Introduction
Hepatitis C virus (HCV) infection is a major health problem affecting approximately 170 million people worldwide and 4 million of people in the USA (Asnis & De La Garza, Reference Asnis and De La Garza2006). Up to 85% of HCV-infected individuals may develop chronic hepatitis C, a disease associated with serious clinical complications, including cirrhosis of the liver and hepatocellular carcinoma. Chronic hepatitis C is the leading cause of liver transplantation in the developed world. It is estimated that there are approximately 5 million carriers of HCV in Europe, 70–80% of whom are likely to develop a chronic infection. In the UK, around 216 000 individuals are chronically infected with hepatitis C; and, as in all countries, the major risk factor for infection is injecting drug use (HPA, 2011). Therefore, it is easy to understand the importance of using appropriate antiviral treatments to successfully induce a viral remission, in order to reduce the morbidity and mortality associated with chronic hepatitis C.
First-line treatment consists of a combination therapy with pegylated IFN-α (pegIFN-α) plus ribavirin. In pegIFN-α, polyethylene glycol is added to make IFN-α's half-life longer, thus allowing once-weekly administration. This combination therapy can reach a sustained viral eradication in 45–95% of patients, according to viral genotype, viral load and treatment adherence. Novel protease inhibitor antiviral drugs will soon enter clinical care, and promise to increase considerably the rate of viral eradication; but they will be added to IFN-α – not be a substitute for it.
IFN-α neuropsychiatric side-effects
Unfortunately, the use of IFN-α is associated with numerous side-effects, both medical and neuropsychiatric. Among the first, there are flu-like symptoms, including fever, chills, malaise, tachycardia, headache, arthralgias and myalgias, occurring in more than 30% of patients, usually at the beginning of the therapy. Neuropsychiatric side-effects are also common, and there are reports of a variety of symptoms, including depressive syndromes, manic and psychotic episodes, and delirium. In a previous review (Quelhas & Lopes, Reference Quelhas and Lopes2009), prospective studies (that excluded patients with a lifetime history of psychiatric disorders) report an incidence of IFN-α-induced neuropsychiatric symptoms of 12–41%, while studies without this exclusion criterion report an incidence of 17–58%. Depression appears to be the most common side-effect in patients with chronic hepatitis C, and the incidence of major depressive episodes (MDEs), diagnosed according to DSM-IV-TR criteria, generally ranges from 12% to 42% (Quelhas & Lopes, Reference Quelhas and Lopes2009). The incidence of mania during IFN-α treatment is unknown; however, several cases have been reported, including cases of mania emerging spontaneously after IFN-α-induced depression and/or after withdrawal of IFN-α, and reports of ‘switches’ from depression to mania during antidepressant therapy (Quelhas & Lopes, Reference Quelhas and Lopes2009). Cognitive side-effects, especially memory disturbances and concentration problems, have also been found in 10–20% of patients receiving IFN-α, with the risk of cognitive decline possibly being higher with a larger cumulative dose of IFN-α and a longer duration of treatment. There are a few case reports of psychotic episodes induced by IFN-α therapy (Quelhas & Lopes, Reference Quelhas and Lopes2009).
Worsening of psychiatric symptoms in IFN-α therapy
Generally, patients with chronic hepatitis C tend to have a significant history of psychopathology (Yovtcheva et al. Reference Yovtcheva, Rifai, Moles and Van Der Linden2001), which has been considered by some authors as a significant cause for treatment withdrawal, due to the theoretical risk of worsening of psychiatric symptomatology induced by IFN-α treatment. In particular, concerns have been raised about worsening or exacerbation of depression or of suicidal ideation, as well as of psychotic symptoms in patients suffering from bipolar disorder and psychotic illnesses. As recently as in the 2012 Recommendations from the USA National Hepatitis C Program Office, it is prescribed that ‘uncontrolled depression or active suicidal ideation is an absolute contraindication to IFN-based therapies’ (Yee et al. Reference Yee, Chang, Pocha, Lim, Ross, Morgan and Monto2012). While we agree with the accepted clinical practice that patients with active psychopathology should be assessed and supported before starting IFN-α, the wording of the recommendation undoubtedly confirms the degree of anxiety by which medical professionals see psychiatric patients in this context. As we have argued already 10 years ago (Pariante et al. Reference Pariante, Landau and Carpiniello2002), withholding IFN-α inappropriately, especially from members of a stigmatized class, raises questions about fairness and discrimination.
