Introduction
Insomnia is characterised by difficulties initiating sleep, maintaining sleep, early morning awakenings, or unrefreshed sleep that cause distress and/or daytime impairment (American Psychiatric Association, 2013). Symptoms of insomnia are common following childbirth (Sivertsen, Hysing, Dorheim, & Eberhard-Gran, Reference Sivertsen, Hysing, Dorheim and Eberhard-Gran2015). A large longitudinal study found 60% of women experienced significant insomnia symptoms at two months postpartum and 41% at two years following childbirth (Sivertsen et al., Reference Sivertsen, Hysing, Dorheim and Eberhard-Gran2015). Although some aspects of sleep improve over time (e.g. sleep duration), other aspects (e.g. sleep continuity and quality) remain compromised beyond the initial postpartum months (Matsumoto, Shinkoda, Kang, & Seo, Reference Matsumoto, Shinkoda, Kang and Seo2003).
Postpartum insomnia symptoms are associated with a wide range of undesirable outcomes including symptoms of depression (Emamian, Khazaie, Okun, Tahmasian, & Sepehry, Reference Emamian, Khazaie, Okun, Tahmasian and Sepehry2019), anxiety (Lawson, Murphy, Sloan, Uleryk, & Dalfen, Reference Lawson, Murphy, Sloan, Uleryk and Dalfen2015), and fatigue (Giallo et al., Reference Giallo, Seymour, Dunning, Cooklin, Loutzenhiser and McAuslan2015). Currently, there is a lack of safe, efficacious, and evidence-based interventions tailored for postpartum insomnia. Existing trials show some benefits of complementary health interventions (i.e. massage, exercise, herbal tea) on maternal sleep quality in the first 10 weeks postpartum (Owais, Chow, Furtado, Frey, & Van Lieshout, Reference Owais, Chow, Furtado, Frey and Van Lieshout2018). Psychoeducation-focused interventions in community samples have shown limited benefits to maternal sleep (Stremler et al., Reference Stremler, Hodnett, Kenton, Lee, Weiss, Weston and Willan2013), likely due to the typically inconsistent early postpartum sleep-wake patterns of infants and new parents (Matsumoto et al., Reference Matsumoto, Shinkoda, Kang and Seo2003).
It is possible that postpartum insomnia may develop from different mechanistic pathways which have implications for intervention. From a cognitive behavioural perspective, early experiences of disturbed sleep during pregnancy and the initial postpartum periods may precipitate unhelpful sleep behaviours (e.g. extending time in bed by sleeping in or attempting sleep too early, taking long daytime naps) and cognitions (e.g. worries related to sleep disturbance, catastrophising sleep loss), potentially perpetuating insomnia symptoms in the latter postpartum period (Swanson, Kalmbach, Raglan, & O'Brien, Reference Swanson, Kalmbach, Raglan and O'Brien2020). From a circadian rhythm perspective, changes in circadian functioning such as phase delays (Sharkey, Pearlstein, & Carskadon, Reference Sharkey, Pearlstein and Carskadon2013) and dampened amplitude (Thomas & Burr, Reference Thomas and Burr2006), have been reported during perinatal periods. These alternations may be related to changes in sleep-wake patterns (e.g. daytime naps, nighttime awakenings; Matsumoto et al., Reference Matsumoto, Shinkoda, Kang and Seo2003) and low levels of light exposure (Tsai, Barnard, Lentz, & Thomas, Reference Tsai, Barnard, Lentz and Thomas2009), and may contribute to further sleep complaints.
