore, fresh Zy-4 metal was likely exposed to the alkaline solution allowing 14C release.

Figure 2 SEM images on irradiated Zy-4 samples: not-immersed in NaOH solution (top) and immersed in NaOH solution for 6 months (bottom).
SEM analyses were also carried out on another irradiated Zy-4 sample originating from the same spent tube and not used in the leaching tests. Similar cracks were also observed on this sample confirming that the observed cracks were not induced by sample immersion in NaOH solution.
Corrosion Rates Estimated from Electrochemical Tests
Electrochemical tests were carried out both on non-irradiated and irradiated Zy-4 samples (after gas and liquid phases were sampled from leaching tests for 14C measurement).
Analyzing the Tafel plots obtained for the non-irradiated Zy-4 after 3 and 7 months of immersion in NaOH solution (Figure 3) it can be observed that the specific curve of the sample immersed for 3 months start from more cathodic potential indicating a slightly higher current value than the specific curve of sample immersed for 7 months.

Figure 3 Tafel plots obtained by LPR method for non-irradiated Zy-4 samples immersed in NaOH solution: for 3 months (red curve) and 7 months (blue curve). (Please see electronic version for color figures.)
The values obtained for corrosion potentials (Ecorr), current densities (icorr), polarization resistance and corrosion rates, calculated from the Tafel plots achieved at applied potentials of ± 10 mV vs. Ecorr, are reported in Table 5.
Table 5 LPR results for non-irradiated Zy-4 samples, at imposed potentials of ± 10mV vs. Ecorr.
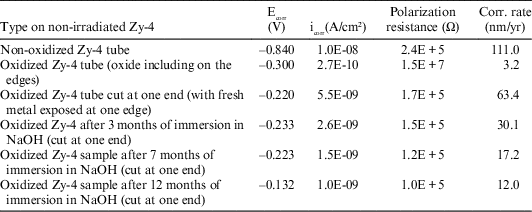
From the data reported in Table 5, it can be said that the highest value for the corrosion rate was obtained for the non-oxidized non-irradiated Zy-4 sample and the smallest one for the completed oxidized (including edges) Zy-4 sample. Reported to the oxidized Zy-4 sample that was cut at one end (exposing fresh metal at one edge), the corrosion rates obtained for the samples immersed in NaOH solution (to have a comparison with the irradiated Zy-4 samples used in the leaching tests) slowly decrease with increasing the immersing time. This behavior suggests that a thin oxide layer was formed on the fresh surface during immersion in NaOH solution, protecting the metal surface at the cutting edge.
SEM investigations were performed on the surface of the non-irradiated sample not immersed in NaOH solution and on the non-irradiated sample immersed for 7 months in NaOH solution and defects or scratches were evidenced on the oxide layers in both cases (Figure 4). Also, from SEM analysis, on an image taken at the edge part of the oxidized sample (the cutting edge) the presence of some cracks (probably resulting from samples machining) was put in evidence (Figure 4, right image).