The evidence
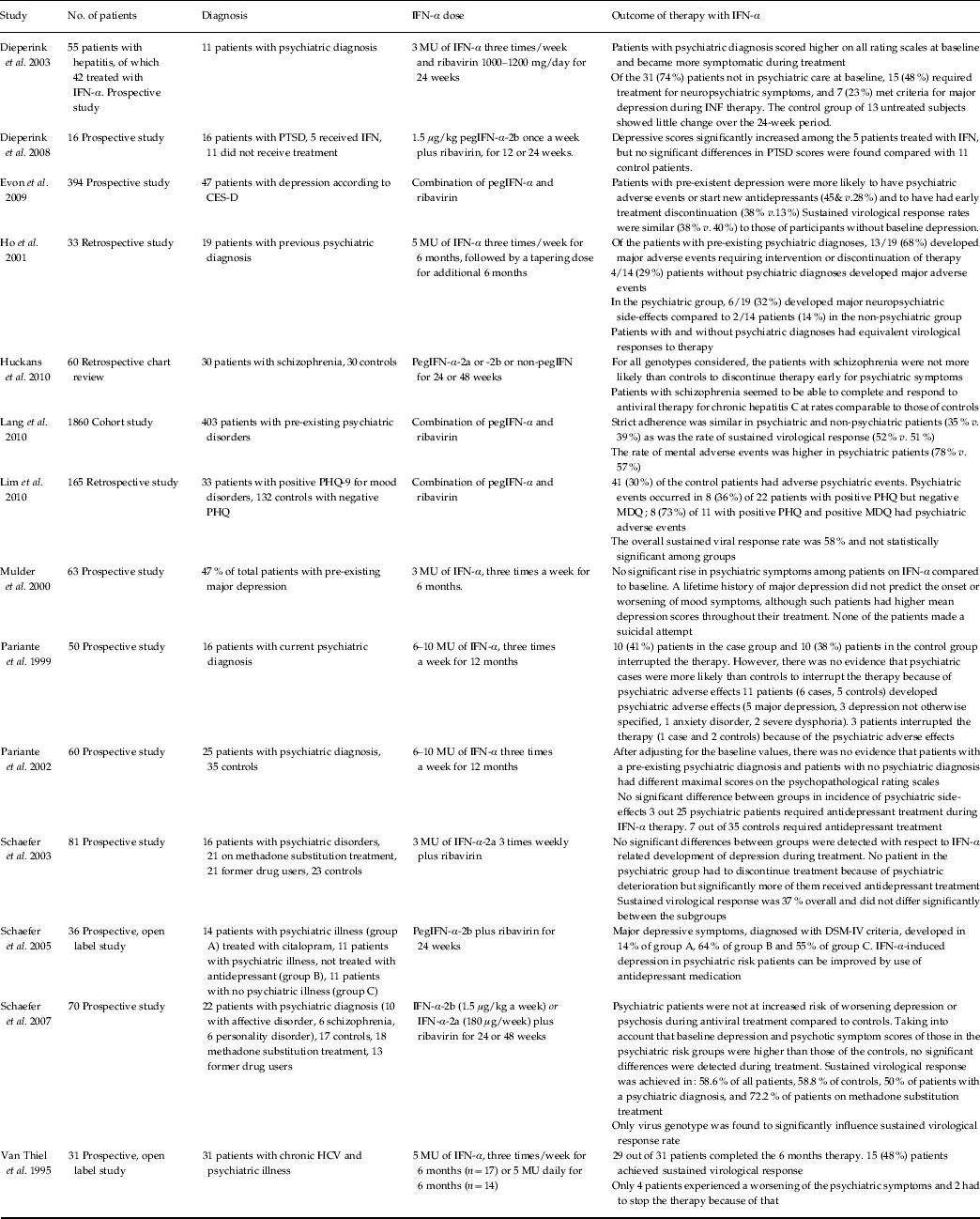
The aim of this editorial is to discuss the existing literature with regard to the administration of IFN-α therapy in patients with chronic hepatitis C and a current psychiatric illness. Studies were identified by using the PubMed database, cross-searching for ‘Interferon-α’, ‘psychiatric symptoms’ and ‘chronic hepatitis C’. Further references were obtained from bibliographies of the reviewed articles. Fourteen studies were considered eligible, and are summarized in Table 1, with details of the findings. Overall, most studies did not find a significantly higher risk of IFN-α-induced neuropsychiatric toxicity in patients with a psychiatric diagnosis, once the higher baseline psychopathology was taken into account. Interestingly, patients with severe mental illnesses, such as schizophrenia, seem also to be resilient during IFN-α. Furthermore, no higher suicidal risk was recorded in these patients. Of particular relevance, and consistently across all studies, is the fact that the rates of completion of therapy and sustained virological response are comparable between psychiatric and non-psychiatric patients.
Table 1. Summary of studies on psychiatric patients taking interferon-α

CESD, Center for Epidemiologic Studies Depression Scale; IFN, interferon; PHQ, Physician Health Questionnaire; PTSD, post-traumatic stress disorder; MDQ, Mood Disorders Questionnaire; MU, million units.
Conclusions
Our analysis of the relatively scant literature highlights that patients with a psychiatric illness have rates of treatment adherence and sustained virological response similar to those of non-psychiatric patients. Moreover, the majority of the studies do not show a significantly higher neurotoxicity of IFN-α therapy in these patients; and even in those studies that find a greater incidence of psychiatric adverse events, psychiatric patients are able to successfully complete the therapy. The lack of an increased risk of suicide in psychiatric patients is also consistent with a recent review on this topic (Sockalingam et al. Reference