Introduction
Biomolecular recognition plays key roles in many fundamental biological processes, including immune response, cellular signal transduction and so on (Nooren and Thornton, Reference Nooren and Thornton2003). Moreover, these processes are implicated in the development of numerous human diseases and serve as important drug targets (Ferreira et al., Reference Ferreira, Oliva and Andricopulo2016; Scott et al., Reference Scott, Bayly, Abell and Skidmore2016). Experimental techniques (Miura, Reference Miura2018) including X-ray crystallography, nuclear magnetic resonance (NMR) and cryo-electron microscopy (cryo-EM) have been applied to determine the bound structures of protein–small molecule, protein–peptide and protein–protein complexes. The number of experimental complex structures are significantly increased in recent years (Sussman et al., Reference Sussman, Lin, Jiang, Manning, Prilusky, Ritter and Abola1998). However, it is still rather time consuming and resource demanding to obtain high-resolution experimental structures. Moreover, the experimental structures often capture static pictures of protein complexes. Intermediate conformational states that could be relevant for drug design are usually difficult to probe using current experimental techniques.
Computational methods have been developed to model biomolecular recognition and predict the binding free energies and/or kinetic rates, including the widely used molecular docking (Morris et al., Reference Morris, Huey, Lindstrom, Sanner, Belew, Goodsell and Olson2009; Wang and Zhu, Reference Wang and Zhu2016; Porter et al., Reference Porter, Xia, Beglov, Bohnuud, Alam, Schueler-Furman and Kozakov2017; Ciemny et al., Reference Ciemny, Kurcinski, Kamel, Kolinski, Alam, Schueler-Furman and Kmiecik2018; Vakser, Reference Vakser2020), Brownian dynamics (Ermak and McCammon, Reference Ermak and McCammon1978; Gabdoulline and Wade, Reference Gabdoulline and Wade2001; Spaar et al., Reference Spaar, Dammer, Gabdoulline, Wade and Helms2006; Wieczorek and Zielenkiewicz, Reference Wieczorek and Zielenkiewicz2008; Votapka and Amaro, Reference Votapka and Amaro2015) and molecular dynamics (MD) simulations (Karplus and McCammon, Reference Karplus and McCammon2002; Basdevant et al., Reference Basdevant, Borgis and Ha-Duong2013; Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019; He et al., Reference He, Paul and Roux2021; Lamprakis et al., Reference Lamprakis, Andreadelis, Manchester, Velez-Vega, Duca and Cournia2021). Molecular docking has been widely used for predicting the holo structures of protein–ligand (Wang and Zhu, Reference Wang and Zhu2016), protein–peptide (Ciemny et al., Reference Ciemny, Kurcinski, Kamel, Kolinski, Alam, Schueler-Furman and Kmiecik2018) and protein–protein complexes (Vakser, Reference Vakser2020). Although significant improvements have been achieved in developments of the molecular docking algorithms, the accuracy of docking could be still limited, due to high system flexibility especially in docking of the peptides and proteins. Recently, deep learning techniques have been introduced into molecular docking to increase accuracy. One successful example is the AlphaFold-multimer (Evans et al., Reference Evans, O’Neill, Pritzel, Antropova, Senior, Green, Žídek, Bates, Blackwell, Yim, Ronneberger, Bodenstein, Zielinski, Bridgland, Potapenko, Cowie, Tunyasuvunakool, Jain, Clancy, Kohli, Jumper and Hassabis2022), which has significantly increased the accuracy of predicting protein–protein complex structures. However, one is still not able to predict biomolecular binding kinetics from molecular docking.
MD is a powerful technique for simulations of biomolecular structural dynamics (Karplus and McCammon, Reference Karplus and McCammon2002). Remarkable advances in computing hardware (e.g., the Anton supercomputer and GPUs) and software developments have significantly increased the accessible time scale of conventional MD (cMD) from hundreds of nanoseconds to hundreds of microseconds (Harvey et al., Reference Harvey, Giupponi and Fabritiis2009; Shaw et al., Reference Shaw, Maragakis, Lindorff-Larsen, Piana, Dror, Eastwood, Bank, Jumper, Salmon, Shan and Wriggers2010; Johnston and Filizola, Reference Johnston and Filizola2011; Lane et al., Reference Lane, Shukla, Beauchamp and Pande2013; Hollingsworth and Dror, Reference Hollingsworth and Dror2018; Shaw et al., Reference Shaw, Adams, Azaria, Bank, Batson, Bell, Bergdorf, Bhatt, Butts, Correia, Dirks, Dror, Eastwood, Edwards, Even, Feldmann, Fenn, Fenton, Forte, Gagliardo, Gill, Gorlatova, Greskamp, Grossman, Gullingsrud, Harper, Hasenplaugh, Heily, Heshmat, Hunt, Ierardi, Iserovich, Jackson, Johnson, Kirk, Klepeis, Kuskin, Mackenzie, Mader, McGowen, McLaughlin, Moraes, Nasr, Nociolo, O’Donnell, Parker, Peticolas, Pocina, Predescu, Quan, Salmon, Schwink, Shim, Siddique, Spengler, Szalay, Tabladillo, Tartler, Taube, Theobald, Towles, Vick, Wang, Wazlowski, Weingarten, Williams and Yuh2021). Notably, the latest Anton3 (Shaw et al., Reference Shaw, Adams, Azaria, Bank, Batson, Bell, Bergdorf, Bhatt, Butts, Correia, Dirks, Dror, Eastwood, Edwards, Even, Feldmann, Fenn, Fenton, Forte, Gagliardo, Gill, Gorlatova, Greskamp, Grossman, Gullingsrud, Harper, Hasenplaugh, Heily, Heshmat, Hunt, Ierardi, Iserovich, Jackson, Johnson, Kirk, Klepeis, Kuskin, Mackenzie, Mader, McGowen, McLaughlin, Moraes, Nasr, Nociolo, O’Donnell, Parker, Peticolas, Pocina, Predescu, Quan, Salmon, Schwink, Shim, Siddique, Spengler, Szalay, Tabladillo, Tartler, Taube, Theobald, Towles, Vick, Wang, Wazlowski, Weingarten, Williams and Yuh2021) has achieved the speed of hundreds of microseconds per day for ATPase and Satellite Tobacco Mosaic Virus (STMV) with total number of atoms ranging from 328 K to 1,067 K, which will significantly facilitate simulations of biomolecular recognition process. The cMD simulations have been widely applied to investigate biomolecular dynamics, including conformational change (Jensen et al., Reference Jensen, Jogini, Borhani, Leffler, Dror and Shaw2012), protein folding (Lindorff-Larsen et al., Reference Lindorff-Larsen, Piana, Dror and Shaw2011) and substrate binding (Shan et al., Reference Shan, Kim, Eastwood, Dror, Seeliger and Shaw2011; Dror et al., Reference Dror, Green, Valant, Borhani, Valcourt, Pan, Arlow, Canals, Lane, Rahmani, Baell, Sexton, Christopoulos and Shaw2013; Robustelli et al., Reference Robustelli, Piana and Shaw2020).
For small-molecule ligand binding, Shan et al. (Reference Shan, Kim, Eastwood, Dror, Seeliger and Shaw2011) observed spontaneous binding of the Dasatinib drug to its target Src kinase during tens of microseconds cMD simulations. However, no dissociation event was observed in the cMD simulations. Pan et al. (Reference Pan, Xu, Palpant and Shaw2017) performed tens of microseconds cMD simulations to successfully characterise repetitive binding and dissociation of six small-molecule fragments to the protein FKBP. Based on the large number of binding and dissociation events in the simulations, they were able to accurately calculate the binding free energies and kinetic rates. Remarkably, the binding free energies calculated from the cMD simulations agreed very well with those predicted from free energy perturbations (FEP) calculations. It is worth noting that the tested fragments were weak binders with affinities ranging from 200 μM to 20 mM. It is still challenging to simulate both binding and dissociation of typical small-molecule ligands of proteins (usually with higher binding affinities and slower dissociation rates) using cMD, although the ligand residence time (or dissociation rate) has recently been recognised to correlate better with drug efficacy (Schuetz et al., Reference Schuetz, De Witte, Wong, Knasmueller, Richter, Kokh, Sadiq, Bosma, Nederpelt, Heitman, Segala, Amaral, Guo, Andres, Georgi, Stoddart, Hill, Cooke, De Graaf, Leurs, Frech, Wade, De Lange, Ap, Muller-Fahrnow and Ecker2017). For protein–protein interactions, tens of microseconds cMD simulations were able to capture barnase binding to barstar (Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019). Accurate barnase binding rate (kon) was predicted based on multiple binding events captured in a total of ~440 μs Anton cMD simulations (Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019). However, it remains challenging to simulate dissociation of the barnase–barstar model system using cMD (Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019).