Cognitive behavioural therapy (CBT) for insomnia and light and dark therapy (LDT) are two non-pharmacological interventions that target these distinct mechanisms to reduce symptoms of insomnia. CBT for insomnia addresses unhelpful thoughts, beliefs and behaviours that perpetuate insomnia through strategies including stimulus control, sleep restriction, cognitive reframing, psychoeducation, relaxation, and sleep hygiene. CBT for insomnia is a highly effective insomnia treatment among diverse populations (Seyffert et al., Reference Seyffert, Lagisetty, Landgraf, Chopra, Pfeiffer, Conte and Rogers2016). Six studies have tested CBT for insomnia in the perinatal periods showing promising results, including randomised controlled trials (RCTs) during pregnancy (Cain et al., Reference Cain, Brumley, Louis-Jacques, Drerup, Stern and Louis2020; Felder, Epel, Neuhaus, Krystal, & Prather, Reference Felder, Epel, Neuhaus, Krystal and Prather2020; Kalmbach et al., Reference Kalmbach, Cheng, O'Brien, Swanson, Sangha, Sen and Drake2020; Manber et al., Reference Manber, Bei, Simpson, Asarnow, Rangel, Sit and Lyell2019; Sweeney, Signal, & Babbage, Reference Sweeney, Signal and Babbage2020). A recent RCT showed that for those with insomnia symptoms during pregnancy, CBT delivered in pregnancy and early postpartum was effective in reducing insomnia symptoms, with long-term benefits across the first two postpartum years (Bei et al., Reference Bei, Pinnington, Quin, Shen, Blumfield, Wiley and Manber2021). In the postpartum, one open pilot study of 12 women (M = 6.5 months postpartum) with comorbid insomnia disorder and depression showed that CBT for insomnia strongly and significantly reduced insomnia symptoms from pre- to post-treatment (Swanson, Flynn, Adams-Mundy, Armitage, & Arnedt, Reference Swanson, Flynn, Adams-Mundy, Armitage and Arnedt2013).
LDT involves the use of specifically-timed light and dark exposure to realign the internal biological clock (circadian rhythm) with the external environment and/or to reduce sleepiness through its alerting effects (Phipps-Nelson, Redman, Dijk, & Rajaratnam, Reference Phipps-Nelson, Redman, Dijk and Rajaratnam2003). Strategies to reduce nighttime light exposure (i.e. dark therapy) may also be provided. LDT has been shown to improve sleep and insomnia outside of the perinatal periods (van Maanen, Meijer, van der Heijden, & Oort, Reference van Maanen, Meijer, van der Heijden and Oort2016). During perinatal periods, however, LDT has almost exclusively been tested as a treatment of depression (Crowley & Youngstedt, Reference Crowley and Youngstedt2012).
The current study aimed to simultaneously evaluate the efficacy of CBT and LDT, two interventions targeting different mechanisms (i.e. cognitive behavioural and circadian rhythm) of postpartum insomnia, to accelerate intervention development. It was hypothesised that compared to a treatment-as-usual (TAU) control group, participants who received either intervention would demonstrate significantly greater reductions from baseline to immediate post-intervention in insomnia symptoms (primary outcome), and symptoms of sleep disturbance, fatigue, sleepiness, depression, and anxiety (secondary outcomes). Fatigue and mood were included as secondary outcomes given observed relationships with maternal sleep disturbance (Giallo et al., Reference Giallo, Seymour, Dunning, Cooklin, Loutzenhiser and McAuslan2015; Lawson et al., Reference Lawson, Murphy, Sloan, Uleryk and Dalfen2015). TAU was selected as a comparator to determine how well interventions worked compared to current perinatal care practices to inform further effectiveness/implementation trials.
Methods
Study design
This was a three-arm, parallel-group, randomised controlled, superiority trial conducted in Australia within a community setting. A detailed protocol has been published (Verma, Rajaratnam, Davey, Wiley, & Bei, Reference Verma, Rajaratnam, Davey, Wiley and Bei2021), and key methodology is summarised in the following. Reporting follows CONSORT 2010 guidelines, including CONSORT-SPI and -PRO, and CONSORT Extensions and Harms (see online Supplementary Tables S2 and S3). Study materials are available publicly online (https://doi.org/10.17605/OSF.IO/FBPH2), including ethics approvals, informed consent form, explanatory statement, study protocol (including risk assessment/management), intervention outlines. The trial was prospectively registered prospectively with Australia and New Zealand Clinical Trials Registry (ACTRN12618000842268). A data safety and monitoring committee was not employed due to limited scale.
Participants
Inclusion criteria were: (a) females who gave birth within the past four to 12 months; (b) age ⩾18 years; (c) presence of insomnia symptoms, operationalised as >7 on the Insomnia Severity Index [(ISI); Bastien, Vallières, & Morin, Reference Bastien, Vallières and Morin2001]; (d) nulliparous (i.e. first-time parents); (e) singleton pregnancy; (f) ability to read and write in English; (g) regular access to telephone, email and the internet.