Sockalingam, Links and Abbey2011), which also found that the evidence for an increased suicide risk during IFN-α is largely anecdotal. In most cases, a combination of psychotropic and psychosocial interventions can be used to both prevent worsening of existing symptoms and to manage patients who show a reactivation of psychiatric problems. In fact, there is reasonably good evidence in favour of use of antidepressants, for both prophylaxis and treatment of anxiety and depressive symptoms induced by IFN-α (Baraldi et al. Reference Baraldi, Hepgul, Mondelli and Pariante2012). In addition, the early involvement of a multidisciplinary therapeutic approach, such as the assessment and follow-up with liaison psychiatrists and psychologists (Neri et al. Reference Neri, Bertino, Petralia, Giancarlo, Rizzotto, Calvagno, Mauceri, Abate, Boemi, Di Pino, Ignaccolo, Vadala', Misseri, Maiorca, Mastrosimone, Judica and Palermo2010), or an interdisciplinary nurse-managed treatment programme, can be used to support patients with pre-existent psychiatric problems undergoing IFN-α therapy (Gardenier et al. Reference Gardenier, Wisnivesky, McGinn, Kronish and McGinn2011). It should be noted that it is important not to underestimate the fact that patients with no psychiatric history have virtually the same risk of developing IFN-α-induced side-effects, and as such they may suffer even more from the lack of management programmes and prophylactic strategies. In summary, while we support the notion of assertive psychosocial and pharmacological prevention in these patients, we find no evidence that patients with a pre-existing psychiatric disorder should not be treated with interferon-α; and withdrawal of such treatment should be considered as discrimination.
Acknowledgements
This paper received funding from The NIHR ‘Biomedical Research Centre for Mental Health’, Institute of Psychiatry and South London and Maudsley NHS Foundation Trust and the Commission of European Communities 7th Framework Programme Collaborative Project Grant Agreement no. 22963 (Mood Inflame).
DECLARATION OF INTEREST
None.