Weighted ensemble (Saglam and Chong, Reference Saglam and Chong2019) and Markov state model (MSM) (Plattner et al., Reference Plattner, Doerr, De Fabritiis and Noé2017) have been developed to improve the prediction of biomolecular binding thermodynamics and kinetics based on a large number of short cMD trajectories. The kinetic binding rate (kon) of the p53 peptide to the MDM2 protein was accurately predicted with weighted ensemble of a total amount of ~120 μs cMD simulations in implicit solvent (Zwier et al., Reference Zwier, Pratt, Adelman, Kaus, Zuckerman and Chong2016). Another weighted ensemble of a total of ~18 μs cMD simulations was able to accurately predict the barnase–barstar binding rate constant (kon) (Saglam and Chong, Reference Saglam and Chong2019). However, it is still challenging to model the slow protein/peptide dissociation processes with weighted ensemble simulations (Zwier et al., Reference Zwier, Pratt, Adelman, Kaus, Zuckerman and Chong2016; Saglam and Chong, Reference Saglam and Chong2019). MSM (Plattner and Noe, Reference Plattner and Noe2015; Paul et al., Reference Paul, Wehmeyer, Abualrous, Wu, Crabtree, Schöneberg, Clarke, Freund, Weikl and Noé2017; Plattner et al., Reference Plattner, Doerr, De Fabritiis and Noé2017) was able to simultaneously predict the binding and dissociation kinetics through longer aggregated cMD simulations. MSM built with 150 μs MD simulation data was used to accurately predict benzamidine–trypsin binding kinetics (Plattner and Noe, Reference Plattner and Noe2015). Based on a total of two millisecond cMD simulations of barnase binding to barstar, MSM was generated to predict intermediate structures, binding energies and kinetic rates that were consistent with experimental data (Plattner et al., Reference Plattner, Doerr, De Fabritiis and Noé2017). However, these calculations required very expensive computational resources.
Coarse-grained MD models have been developed to reduce the demand of computational resources and extend simulation time scales (Souza et al., Reference Souza, Thallmair, Conflitti, Ramirez-Palacios, Alessandri, Raniolo, Limongelli and Marrink2020, Reference Souza, Alessandri, Barnoud, Thallmair, Faustino, Grünewald, Patmanidis, Abdizadeh, Bruininks, Wassenaar, Kroon, Melcr, Nieto, Corradi, Khan, Domański, Javanainen, Martinez-Seara, Reuter, Best, Vattulainen, Monticelli, Periole, Tieleman, De Vries and Marrink2021). Souza et al. (Reference Souza, Thallmair, Conflitti, Ramirez-Palacios, Alessandri, Raniolo, Limongelli and Marrink2020) performed millisecond cMD simulations to capture the binding of diverse protein–ligand systems. Accurate binding free energies were predicted through the cMD simulations without a priori information (Souza et al., Reference Souza, Thallmair, Conflitti, Ramirez-Palacios, Alessandri, Raniolo, Limongelli and Marrink2020). Millisecond MD simulations with a useful coarse-grained model (PACE) were performed to characterise the binding mechanism of the intrinsically disordered Aβ peptides (Aβ17–42) to form Aβ fibril (Han and Schulten, Reference Han and Schulten2014). In addition, coarse-grained models could be incorporated into multiscale computational approaches to improve the efficiency and accuracy of ligand binding thermodynamics and kinetics calculations (Elber, Reference Elber2020; Jagger et al., Reference Jagger, Ojha and Amaro2020; Huang, Reference Huang2021). For example, simulation enabled estimation of kinetic rates (SEEKR; Votapka and Amaro, Reference Votapka and Amaro2015; Jagger et al., Reference Jagger, Ojha and Amaro2020) is a multiscale simulation approach combining MD, Brownian dynamics and milestoning for calculating receptor−ligand binding and dissociation rates. SEEKR has been shown to estimate binding kinetic rates with up to a factor of 10 less simulation time (Jagger et al., Reference Jagger, Ojha and Amaro2020).
Enhanced sampling methods have been developed to efficiently simulate biomolecular recognition. They could be generally divided into two categories depending on the usage of collective variables (CVs). The CV-based methods include the widely used steered MD (Kingsley et al., Reference Kingsley, Esquivel-Rodríguez, Yang, Kihara and Lill2016), umbrella sampling (Gumbart et al., Reference Gumbart, Roux and Chipot2013; Kingsley et al., Reference Kingsley, Esquivel-Rodríguez, Yang, Kihara and Lill2016; Joshi and Lin, Reference Joshi and Lin2019b), metadynamics (Antoszewski et al., Reference Antoszewski, Feng, Vani, Thiede, Hong, Weare, Tokmakoff and Dinner2020; Banerjee and Bagchi, Reference Banerjee and Bagchi2020), adaptive biasing force (ABF; Darve and Pohorille, Reference Darve and Pohorille2001; Darve et al., Reference Darve, Rodríguez-Gómez and Pohorille2008) and so on. These methods often use predefined CVs to effectively guide simulations. Thus, a priori knowledge of the system is required in CV-based enhanced sampling. Alternatively, when it is difficult to predefine CVs, CV-free enhanced sampling methods could be useful (Kamenik et al., Reference Kamenik, Linker and Riniker2022). These methods include replica exchange MD (Sugita and Okamoto, Reference Sugita and Okamoto1999; Sugita et al., Reference Sugita, Kamiya, Oshima, Re, Bonomi and Camilloni2019; Siebenmorgen and Zacharias, Reference Siebenmorgen and Zacharias2020), random acceleration molecular dynamics (RAMD; Nunes-Alves et al., Reference Nunes-Alves, Kokh and Wade2021), tempered binding (Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019), integrated tempering sampling (ITS; Yang et al., Reference Yang, Liu, Shao, Zhang and Gao2015; Shao and Zhu, Reference Shao and Zhu2019), scaled MD (Deb and Frank, Reference Deb and Frank2019), accelerated MD (aMD; Hamelberg et al., Reference Hamelberg, Mongan and McCammon2004), Gaussian accelerated MD (GaMD; Miao et al., Reference Miao, Feher and McCammon2015b; Wang et al., Reference Wang, Arantes, Bhattarai, Hsu, Pawnikar, Huang, Palermo and Miao2021) and so on. The above-mentioned methodological advances have enabled simulations of millisecond or even longer time scale processes. Here, we will briefly review recent efforts in modelling biomolecular recognition, especially characterisation of binding thermodynamics and kinetics.
Collective variable-based enhanced sampling
During CV-based enhanced sampling simulations, a potential or force bias is applied along certain CVs to facilitate energy barrier crossing events among different conformational states. Typical CVs include distances, angle, dihedral, path, eigenvectors generated from the principal component analysis, root-mean square deviation (RMSD) relative to a reference conformation (Bouvier and Grubmuller, Reference Bouvier and Grubmuller2007) and so on. The bias potential applied to the system is usually around several kcal/mol. Thus one is able to accurately recover the original free energy profiles.