Exclusion criteria were: (a) current pregnancy; (b) nighttime shift work; (c) current severe non-insomnia sleep disorders (e.g. narcolepsy, sleep apnoea, restless legs syndrome, circadian rhythm disorders); (d) use of medication or reported medical conditions that directly affect sleep; (e) current severe psychopathology (e.g. bipolar disorder, psychosis) or risk of harm to self/others; (f) reported infants having medical conditions that affect maternal sleep; (g) epilepsy, photosensitivity, or having been recommended by a health professional to avoid bright light; (h) reported current unsettled infant sleep behaviour, operationalised as infant waking on average more than three times per night and requiring parental assistance to re-initiate sleep. Criteria (c) and (d) were assessed through structured interview via telephone following completion of the baseline questionnaire.
Randomisation and blinding
Eligible participants were randomly allocated through REDCap to one of three groups (CBT, LDT, or TAU) at a 1:1:1 ratio using variable blocks of size three, six, or nine, with randomisation scheme generated in advance by a research staff who is not involved in recruitment or delivery of intervention. Randomisation was stratified by baseline ISI (⩽14 and >14) and infant age (<8 months and ⩾8 months; mid-point of the included infant age of 4–12 months). Group allocation was concealed until the time of randomisation. Principal investigators, participants, and the investigator who undertook interview reliability assessments were unblinded to group allocation. Participants were asked to withhold disclosure of group allocation from blinded research assistants conducting outcome assessment interviews.
Procedures
Participants were recruited via social media posts (e.g. on parent groups on Facebook, posts made by perinatal organisations/services) and flyers at community maternal and child health centres in Melbourne, Victoria. After providing informed consent, participants completed the baseline questionnaire, followed by a telephone screening interview to determine eligibility. Eligible participants were then randomised. All participants were asked to complete outcome assessments via online questionnaires at baseline (Week 0; T1), midpoint-intervention (Week 3; T2), post-intervention (Week 6; T3), and 1-month follow-up (Week 10; T4). Insomnia disorder diagnostic status was assessed via structured telephone interview at baseline and post-intervention.
Interventions
Intervention development is detailed in the protocol (Verma et al., Reference Verma, Rajaratnam, Davey, Wiley and Bei2021), with qualitative feedback from four birthing parents incorporated prior to delivery. All participants received standard perinatal care. To increase scalability, we used remote intervention delivery via telephone-based therapist assistance and internet-delivered self-help materials. This allowed participants to undertake the intervention from any location in which they had phone and internet access (e.g. from home). A meta-analysis of RCTs found that self-help CBT for insomnia is effective at reducing insomnia symptoms, and outcomes were enhanced with therapist assistance (Ho et al., Reference Ho, Chung, Yeung, Ng, Kwan, Yung and Cheng2015). Interventions were manualised and delivered through telephone calls and emails. Participants in intervention groups received two telephone calls: (1) The first call (60 min for CBT, 45 min for LDT) explained key intervention components, personalised recommendations, and encouraged intervention adherence. (2) The second call (30 min) occurred in Week 3 (i.e. midpoint-intervention) to encourage intervention adherence and address barriers to applying strategies. Those in the TAU group were thanked for their involvement and reminded of project logistics during both telephone calls (~10 min).
Cognitive behavioural therapy
The CBT intervention materials were based on CBT for insomnia adapted for perinatal populations (Bei et al., Reference Bei, Pinnington, Quin, Shen, Blumfield, Wiley and Manber2021; Manber et al., Reference Manber, Bei, Simpson, Asarnow, Rangel, Sit and Lyell2019). Strategies were delivered via brief, easy-to-read emails using online software that automated the timing of delivery. Components of the CBT intervention included: psychoeducation about sleep, stimulus control, healthy sleep attitudes and behaviours, sleep hygiene, relaxation, managing unhelpful thoughts and worries, fatigue management, prioritising rest and self-care, enlisting social support, and infant settling. In total, 21 emails were delivered: five in Week 1, four in Week 2, followed by three emails per week in Weeks 3–6.