Figure 4 SEM images of non-irradiated Zy-4 samples oxidized and cut at one end: non-immersed (left), immersed for 7 months in NaOH solution (middle) and on the cut part of oxidized Zy-4 sample (right).
For the irradiated Zy-4 samples, after 6, 8, 10, 12, and 18 months of immersion in NaOH solution, the values obtained for corrosion potentials (Ecorr), current densities (icorr), polarization resistance and corrosion rates, calculated from the Tafel plots (Figure 5) achieved at applied potentials of ± 10 mV vs. Ecorr, are reported in Table 6. The values for mass loss rate (MR), computed using Equation (2), are also reported in Table 6.
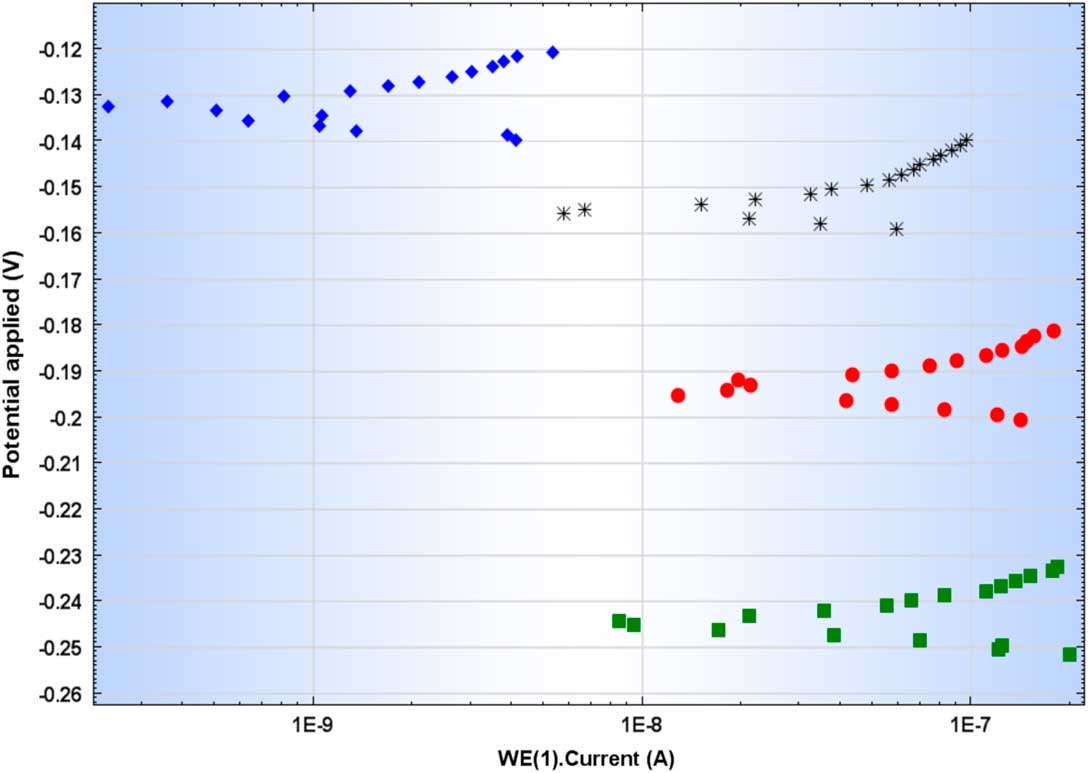
Figure 5 Tafel plots obtained by LPR method for irradiated Zy-4 samples.
Table 6 LPR results for irradiated Zy-4 samples, at imposed potentials of ± 10mV vs. Ecorr.

For all irradiated Zy-4 samples immersed in alkaline solution, the corrosion rates are higher than those obtained for non-irradiated Zy-4 (see Table 5), and for the sample immersed for 6 months in alkaline solution, the corrosion rate is similar with that obtained for non-irradiated and non-oxidized sample.
As for the non-irradiated Zy-4 samples, the corrosion rate decreased with increasing the immersion time. The lower value of the corrosion rate obtained after 12 and 18 months of immersion in alkaline solution may indicate that only after a longer period the fresh metal exposed on the edge and in the cracks generated by samples cutting are oxidized.
As observed by SEM investigations carried out on the irradiated Zy-4 samples (Figure 2), the oxide surface of the irradiated samples presents large cracks, which could have initiated because of the samples machining. The highest corrosion rates obtained for the irradiated Zy-4 samples after 6 months of static leaching in NaOH solution could be due to these cracks, by exposing Zy-4 alloy to the solution.
All values obtained by polarizing at ± 25mV vs. Ecorr were much higher than those obtained by polarization at ± 10mV vs. Ecorr (around one order of magnitude higher) and are not presented in this paper.
CONCLUSIONS
The irradiated Zy-4, originating from a CANDU Spent Fuel element, presents a 14C inventory measured experimentally of around 2E + 04 Bq per gram of Zy-4, mainly as organic compounds. This content is similar with the value determined by ORIGEN simulation for an average burn-up of 7 MWd/kgU.
The static leaching tests carried out on irradiated Zy-4 samples in alkaline solution (NaOH 0.1 M) indicate that a very small amount of 14C is available as fast release, both in gas and liquid phase, representing around 0.077% (considering both soluble and gaseous 14C species) of the initial 14C content in irradiated Zy-4. After 18 months of immersion in alkaline solution, the amount of 14C released in solution (as well as in gas phase) is similar with the values obtained after 18 days, and the 14C release is likely due to the cracks observed on the surface of the oxide layer (leading to the metal exposure to the leaching solution).
Linear polarization measurements indicated that the irradiated Zy-4 samples are characterized by higher corrosion rates than the non-irradiated samples, with corrosion rates between 46 and 113 nm/yr. In addition, these values decreased as the immersion time increases. Also, these high corrosion rates are assigned to the defects and cracks found in the oxides developed on the samples surface during irradiation.
ACKNOWLEDGMENTS
The results presented in this paper were achieved in the frame of CArbon-14 Source Term project funded by the European Union’s European Atomic Energy Community’s (EURATOM) Seventh Framework Programme FP7/2007-2013 under grant agreement no. 604779, the CAST project.