Umbrella sampling has been applied to predict the ligand/peptide/protein binding and/or dissociation pathways and map the associated free energy landscapes (Gumbart et al., Reference Gumbart, Roux and Chipot2013; Joshi and Lin, Reference Joshi and Lin2019a; Sieker et al., Reference Sieker, Straatsma, Springer and Zacharias2008; You et al., Reference You, Tang and Chang2019). Metadynamics has been applied to investigate ligand/peptide/protein binding in terms of the binding kinetic rates (Casasnovas et al., Reference Casasnovas, Limongelli, Tiwary, Carloni and Parrinello2017; Sun et al., Reference Sun, Li, Shen, Li, Kang and Hou2017) and free energies (Saleh et al., Reference Saleh, Ibrahim, Saladino, Gervasio and Clark2017; Banerjee et al., Reference Banerjee, Mondal and Bagchi2018; Raniolo and Limongelli, Reference Raniolo and Limongelli2020; Wang et al., Reference Wang, Ishchenko, Zhang, Razavi and Langley2022a). Metadynamics simulations (Limongelli et al., Reference Limongelli, Bonomi and Parrinello2013; Tiwary and Parrinello, Reference Tiwary and Parrinello2013) have also been applied to investigate the thermodynamics and kinetics of benzamidine inhibitor binding to trypsin. Multiple metadynamics trajectories with a total of 5 μs simulations were obtained to predict the ligand unbinding pathways and dissociation rate constant (k off). The predicted k off (9.1 ± 2.5 s−1) was smaller than the experimental value (600 ± 300 s−1). Separate funnel metadynamics simulations predicted accurate of ligand binding free energies (−8.5 ± 0.7 kcal/mol) for the same system (Limongelli et al., Reference Limongelli, Bonomi and Parrinello2013). Infrequent metadynamics simulations with three carefully chosen CVs have successfully predicted the peptide binding and dissociation rates for the system of p53-MDM2 -(Zou et al., Reference Zou, Zhou, Wang, Kuang, Ågren, Wu and Tu2020). Although these methods have shown remarkable improvements in capturing rare events that happen over exceedingly long timescales, users often face a challenge for defining CVs, which requires expert knowledge of the studied systems (Abrams and Bussi, Reference Abrams and Bussi2014; Zuckerman, Reference Zuckerman2011). Additionally, the predefined CVs could constrain the sampling space, leading to slow convergence of the simulations and suffering from “hidden energy barrier” once important CVs were missed during the simulation setup (Bešker and Gervasio, Reference Bešker, Gervasio and Baron2012). To accelerate the convergence of simulations, replica exchange or parallel tempering methods have been incorporated into metadynamics. For example, bias-exchange metadynamics simulations with eight CVs have been performed to predict accurate binding free energy of the p53 peptide to the MDM2 protein. Parallel tempering metadynamics simulations with well-tempered ensemble (PTMetaD-WTE) successfully captured the binding and dissociation processes of insulin dimer (Antoszewski et al., Reference Antoszewski, Feng, Vani, Thiede, Hong, Weare, Tokmakoff and Dinner2020). In summary, by carefully defining reaction coordinates, the CV-based enhanced sampling methods could efficiently and accurately predict binding free energies and kinetic rates.
Enhanced sampling without predefined collective variables
In CV-free enhanced sampling methods, bias is often applied on generalised properties of the system (such as the potential energy and atomic forces) in the simulations. Repetitive benzamidine binding and unbinding in trypsin were captured using the selective ITS method (Yang and Qin Gao, Reference Yang and Qin Gao2009; Yang et al., Reference Yang, Liu, Shao, Zhang and Gao2015; Shao and Zhu, Reference Shao and Zhu2019). Pan et al. (Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019) developed the tempered binding method, which significantly accelerates the slow protein dissociation process by dynamically adjusting electrostatic and van der Waals interactions between different groups of protein atoms by a factor λ. The tempered binding simulations have successfully captured repetitive binding and dissociation events for five diverse protein–protein systems (Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019). In the scaled MD simulations (Sinko et al., Reference Sinko, Miao, De Oliveira and McCammon2013), a scale factor ranging from 0 to 1 is introduced to smoothen the potential energy surface. Schuetz et al. (Reference Schuetz, Bernetti, Bertazzo, Musil, Eggenweiler, Recanatini, Masetti, Ecker and Cavalli2018) performed scaled MD simulations to accurately predict the residence time and drug dissociation pathways of different inhibitors of heat shock protein 90 (Hsp90). In a recent study, Bianciotto et al. (Reference Bianciotto, Gkeka, Kokh, Wade and Minoux2021) used scaled MD simulations to predict the residence time and ligand unbinding pathways for a set of 27 ligands of Hsp90 protein, being highly consistent with experimental data. Deb and Frank (Reference Deb and Frank2019) developed a selective scaled MD simulation method, where specific energy terms are scaled to promote dissociation of bound ligands from the protein. Particularly, ligand–water interactions are scaled to help the ligands dissociate from its bound state. Selective scaled MD predict accurate residence times and associated free energy change of three inhibitor drugs bound to cyclin-dependent kinase protein complexes. Hence, selective scaled MD proves to be an important enhanced sampling method for modelling biomolecular dissociation process.
In RAMD, an additional random force is applied on the ligand to promote especially the dissociation. In one recent study, Nunes-Alves et al. (Reference Nunes-Alves, Kokh and Wade2021) performed RAMD simulations to predict ligand dissociation rates of T4 lysozyme. The predicted kinetic rates agreed well with experimental values for various systems with different ligands, temperatures and protein mutations. Moreover, a ligand with complex dissociation pathways was often associated with longer residence time. In another study, the same group (Kokh and Wade, Reference Kokh and Wade2021) performed RAMD simulations to explore ligand dissociation pathways and kinetics of two GPCRs, i.e., the β2 adrenergic receptor (β2AR) and M2 muscarinic acetylcholine receptor (M2R). The ligand dissociation pathways observed in the RAMD simulations were similar to those in long cMD and metadynamics simulations. Additionally, RAMD revealed an allosteric modulation mechanism of the LY2119620 PAM in the M2R. Dissociation of the iperoxo agonist was blocked from one of the possible pathways and hence had increased residence time, being consistent with the experimental data.
The aMD enhanced sampling technique works by adding a non-negative boost potential to smooth the system potential energy surface (Voter, Reference Voter1997; Hamelberg et al., Reference Hamelberg, Mongan and McCammon2004). The boost potential (ΔV) decreases the energy barrier to facilitate the system cross different conformational states (Hamelberg et al., Reference Hamelberg, Mongan and McCammon2004, Reference Hamelberg, De Oliveira and McCammon2007). In one study, Kappel et al. (Reference Kappel, Miao and McCammon2015) performed aMD simulations to study ligand binding to M3 muscarinic receptor (M3R). Three ligands of the receptor: full agonist Ach, partial agonist arecoline (Arc) and antagonist tiotropium (TTP) were used to perform the aMD simulations. Starting from the bulk solvent, aMD captured the binding of Ach to the M3R orthosteric site in significantly less time as compared to the cMD simulations. The Arc was also observed binding to the orthosteric site whereas the TTP molecule bound to the extracellular vestibule of the receptor. Moreover, all ligands could bind to the extracellular vestibule of the receptor, suggesting the vestibule as metastable binding site for orthosteric ligands. However, aMD suffers from large energetic noise during reweighting as the boost potential is typically on the order of tens to hundreds of kcal/mol (Shen and Hamelberg, Reference Shen and Hamelberg2008).
GaMD is developed to apply a harmonic boost potential to enhance sampling with significantly reduced energetic noise. The boost potential normally exhibits a near Gaussian distribution, which enables proper reweighting of the free energy profiles through cumulant expansion to the second order (Miao et al., Reference Miao, Feher and McCammon2015b; Wang et al., Reference Wang, Arantes, Bhattarai, Hsu, Pawnikar, Huang, Palermo and Miao2021). GaMD has been successfully applied to simulate important biomolecular processes, including ligand/protein/RNA binding (Miao et al., Reference Miao, Caliman and McCammon2015a, Reference Miao, Huang, Walker, McCammon and Chang2018b; Miao and McCammon, Reference Miao and McCammon2016; Pang et al., Reference Pang, Miao, Wang and McCammon2017; Wang and Chan, Reference Wang and Chan2017; Chuang et al., Reference Chuang, Chiou, Cheng and Wang2018; Liao and Wang, Reference Liao and Wang2019; Wang et al., Reference Wang, Lan, Wu, Xu and Miao2022b), protein folding (Miao et al., Reference Miao, Caliman and McCammon2015a; Pang et al., Reference Pang, Miao, Wang and McCammon2017) and protein conformational changes (Miao and McCammon, Reference Miao and McCammon2016; Salawu, Reference Salawu2018; Zhang et al., Reference Zhang, Wang, Miao, Hauser, McCammon, Rappel and Schroeder2018). However, it remained challenging to simulate repetitive substrate binding and dissociation through normal GaMD (Miao and McCammon, Reference Miao and McCammon2018; Wang et al., Reference Wang, Arantes, Bhattarai, Hsu, Pawnikar, Huang, Palermo and Miao2021).