Light dark therapy
Participants in the LDT group were provided with light glasses (Luminette 2) and asked to wear them for 20 min each morning at a prescribed wake-time personalised for each participant immediately upon awakening for most participants). Light glasses provided blue-enriched white light (1250 lux directed at each eye, 813 melanopic lux). Participants also received light hygiene strategies including seeking natural bright light to promote alertness during the day and avoidance of bright light in the hours prior to nighttime sleep, especially of blue-enriched light from hand-held electronic devices. To further minimise nighttime light exposure, a small LED night light (Dreambaby Auto Sensor Swivel Light) was provided to participants for use during nighttime awakenings. One brief email per week was sent from Week 1 to Week 6 to remind participants of pertinent strategies and encourage intervention adherence. Following the first phone call, LDT participants were provided with a two-page summary of key points. Upon LDT participants had completed the study by completing their final questionnaire, they were offered the suite of CBT intervention emails.
TAU control
Upon completion of the study, TAU participants received CBT intervention emails either altogether or on a week-to-week basis according to their preference.
Therapist assistance
Intervention telephone calls were conducted by a provisional psychologist undertaking doctoral level training in clinical psychology (SV) trained and supervised by a clinical psychologist (BB). All intervention calls were recorded and an independent rater (NQ) assessed intervention fidelity on 7% of randomly selected participants. Intervention fidelity was high for both CBT (95.8%) and LDT (100%) groups.
Outcomes
Primary outcome
The ISI is a 7-item measure of insomnia symptoms (Bastien et al., Reference Bastien, Vallières and Morin2001) and was used as the primary outcome. Total scores range from 0 to 28, with higher scores indicating more severe insomnia symptoms. The ISI has been shown to have high internal consistency and a cut-off score of ⩾8 has a sensitivity 96–99% and specificity of 78–92% in community and clinical populations respectively (Morin, Belleville, Bélanger, & Ivers, Reference Morin, Belleville, Bélanger and Ivers2011). In this study, internal consistency (Cronbach's α) of the ISI across 4 time points ranged 0.80–0.84.
Secondary outcomes
Maternal sleep disturbance was measured using PROMIS Sleep Disturbance – Short Form – 8a (PROMIS Sleep Disturbance; Yu et al., Reference Yu, Buysse, Germain, Moul, Stover, Dodds and Pilkonis2011); α ranged 0.85–0.90 in this study. Participants also self-reported sleep timing and duration via a modified version of the Consensus Sleep Diary (Carney et al., Reference Carney, Buysse, Ancoli-Israel, Edinger, Krystal, Lichstein and Morin2012), from which total sleep time and sleep efficiency (percentage of total sleep time to time spent in bed attempting to sleep) were derived. Fatigue was measured using a 5-item version of the Fatigue Assessment Scale (FAS) adapted for use in the postpartum population (Wilson, Wynter, Fisher, & Bei, Reference Wilson, Wynter, Fisher and Bei2018); α ranged 0.85–0.86 in this study. Maternal mood was measured using PROMIS Depression – Short Form – 8a (PROMIS Depression) and PROMIS Anxiety – Short Form – 8a (PROMIS Anxiety), both 8-item scales of emotional distress over the past seven days (Pilkonis et al., Reference Pilkonis, Choi, Reise, Stover, Riley and Cella2011); α ranged 0.88–0.93 across both scales over time in this study. All PROMIS scales result in a T-score with a population mean of 50 and standard deviation of 10. The Epworth Sleepiness Scale (ESS; Johns, Reference Johns1991 alpha ranged 0.73–0.79 in this study) and the single-item Karolinska Sleepiness Scale (KSS; Åkerstedt & Gillberg, Reference Åkerstedt and Gillberg1990) measured trait and state levels of sleepiness respectively.
Diagnostic status of DSM-5 insomnia disorder was assessed using the Insomnia module of the Duke Structured Interview of Sleep Disorders (Edinger et al., Reference Edinger, Wyatt, Olsen, Stechuchak, Carney, Chiang and Radtke2009) during screening telephone interview. All telephone interviews were recorded and rated for reliability by an independent rater (NQ), with discrepancies reconciled via discussion.