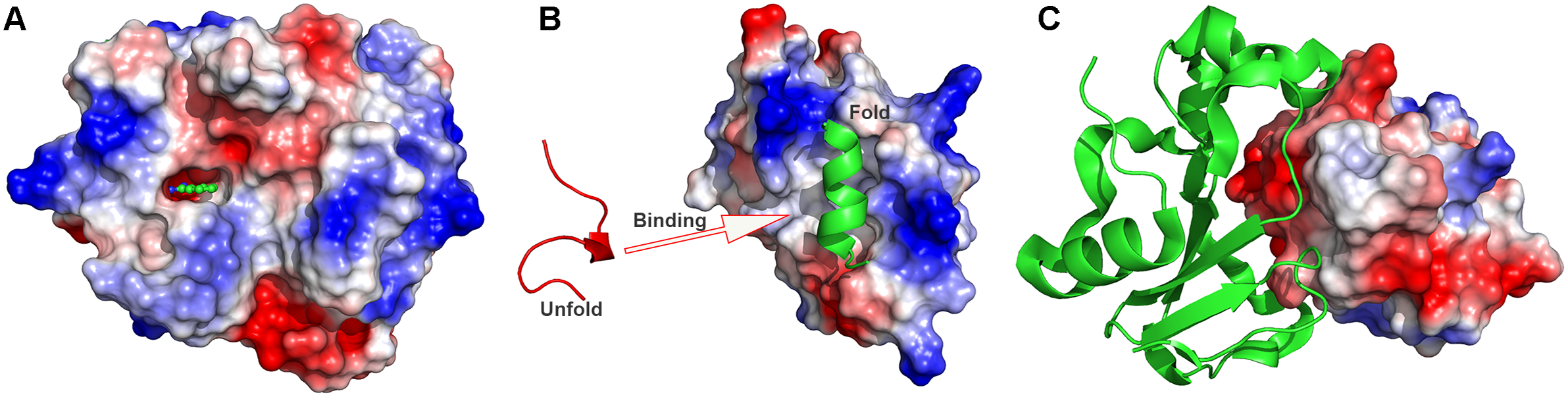
Recently, “selective GaMD” algorithms have been developed to allow for more efficient enhanced sampling of biomolecular binding and dissociation processes, including the Ligand GaMD (LiGaMD) (Miao et al., Reference Miao, Bhattarai and Wang2020), Peptide GaMD (Pep-GaMD; Wang and Miao, Reference Wang and Miao2020) and protein–protein interaction – GaMD (PPI-GaMD; Wang and Miao, Reference Wang and Miao2022). For simulations of biomolecular binding, the system contains substrate L (e.g. small-molecule ligands, peptides or ligand protein), protein P and the biological environment E. Therefore, the potential energy of system could be decomposed into the following terms:
![]() $ V(r)={V}_{P,b}\left({r}_P\right)+{V}_{L,b}\left({r}_L\right)+{V}_{E,b}\left({r}_E\right)+{V}_{PP, nb}\left({r}_P\right)+{V}_{LL, nb}\left({r}_L\right)+{V}_{EE, nb}\left({r}_E\right)+{V}_{PL, nb}\left({r}_{PL}\right)+{V}_{PE, nb}\left({r}_{PE}\right)+{V}_{LE, nb}\left({r}_{LE}\right) $
, where
$ V(r)={V}_{P,b}\left({r}_P\right)+{V}_{L,b}\left({r}_L\right)+{V}_{E,b}\left({r}_E\right)+{V}_{PP, nb}\left({r}_P\right)+{V}_{LL, nb}\left({r}_L\right)+{V}_{EE, nb}\left({r}_E\right)+{V}_{PL, nb}\left({r}_{PL}\right)+{V}_{PE, nb}\left({r}_{PE}\right)+{V}_{LE, nb}\left({r}_{LE}\right) $
, where
![]() $ {V}_{P,b} $
,
$ {V}_{P,b} $
,
![]() $ {V}_{L,b} $
and
$ {V}_{L,b} $
and
![]() $ {V}_{E,b} $
are the bonded potential energies in protein P, substrate L and environment E, respectively.
$ {V}_{E,b} $
are the bonded potential energies in protein P, substrate L and environment E, respectively.
![]() $ {V}_{PP, nb} $
,
$ {V}_{PP, nb} $
,
![]() $ {V}_{LL, nb} $
and
$ {V}_{LL, nb} $
and
![]() $ {V}_{EE, nb} $
are the self non-bonded potential energies in protein P, substrate L and environment E, respectively.
$ {V}_{EE, nb} $
are the self non-bonded potential energies in protein P, substrate L and environment E, respectively.
![]() $ {V}_{PL, nb} $
,
$ {V}_{PL, nb} $
,
![]() $ {V}_{PE, nb} $
and
$ {V}_{PE, nb} $
and
![]() $ {V}_{LE, nb} $
are the non-bonded interaction energies between P-L, P-E and L-E, respectively. In order to facilitate the ligand/peptide/protein binding (Fig. 1), a boost potential is selectively added on the essential energy terms (
$ {V}_{LE, nb} $
are the non-bonded interaction energies between P-L, P-E and L-E, respectively. In order to facilitate the ligand/peptide/protein binding (Fig. 1), a boost potential is selectively added on the essential energy terms (
![]() $ {V}_{select}(r) $
) in the LiGaMD, Pep-GaMD and PPI-GaMD, respectively. Presumably, ligand binding mainly involves the non-bonded interaction energies of the ligand. LiGaMD thus selectively boosts on the energy terms of
$ {V}_{select}(r) $
) in the LiGaMD, Pep-GaMD and PPI-GaMD, respectively. Presumably, ligand binding mainly involves the non-bonded interaction energies of the ligand. LiGaMD thus selectively boosts on the energy terms of
![]() $ {V}_{select}(r)={V}_{LL, nb}\left({r}_L\right)+{V}_{PL, nb}\left({r}_{PL}\right)+{V}_{LE, nb}\left({r}_{LE}\right) $
. In comparison, peptide binding involves in both the bonded and non-bonded interaction energies of the peptide since peptides often undergo large conformational changes during binding to the target proteins. Thus, the essential energy term in Pep-GaMD is
$ {V}_{select}(r)={V}_{LL, nb}\left({r}_L\right)+{V}_{PL, nb}\left({r}_{PL}\right)+{V}_{LE, nb}\left({r}_{LE}\right) $
. In comparison, peptide binding involves in both the bonded and non-bonded interaction energies of the peptide since peptides often undergo large conformational changes during binding to the target proteins. Thus, the essential energy term in Pep-GaMD is
![]() $ {V}_{select}(r)={V}_{LL,b}\left({r}_L\right)+{V}_{LL, nb}\left({r}_L\right)+{V}_{PL, nb}\left({r}_{PL}\right)+{V}_{LE, nb}\left({r}_{LE}\right). $
While protein–protein binding and unbinding processes mainly involve the non-bonded interaction energies between protein partners, one can apply a selective boost to the essential energy term
$ {V}_{select}(r)={V}_{LL,b}\left({r}_L\right)+{V}_{LL, nb}\left({r}_L\right)+{V}_{PL, nb}\left({r}_{PL}\right)+{V}_{LE, nb}\left({r}_{LE}\right). $
While protein–protein binding and unbinding processes mainly involve the non-bonded interaction energies between protein partners, one can apply a selective boost to the essential energy term
![]() $ {V}_{select}(r)={V}_{PL, nb} $
in PPI-GaMD. In addition to selectively boost the essential energy term
$ {V}_{select}(r)={V}_{PL, nb} $
in PPI-GaMD. In addition to selectively boost the essential energy term
![]() $ {V}_{select}(r) $
, another boost potential could be applied on the remaining energy of the system to facilitate substrate rebinding in a dual-boost scheme. These new algorithms have been implemented in the GPU version of AMBER22 (Case et al. Reference Case, Cheatham, Darden, Duke, Giese, Gohlke, Goetz, Greene, Homeyer, Izadi, Kovalenko, Lee, Legrand, Li, Lin, Liu, Luchko, Luo, Mermelstein, Merz, Monard, Nguyen, Omelyan, Onufriev, Pan, Qi, Roe, Roitberg, Sagui, Simmerling, Botello-Smith, Swails, Walker, Wang, Wang, Wolf, Wu, Xiao, York and Kollman2022).
$ {V}_{select}(r) $
, another boost potential could be applied on the remaining energy of the system to facilitate substrate rebinding in a dual-boost scheme. These new algorithms have been implemented in the GPU version of AMBER22 (Case et al. Reference Case, Cheatham, Darden, Duke, Giese, Gohlke, Goetz, Greene, Homeyer, Izadi, Kovalenko, Lee, Legrand, Li, Lin, Liu, Luchko, Luo, Mermelstein, Merz, Monard, Nguyen, Omelyan, Onufriev, Pan, Qi, Roe, Roitberg, Sagui, Simmerling, Botello-Smith, Swails, Walker, Wang, Wang, Wolf, Wu, Xiao, York and Kollman2022).

Figure 1. Schematic illustration of biomolecular recognition: (a) Small-molecule ligand binding, (b) peptide binding and (c) protein–protein interactions (PPIs).
Repetitive binding and dissociation of small-molecule ligands were captured in the LiGaMD simulations of host–guest and protein–ligand binding model systems (Miao et al., Reference Miao, Bhattarai and Wang2020), which enabled us to calculate ligand binding thermodynamics and kinetics calculations. Repetitive guest binding and dissociation in the β-cyclodextrin host were observed in hundreds-of-nanoseconds LiGaMD simulations. The binding free energies of guest molecules predicted from LiGaMD simulations agreed excellently with experimental data (< 1.0 kcal/mol error). In comparison with previous microsecond-timescale cMD simulations, accelerations of ligand kinetic rate constants in LiGaMD simulations were properly estimated using Kramers’ rate theory. Furthermore, microsecond LiGaMD simulations observed repetitive benzamidine binding and dissociation in trypsin. Trypsin–benzamidine ligand binding free energy was calculated from the 3D PMF profile to be −6.13 ± 0.35 kcal/mol, being highly consistent with the experimental value of −6.2 kcal/mol (Guillain and Thusius, Reference Guillain and Thusius1970). Similarly, the ligand binding and dissociation time periods were recorded to calculate the reweighted kon and koff values to be 1.15 ± 0.79 × 107 M−1·s−1 and 3.53 ± 1.41 s−1, respectively. These data were comparable to the values calculated from experiments (Guillain and Thusius, Reference Guillain and Thusius1970).
Pep-GaMD (Wang and Miao, Reference Wang and Miao2020) has been demonstrated on binding of three model peptides to the SH3 domains (Ball et al., Reference Ball, Kuhne, Schneider-Mergener and Oschkinat2005; Ahmad and Helms, Reference Ahmad and Helms2009), including “PAMPAR” (PDB: 1SSH), “PPPALPPKK” (PDB: 1CKA) and “PPPVPPRR” (PDB: 1CKB). Repetitive dissociation and binding of the three peptides were successfully captured in each of the 1 microsecond Pep-GaMD simulations. The peptide binding free energies calculated from Pep-GaMD simulations were in excellent agreements with those from the experiments. For the 1CKA system, the calculated peptide binding free energy value was −7.72 ± 0.54 kcal/mol, being highly consistent with the experimental value of −7.84 kcal/mol (Wu et al., Reference Wu, Knudsen, Feller, Zheng, Sali, Cowburn, Hanafusa and Kuriyan1995). For the 1CKB system, the predicting binding free energy was −6.84 ± 0.14 kcal/mol, being closely similar to the experimental value of −7.24 kcal/mol (Wu et al., Reference Wu, Knudsen, Feller, Zheng, Sali, Cowburn, Hanafusa and Kuriyan1995). In addition, the Pep-GaMD predicted the kon and koff of 1CKA as 4.06 ± 2.26 × 1010 M−1⋅s−1 and 1.45 ± 1.07 × 103 s−1, respectively. They were comparable to the experimental data (Xue et al., Reference Xue, Yuwen, Zhu and Skrynnikov2014) of
![]() $ {k}_{on}^{exp} $
= 1.5 × 109 M−1⋅s−1 and
$ {k}_{on}^{exp} $
= 1.5 × 109 M−1⋅s−1 and
![]() $ {k}_{off}^{exp} $
= 8.9 × 103 s−1.
$ {k}_{off}^{exp} $
= 8.9 × 103 s−1.
More recently, Pep-GaMD simulations were combined with complementary biochemical experiments to elucidate mechanism of tripeptide trimming of amyloid β-peptide (Aβ peptide) by γ-secretase (Bhattarai et al., Reference Bhattarai, Devkota, Do, Wang, Bhattarai, Wolfe and Miao2022). The active model of γ-secretase for ε cleavage was extracted from previous study (Bhattarai et al., Reference Bhattarai, Devkota, Bhattarai, Wolfe and Miao2020) and used as the starting structure for Pep-GaMD simulations. 600 ns Pep-GaMD simulations were able to capture the ζ cleavage activation starting from the ε cleavage activated model, which was suggested to carry out in timescale of minutes (Kamp et al., Reference Kamp, Winkler, Trambauer, Ebke, Fluhrer and Steiner2015). During activation, coordinated hydrogen bonds were formed between carbonyl oxygen of Aβ49 Val46 and enzyme catalytic Asp257. The two catalytic aspartates, Asp257 and Asp385 in the active site of the enzyme both formed hydrogen bonds with the water molecule aligned in between them. This activated enzyme conformation was well oriented for the ζ cleavage of amide between Val46 and Ile47 of the Aβ49. Three low energy states including “Final”, “Intermediate” and “Initial” were identified from the Pep-GaMD simulations ( Fig. 2a ). The Final state denoted the activated enzyme conformation for ζ cleavage where the Asp257–Asp385 distance was ~7–8 Å and the Asp257–Aβ49 Val46 distance was ~3 Å (hydrogen bond). The Initial and Intermediate low energy states denoted the starting and transitional conformation during the activation process. Furthermore, Pep-GaMD simulations were performed for three additional FAD mutant Aβ49 bound enzyme systems. Similar to the wildtype system, Pep-GaMD simulations of I45F, A42T and V46F mutant Aβ49 bound enzyme systems were able to capture the ζ cleavage activation starting from the ε cleavage activated model. Free energy profiles of the FAD mutant systems were similar to the wildtype system ( Fig. 2b–d). In the I45F mutant system, two low energy states were identified including “Initial” and “Final” (Fig. 2b). The A42T mutant was the most dynamic enzyme system with four distinct low energy states identified in a larger area covered free energy profile including “Initial”, “Final”, “Inhibited-1” and “Inactive” (Fig. 2c). The catalytic aspartates of the “Inhibited-1” conformational state were too close for activation and hence was inhibited. In contrast, the aspartates were too far for their catalytic activity in the “Inactive” low energy state of the enzyme. In the V46F mutant γ-secretase system, two low energy states were identified in the free energy profile including “Final” and “Inhibited-2” (Fig. 2d). The structures were compared between the “Initial” and “Final” low energy conformational states of the enzyme as identified from the free energy profiles (Fig. 2e–g). The enzyme moved from Initial to Final conformational state, the Aβ49 substrate tilted by ~50° (Fig. 2f). Unwinding of helix was observed in the C-terminus of Aβ49 where residues Val44 and Ile45 were observed changing their conformation from helix to a loop (Fig. 2f). Similarly, in the active site of the enzyme, the protonated Asp257 in the Final state was observed moving forward towards the substrate scissile amide bond by 3 Å in comparison to the Initial state (Fig. 2g). In contrast, the deprotonated Asp385 in the Final state and the Initial state were observed in a similar conformation (Fig. 2g). The simulation findings were highly consistent with biochemical experimental data. Taken together, complementary biochemical experiments and Pep-GaMD simulations have enabled elucidation of the mechanism of tripeptide trimming of Aβ49 by γ-secretase.

Figure 2. Mechanism of tripeptide trimming of amyloid β-peptide 49 by γ-secretase. 2D free energy profiles calculated regarding Asp257 - Asp 385 distance and Asp257 – Aβ49 Val46 distance calculated from Pep-GaMD simulations of (a) wildtype Aβ49 bound γ-secretase, (b) I45F mutant Aβ49 bound γ-secretase, (c) A42T mutant Aβ49 bound γ-secretase and (d) V46F mutant Aβ49 bound γ-secretase systems. (e) Structures of catalytic subunit PS1 bound to APP and Aβ49 substrates representing the “Initial” and “Final” conformational states, respectively. (f) Conformational changes in (f) Aβ49 and (g) active site of the enzyme during transition from Initial to Final activated state for ζ cleavage. Adapted with permission from Bhattari A, Devkota S, Do HN, Wang J, Bhattarai S, Wolfe MS and Miao Y. Journal of the American Chemical Society. 10.1021/jacs.1c10533. Copyright 2022 American Chemical Society.