Other measures
Participants' perceived credibility and expectancy of treatment were assessed using the Credibility Expectancy Questionnaire (Devilly & Borkovec, Reference Devilly and Borkovec2000). Project satisfaction was measured with the [Client Satisfaction Questionnaire (CSQ); Attkisson & Zwick, Reference Attkisson and Zwick1982]; scores were transformed to range from 25 to 100; Cronbach's α 0.93 in this study. Experiences of adverse events throughout the trial were assessed via self-report formally in post-intervention and follow-up questionnaires, or informally if they arose as detailed in the protocol (Verma et al., Reference Verma, Rajaratnam, Davey, Wiley and Bei2021); adverse events were documented and monitored on a tracking sheet. Part of data collection coincided with the COVID-19 pandemic. Participants who enrolled after February 2020 were asked whether the pandemic had impacted on participation; no protocol changes were made as all prior procedures continued to be applicable during the pandemic.
Statistical analysis
This study was powered for the primary outcome (i.e. ISI scores) based on a priori power analysis. Assuming 10% missing data at midpoint-intervention and 15% missing data at post-intervention (Bei, Milgrom, Ericksen, & Trinder, Reference Bei, Milgrom, Ericksen and Trinder2010), a sample size of 90 was powered adequately at 82% (two-tailed α = 0.05) to detect a medium effect size (f = 0.25), which was observed in a recent CBT for insomnia trial on prenatal insomnia (Manber et al., Reference Manber, Bei, Simpson, Asarnow, Rangel, Sit and Lyell2019). Statistical significance for secondary outcomes (PROMIS Sleep Disturbance, ESS, KSS, FAS, PROMIS Depression and Anxiety) were conservatively set at two-tailed α = 0.01 due to multiple comparisons.
All analyses were intention-to-treat and carried out using R 4.1.0 (R Core Team, 2021) and Mplus 8.3 (Muthén & Muthén, Reference Muthén and Muthén1998–2020) via MplusAutomation (Hallquist & Wiley, Reference Hallquist and Wiley2018). Changes in primary and secondary outcomes were examined using piecewise latent growth models allowing for different rates of change: Slope 1 starts from baseline, through midpoint-intervention, to immediate post-intervention; it captured change from baseline to post-intervention as the primary endpoint for all outcomes. Loadings for Slope 1 were fixed at 0 for T1, freely estimated for T2, and fixed at 1 for T3 and T4. Loading was freely estimated for T2 to (1) allow non-linear symptom changes often during CBT treatments (Bei et al., Reference Bei, Asarnow, Krystal, Edinger, Buysse and Manber2018), and (2) allow mid-treatment symptom levels to be estimated. Slope 2 starts from immediate post-intervention to one-month follow-up; it captured maintenance of intervention effects over the one-month follow-up and was an exploratory endpoint. Loadings for Slope 2 were fixed at 0 for T1, T2, and T3, and fixed at 1 for T4.
The residual variance was constrained to be independent and equal across time (i.e. an independent, homogenous residual structure). Intercepts of the outcomes were constrained to 0 to allow estimation of the latent random intercept mean. The intercept and Slope 1 variances and covariance were freely estimated. The variance of Slope 2 and its covariance with both the intercept and Slope 1 were freely estimated. The two stratification factors, ISI (⩽14 and >14) and infant age (<8 months and ⩾8 months) formed four grouping conditions, which were dummy coded with ‘Early Postpartum Low ISI’ as the reference group. These dummy codes were included as covariates to adjust for effect on the random intercept (Kahan & Morris, Reference Kahan and Morris2012).
Group was dummy coded and entered as a predictor of the intercept, Slope 1, and Slope 2, with the effect constrained to 0 for the intercept (due to randomisation) to implement constrained longitudinal data analysis, which provides a more accurate estimate of treatment effects from RCTs with repeated measures (Coffman, Edelman, & Woolson, Reference Coffman, Edelman and Woolson2016). These models test whether the change in outcomes over time differed by group (Fig. 1).