PPI-GaMD (Wang and Miao, Reference Wang and Miao2022) has been demonstrated on a model system of the ribonuclease barnase binding to barstar. Six independent 2 μs PPI-GaMD simulations have successfully captured repetitive barstar dissociation and rebinding events (Fig. 3a). Five binding and six dissociation events were observed in both Sim1 and Sim3. In Sim2, three binding and four dissociation events were captured. For the remaining simulations (Sim4–Sim6), three binding and three dissociation events were observed (Fig. 3a). The barstar binding free energy predicted from PPI-GaMD was −17.79 kcal/mol with a standard deviation of 1.11 kcal/mol, being highly consistent with the experimental value of −18.90 kcal/mol (Schreiber and Fersht, Reference Schreiber and Fersht1993). In addition, the PPI-GaMD simulations allowed us to calculate the protein binding kinetics. The average reweighted kon and koff were predicted as 21.7 ± 13.8 × 108 M−1⋅s−1 and 7.32 ± 4.95 × 10−6 s−1, being highly consistent with the corresponding experimental values of 6.0 × 108 M−1⋅s−1 and 8.0 × 10−6 s−1, respectively. Furthermore, PPI-GaMD simulations have provided mechanistic insights into barstar binding to barnase, which involve long-range electrostatic interactions and multiple binding pathways (Fig. 3c–f), being consistent with previous experimental and computational findings of this model system. It is worth noting that at least three independent replicas of selective GaMD simulations with longer simulation lengths (e.g., microsecond) are required to obtain sufficient statistics for ligand binding, peptide binding and protein–protein interactions. In order to calculate accurate binding free energy and kinetic rates, the length of each simulation should be long enough to capture ≥3 binding and dissociation events as suggested by LiGaMD (Miao et al., Reference Miao, Bhattarai and Wang2020), Pep-GaMD (Wang and Miao, Reference Wang and Miao2020) and PPI GaMD (Wang and Miao, Reference Wang and Miao2022) studies.

Figure 3. PPI-GaMD simulations of barnase binding/dissociation to barstar. (a) Time courses of protein–protein interface distance calculated from six independent 2 μs PPI-GaMD simulations. (b) Original (reweighted) and modified (no reweighting) PMF profiles of the protein interface distance averaged over six PPI-GaMD simulations. Error bars are standard deviations of the free energy values calculated from six PPI-GaMD simulations. (c) 2D PMF profiles regarding the interface RMSD and the distance between the CZ atom of barnase Arg59 and CG atom of barstar Asp39. (d) 2D PMF profiles regarding the interface RMSD and the distance between the center of masses (COMs) of barnase residues Ala37-Ser38 and barstar residues Gly43-Trp44. (e,f) Low-energy conformations as identified from the 2D PMF profiles of the (e) intermediate “I1”, (f) intermediate “I2”. Strong electrostatic interactions are shown in red dash lines with their corresponding distance values labelled in the intermediate “I1” (e) and “I2” (f). Adapted with permission from Wang J, Miao Y. Journal of Chemical Theory and Computation. 10.1021/acs.jctc.1c00974. Copyright 2022 American Chemical Society.
Machine learning
Machine learning (ML) has been applied to improve computational docking, especially in the scoring functions (Khamis et al., Reference Khamis, Gomaa and Ahmed2015). A scoring function in molecular docking refers to a mathematical predictive model that outputs a representative score of the binding free energy of a bound conformation. Scoring of a docked complex is the final step of the three essential components in molecular docking, with the first two being chemical molecule representation and pose generation (Khamis et al., Reference Khamis, Gomaa and Ahmed2015). A reliable scoring function should have a good scoring power (the ability to produce scores for different binding poses), ranking power (the ability to correctly rank a given set of ligands with known binding poses when bound to a common protein) and docking power (the ability to identify the best binding pose of a given ligand from a set of computationally generated poses when bound to a specific protein; Ashtaway and Mahapatra, Reference Ashtaway and Mahapatra2012). Kinnings et al. (Reference Kinnings, Liu, Tonge, Jackson, Xie and Bourne2011) used a support vector machine (SVM) to derive a unique set of weights for each individual protein family – the wi’s in the following equation:
This was shown to improve the binding affinity prediction of the electronic high throughput screening (eHiTS) molecular docking software (Zsoldos et al., Reference Zsoldos, Reid, Simon, Sadjad and Johnson2007) compared with empirical knowledge-based scoring functions (Khamis et al., Reference Khamis, Gomaa and Ahmed2015). Similarly, a force field scoring function can be trained to derive a unique set of parameters for each individual protein family – the Aij’s and Bij’s in the following equation:
 $$ {E}_{binding}\hskip0.35em =\hskip0.35em \sum \limits_{i=1}^{ligand}\sum \limits_{j=1}^{protein}\left(\frac{A_{ij}}{r_{ij}^a}-\frac{B_{ij}}{r_{ij}^b}+332\frac{q_i{q}_j}{Dr_{ij}}\right) $$
$$ {E}_{binding}\hskip0.35em =\hskip0.35em \sum \limits_{i=1}^{ligand}\sum \limits_{j=1}^{protein}\left(\frac{A_{ij}}{r_{ij}^a}-\frac{B_{ij}}{r_{ij}^b}+332\frac{q_i{q}_j}{Dr_{ij}}\right) $$
ML could also be used to predict the binding affinity based on a number of features of the protein–ligand complex, including geometric features, physical force field energy terms, pharmacophore features, etc. Specifically, ML could learn the relationship between these features and corresponding known binding affinity to predict the binding affinity of new complexes (Khamis et al., Reference Khamis, Gomaa and Ahmed2015). Recently, Ballester and Mitchell (Reference Ballester and Mitchell2010) applied non-parametric ML techniques to generate the functional form of scoring functions given molecular databases. The authors used random forest (RF; Breiman, Reference Breiman2001) to learn the relationship between the atomic-level description of the complex and the experimental binding affinity. Here, the Kd and Ki measurements were merged into a single binding constant K to represent the experimental binding affinity. The atomic-level description used was of geometric nature and was the occurrence count of nine common elemental atoms (C, N, O, F, P, S, Cl, Br, I) type pair. Even though they completely neglected the energy terms induced by protein–ligand interactions, Ballester and Mitchell (Reference Ballester and Mitchell2010) were able to achieve Pearson correlation coefficient of 0.774 on the PDBbind v2007 core set (195 complexes).
Very recently, deep learning (DL) methods, including RoseTTAFold (Baek et al., Reference Baek, Dimaio, Anishchenko, Dauparas, Ovchinnikov, Lee, Wang, Cong, Kinch and Schaeffer2021) and AlphaFold (Jumper et al., Reference Jumper, Evans, Pritzel, Green, Figurnov, Ronneberger, Tunyasuvunakool, Bates, Žídek and Potapenko2021), were developed to achieve structure prediction accuracies far beyond those from classical force-field-based methods (Baek and Baker, Reference Baek and Baker2022). These methods have millions of parameters, much more than the hundreds of parameters in classical approaches, thus better sample the large conformational space of proteins. Furthermore, they make no assumptions about the functional form of the interactions between atoms. In fact, the two DL-based methods learn millions of parameters directly to generate correct 3D structures from input amino acid sequences (Baek and Baker, Reference Baek and Baker2022; Baek et al., Reference Baek, Dimaio, Anishchenko, Dauparas, Ovchinnikov, Lee, Wang, Cong, Kinch and Schaeffer2021; Jumper et al., Reference Jumper, Evans, Pritzel, Green, Figurnov, Ronneberger, Tunyasuvunakool, Bates, Žídek and Potapenko2021). AlphaFold and RoseTTAFold are trained to predict structures from alignments of homologous amino acid sequences. In particular, the two DL-based approaches learn to extract rich structural information through a three-track network where information at the 1D sequence level, 2D distance map, and 3D coordinate level is successively transformed and integrated (Baek et al., Reference Baek, Dimaio, Anishchenko, Dauparas, Ovchinnikov, Lee, Wang, Cong, Kinch and Schaeffer2021; Jumper et al., Reference Jumper, Evans, Pritzel, Green, Figurnov, Ronneberger, Tunyasuvunakool, Bates, Žídek and Potapenko2021). They were also shown to predict protein structures very accurately from single amino acid sequences (Baek and Baker, Reference Baek and Baker2022; Baek et al., Reference Baek, Dimaio, Anishchenko, Dauparas, Ovchinnikov, Lee, Wang, Cong, Kinch and Schaeffer2021; Jumper et al., Reference Jumper, Evans, Pritzel, Green, Figurnov, Ronneberger, Tunyasuvunakool, Bates, Žídek and Potapenko2021).
MD simulations could generate very large data in terms of conformation frames and number of simulated atoms. For example, weighted ensemble of the COVID19 spike protein’s closed-to-open state generated over 100 terabytes of data (Casalino et al., Reference Casalino, Dommer, Gaieb, Barros, Sztain, Ahn, Trifan, Brace, Bogetti, Clyde, Ma, Lee, Turilli, Khalid, Chong, Simmerling, Hardy, Maia, Phillips, Kurth, Stern, Huang, McCalpin, Tatineni, Gibbs, Stone, Jha, Ramanathan and Amaro2021). This brings a challenge to identify proper CVs to differentiate conformational states from the raw simulation data and to identify corresponding biologically transitions between such states (e.g., open/closed states of spike). In this regard, the ML/deep learning has been applied to identify appropriate CV to analysis MD simulation trajectories (Noé, Reference Noé2020; Wang et al., Reference Wang, Ribeiro and Tiwary2020; Glielmo et al., Reference Glielmo, Husic, Rodriguez, Clementi, Noé and Laio2021; Sun et al., Reference Sun, Vandermause, Batzner, Xie, Clark, Chen and Kozinsky2022). These linear, non-linear and hybrid ML approaches cluster the simulation data along a small number of latent dimensions to identify conformational transitions between states (Bernetti et al., Reference Bernetti, Bertazzo and Masetti2020; Ramanathan et al., Reference Ramanathan, Savol, Burger, Quinn, Agarwal and Chennubhotla2012). Another benefit of MD-coupled ML approaches is that the information learned from ML can be used to iteratively guide the MD sampling (Wang et al., Reference Wang, Ribeiro and Tiwary2019). Based on the predictive information bottleneck, Wang et al. (Reference Wang, Ribeiro and Tiwary2019) developed an approach to identify system reaction coordinates and calculate the free energy and kinetic rates in biomolecules. The algorithm was demonstrated on conformational transitions in the alanine dipeptide model system and ligand dissociation from the L99A T4lysome. Thermodynamic and kinetic quantities calculated from short enhanced MD simulations for slow biomolecular processes were in good agreement with the experiments and long unbiased MD simulations.
Recently, we have integrated the GaMD, Deep Learning and free energy prOfiling Workflow (GLOW) to predict important reaction coordinates and map free energy profiles of biomolecules (Do et al., Reference Do, Wang, Bhattarai and Miao2022). First, GaMD simulations are performed on the target biomolecules (Fig. 4a). The residue contact map is then calculated for each GaMD simulation frame and transformed into images (Fig. 4b). The specialised type of neural network for image classification, two-dimensional (2D) convolutional neural network (CNN), is employed to classify the residue contact maps of target biomolecules, from which important residue contacts are identified by classic gradient-based pixel attribution (Fig. 4c). Finally, the free energy profiles of these reaction coordinates are calculated through reweighting of GaMD simulations to characterise the biomolecular systems of interest (Fig. 4d; Do et al., Reference Do, Wang, Bhattarai and Miao2022). GLOW was successfully demonstrated on characterisation of activation and allosteric modulation of a GPCR, using the adenosine A1 receptor (A1AR) as a model system. Characterisation of the A1AR activation was achieved by classification of the A1AR bound by “Antagonist”, “Agonist” and “Agonist-Gi”. GLOW achieved an overall accuracy of 99.34% and loss of 1.85%, respectively, on the validation data set after 15 epochs. Meanwhile, characterisation of A1AR allosteric modulation was achieved by classification of the A1AR bound by “Agonist-Gi” and “Agonist-Gi-PAM”. GLOW achieved an overall accuracy of 99.27% and loss of 1.78%, respectively, on the validation data set after 15 epochs. GLOW identified characteristic residue contacts that were highly consistent with previous studies to the residue levels for both A1AR activation and allosteric modulation. In particular, the ligand-binding extracellular domains (ECL1–ECL3) and intracellular G-protein binding domains (TM3, TM5, TM6 and TM7) were found to be loosely coupled in the GPCR activation. Furthermore, it showed that ECL2 played a critical role in the allosteric modulation of A1AR, being consistent with previous mutagenesis, structure and molecule modelling studies (Avlani et al., Reference Avlani, Gregory, Morton, Parker, Sexton and Christopoulos2007; Peeters et al., Reference Peeters, Wisse, Dinaj, Vroling, Vriend and Ijzerman2012; Nguyen et al., Reference Nguyen, Vecchio, Thomas, Nguyen, Aurelio, Scammells, White, Sexton, Gregory, May and Christopoulos2016; Miao et al., Reference Miao, Bhattarai, Nguyen, Christopoulos and May2018a; Draper-Joyce et al., Reference Draper-Joyce, Bhola, Wang, Bhattarai, Nguyen, O’Sullivan, Chia, Venugopal, Valant and Thal2021). GLOW revealed that binding of a PAM (MIPS521) to the agonist-Gi-A1AR complex biased the receptor conformational ensemble, especially in the ECL1 and ECL2 regions. PAM binding stabilised agonist binding within the orthosteric pocket of A1AR, which confined the extracellular mouth of the receptor Furthermore, PAM binding disrupted the N148ECL2-V152ECL2 α-helical hydrogen bond and distorted this portion of the ECL2 helix (Do et al., Reference Do, Wang, Bhattarai and Miao2022).
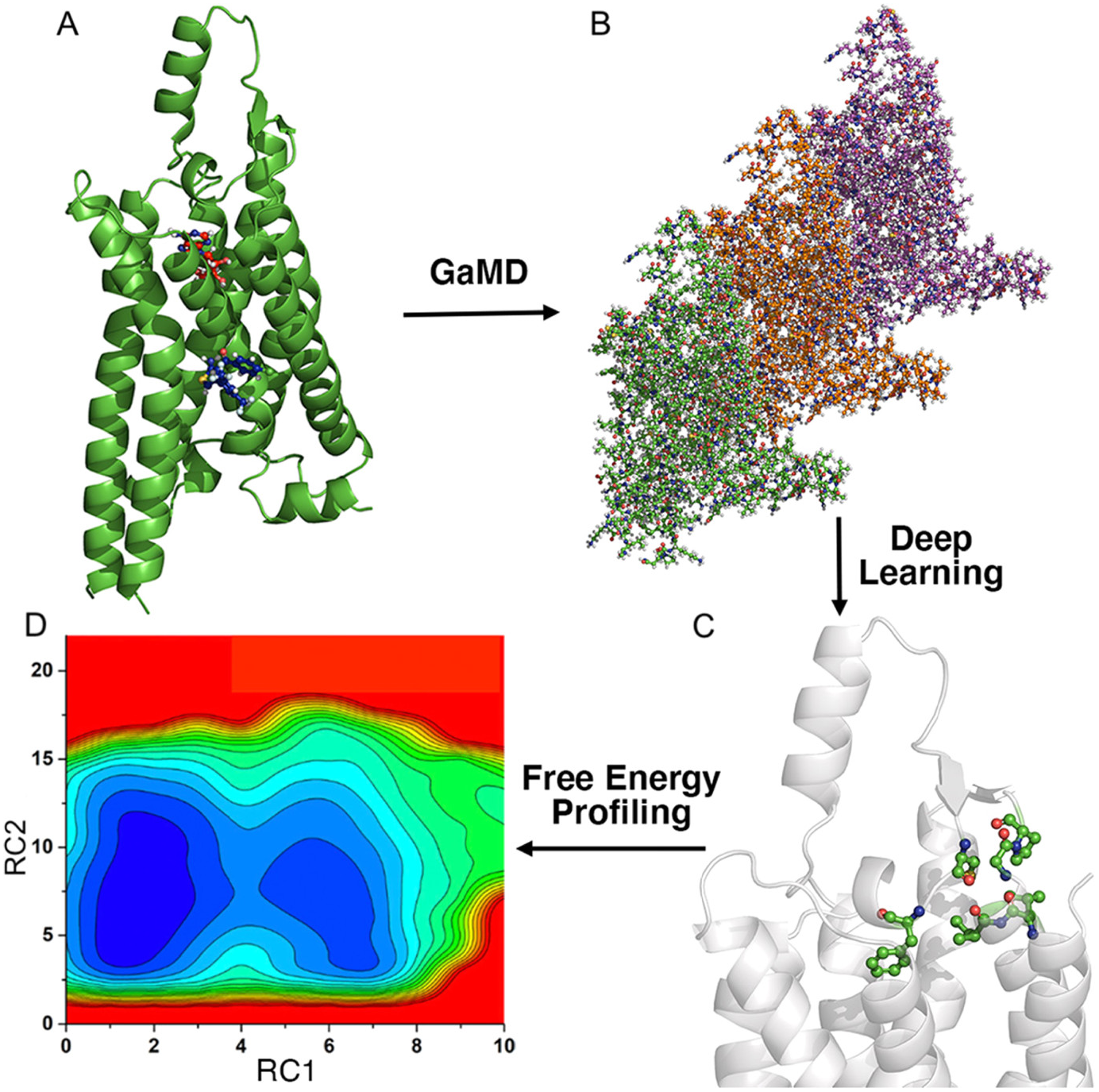
Figure 4. Overview of the Gaussian accelerated molecular dynamics (GaMD), deep learning (DL) and Free Energy PrOfiling Workflow (GLOW). (a) With structures of our interest, GaMD simulations are applied for enhanced sampling of the system dynamics. (b) DL models are then built with GaMD trajectories of residue contact maps transformed into image representations. (c) The DL analysis allows us to identify important residue contacts and system reaction coordinates (RCs). (d) Free energy profiles of the RCs are finally calculated through reweighting of GaMD simulations to characterise the system dynamics. Adapted with permission from Do HN, Wang J, Bhattari A and Miao Y. Journal of Chemical Theory and Computation. 10.1021/acs.jctc.1c01055. Copyright 2022 American Chemical Society.
In addition, DL has been widely applied to optimise force field (Poltavsky and Tkatchenko, Reference Poltavsky and Tkatchenko2021; Unke et al., Reference Unke, Chmiela, Sauceda, Gastegger, Poltavsky, Schütt, Tkatchenko and Müller2021; Chatterjee et al., Reference Chatterjee, Sengul, Kumar and Mackerell2022), binding free energy calculations (Jiang et al., Reference Jiang, Hsieh, Wu, Kang, Wang, Wang, Liao, Shen, Xu, Wu, Cao and Hou2021; Jones et al., Reference Jones, Kim, Zhang, Zemla, Stevenson, Bennett, Kirshner, Wong, Lightstone and Allen2021; Chen et al., Reference Chen, Liu, Feng, Fu, Cai, Shao and Chipot2022) and binding pathway identification (Motta et al., Reference Motta, Callea, Bonati and Pandini2022).
Conclusions and outlook
With remarkable advances in both computer hardware and software, computational approaches have achieved significant improvement to characterise biomolecular recognition, including molecular docking, MD simulations and ML. ML has been incorporated into both molecular docking and MD simulations to improve the docking accuracy, simulation efficiency and trajectory analysis, e.g., AlphaFold-Multimer and GLOW. MD simulations have enabled characterisation of biomolecular binding thermodynamics and kinetics, attracting increasing attention in recent years. Long time scale cMD simulations have successfully captured biomolecular binding processes, although slow dissociation of biomolecules are still often difficult to simulate using cMD.
Enhanced sampling methods have greatly reduced the computational cost for calculations of biomolecular binding thermodynamics and kinetics. Higher sampling efficiency could be generally obtained using the CV-based methods than using the CV-free methods. However, CV-based enhanced sampling methods require predefined CVs, which is often challenging for simulations of complex biological systems. Nevertheless, ML techniques have proven useful to identify proper CVs or reaction coordinates. Alternatively, CV-free methods are usually easy to use without requirement of a priori knowledge of the studied systems. Additionally, the CV-based and CV-free methods could be combined to be more powerful. The CV-free methods can enhance the sampling to potentially overcome the hidden energy barriers in orthogonal degrees of freedom relative to the CVs predefined in the CV-based methods, which could enable faster convergence of the MD simulations. Newly developed algorithms in this direction include integration of replica exchange umbrella sampling with GaMD (GaREUS; Oshima et al., Reference Oshima, Re and Sugita2019), replica exchange of solute tempering with umbrella sampling (gREST/REUS; Kamiya and Sugita, Reference Kamiya and Sugita2018; Re et al., Reference Re, Oshima, Kasahara, Kamiya and Sugita2019), replica exchange of solute tempering with well-tempered Metadynamics (ST-MetaD; Mlýnský et al., Reference Mlýnský, Janeček, Kührová, Fröhlking, Otyepka, Bussi, Banáš and Šponer2022) and temperature accelerated molecular dynamics (TAMD) with integrated tempering sampling (ITS/TAMD; Xie et al., Reference Xie, Shen, Chen and Yang2017).
Recent years have seen an increasing number of techniques that introduce “selective” boost in the CV-free enhanced sampling methods, including the selective ITS, selective scaled MD, selective aMD and selective LiGaMD, Pep-GaMD and PPI-GaMD. In these methods, only essential energy terms are selectively boosted to further increase the sampling efficiency. Additionally, compatible enhanced sampling methods could be combined to be more powerful. For example, GaMD has been combined with Umbrella Sampling to achieve significantly improved efficiency (Oshima et al., Reference Oshima, Re and Sugita2019; Wang et al., Reference Wang, Arantes, Bhattarai, Hsu, Pawnikar, Huang, Palermo and Miao2021). Besides enhanced sampling, the accuracy of force fields and water models play a critical role in predicting the biomolecular binding affinities and kinetics. For example, the TIP4P2015 water model was shown to be more accurate than the TIP3P water model in calculating the kinetics of barnase–barstar binding in cMD simulations (Pan et al., Reference Pan, Jacobson, Yatsenko, Sritharan, Weinreich and Shaw2019). Nevertheless, biomolecular recognition in systems of increasing sizes (such as viruses and cells) and accurate calculations of binding thermodynamics and kinetics of large biomolecular complexes present grand challenges for computational modelling and enhanced sampling simulations. Further innovations in both computing hardware and method developments may help us to address these challenges in the future.
Acknowledgements
This work used supercomputing resources with allocation awards TG-MCB180049 and BIO210039 through the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation Grant no. ACI-1548562 and project M2874 through the National Energy Research Scientific Computing Center (NERSC), which is a U.S. Department of Energy Office of Science User Facility operated under Contract No. DE-AC02-05CH11231. It also used computational resources provided by the Research Computing Cluster at the University of Kansas. This work was supported in part by the National Institutes of Health (R01GM132572), National Science Foundation (2121063) and the startup funding in the College of Liberal Arts and Sciences at the University of Kansas.
Open Peer Review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/qrd.2022.11.







Comments
Comments to Author: In this manuscript, the authors introduce recent enhanced-sampling methods for accelerating association and dissociation events of protein-ligand, protein-peptide, and protein-protein complexes. The authors classify the method into the three types: Collective-variable (CV) based methods, CV-free methods, and the methods combined with machine learning (ML) techniques. In CV-based methods, bias potentials are applied to the system along the predefined CVs. Umbrella sampling or metadynamics are applied to binding problems to investigate binding affinities, pathways, and kinetics. In CV-free methods, bias potentials do not depend on the CVs. The authors mainly introduce Gaussian accelerated MD (GaMD), which was developed by themselves. In particular, selective GaMD methods are efficient for binding and unbinding simulations, because they can apply the boosting potentials to the selective regions of interest in the system. Due to sufficient statistics for binding and unbinding events, the free-energy changes as well as the kinetics (k_on and k_off) can be estimated with high accuracy. In the methods with ML, ML or deep learning (DL) improves the scoring function for docking simulations and achieves the structure prediction, such as AlphaFold and RoseTTAFold. The authors also combine DL with GaMD. DL extracts the important interactions between residues and the CVs from GaMD trajectories, which enables to obtain the accurate free-energy profiles. This manuscript is well written and concisely summarizes recent works of enhanced sampling methods. I recommend the publication of this manuscript after minor revisions, considering the points below.
The authors separately discuss about CV-based and CV-free methods, but their combination should be important for more efficient sampling. In fact, in the last paragraph of Sec.5, the authors mention that compatible enhanced methods could be combined to be more powerful. Even if the hidden energy barriers exist in the orthogonal degrees of freedom for the predefined CVs in the CV-based method, the CV-free method can enhance the sampling in the orthogonal CV spaces. Several combinations of CV-based and CV-free methods have been already proposed. For examples, GaREUS (https://doi.org/10.1021/acs.jctc.9b00761), gREST/REUS (https://doi.org/10.1063/1.5016222; https://doi.org/10.1073/pnas.1904707116), ST-MetaD (https://doi.org/10.1021/acs.jctc.1c01222), ITS/TAMD (https://doi.org/10.1063/1.4973607), etc. The authors should discuss more about the combinations of enhanced sampling methods.
GaMD boosts the motion and flexibility of biomolecules and enhances the sampling in the conformational space, resulting in the reduction of the simulation time. However, even if GaMD is used, many independent GaMD simulations or long GaMD simulations are required to obtain sufficient statistics for protein-peptide binding or binding between large biomolecules. We suggest the authors to discuss convergence issues of GaMD in more details.
GaMD successfully reproduces the binding affinities and kinetics with very high accuracy. However, even if the binding and unbinding events are sufficiently sampled, the affinities and kinetics would strongly depend on the force-field parameters of proteins and ligands and the water model. The author had better explain the relationship between the force-field parameters and enhanced conformational sampling methods.
Minor comments
1. Page 11, Fifth paragraph of Section 3: V_{PP,nb}(r_P) + V_{LL,nb}(r_L) + V_{EE,nb}(r_E) duplicates in V(r). Please modify the duplication.