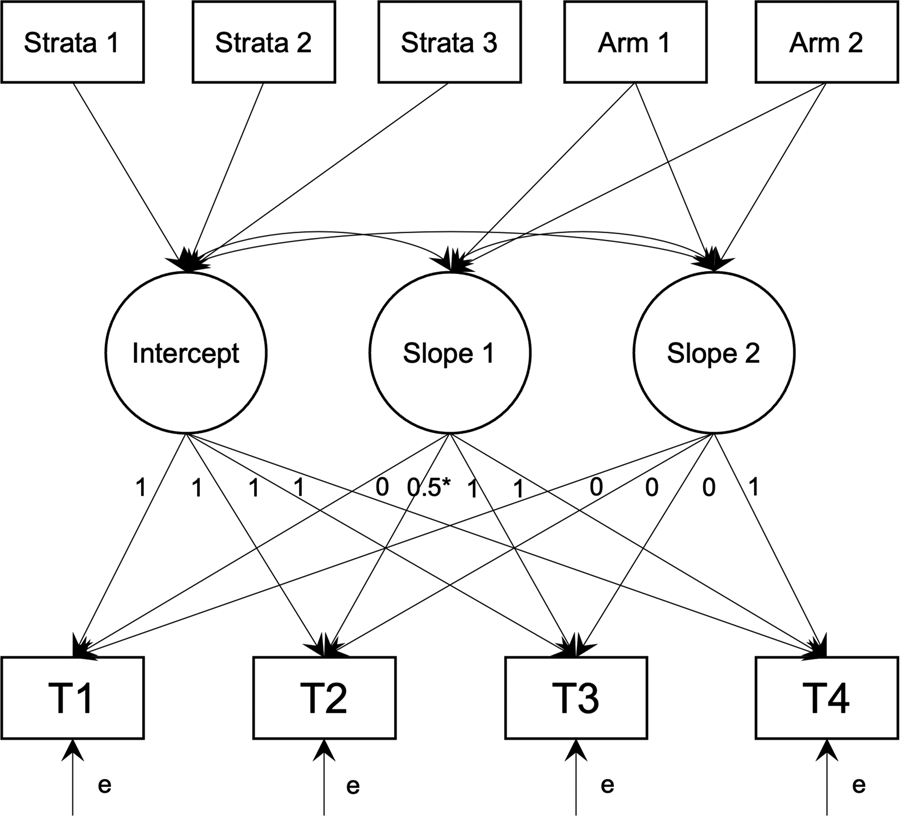
Fig. 1. Path diagram of latent growth models. T1–T4 are outcome values at each time point. Strata 1–3 are the dummy codes for the 4 strata, and Arm 1–2 are the dummy codes for the three randomised arms.
Effect sizes of the group difference at each time point also were calculated as adjusted, standardised mean differences by taking the model estimated difference between groups at each time point and standardising by the combined residual and random intercept variance. Missing data were addressed using full information maximum likelihood. An estimator robust to non-normal data (Yuan & Bentler, Reference Yuan and Bentler2000) was used to derive confidence intervals and statistical inference.
Results
Recruitment spanned the 7 September 2018 to the 23 March 2020. 549 participants expressed interest, 376 (68.5%) gave informed consented, 196 (35.7%) completed screening telephone interviews, and 114 (20.8%) eligible participants were randomised to CBT (n = 39), LDT (n = 36) or TAU (n = 39). All randomised participants were included in analyses. The trial stopped due to successful achievement of recruitment target. See Fig. 2 for detailed reasons for exclusion.

Fig. 2. CONSORT trial profile. T1, time-point 1 (Week 0); ISI, Insomnia Severity Index; PTSD, posttraumatic stress disorder; CBT, cognitive behavioural therapy group; LDT, light dark therapy group; TAU, treatment-as-usual control group.
Detailed sample characteristics at baseline are in Table 1. Participants on average were aged 32.20 years (s.d. = 4.62), mostly white (89.4%), married to male partners (99.1%), not working at baseline (73.7%), and had completed university education (79%). In terms of diagnosed mental health conditions, 42.1% reported no history, 36.8% reported past but not current, and 21.1% reported current mental health conditions (of these, 85.7% an anxiety disorder, 42.6% a depressive disorder, and 28.6% post-traumatic stress disorder). The sample reported poor sleep at baseline: mean ISI score fell just under the ‘clinical insomnia’ threshold of 14, mean sleep efficiency was low at 65.5%, and 76.3% met DSM-5 insomnia disorder diagnosis criteria, excluding the three-month duration criteria. Group differences in baseline characteristics, including demographics, treatment credibility and expectancy, and primary and secondary outcomes were non-significant (p values range: 0.10–0.95).
Table 1. Descriptive statistics of sample characteristics at baseline

CBT, cognitive behavioural therapy group; LDT, light dark therapy group; TAU, treatment-as-usual control group; Means (M) and standard deviations (s.d.) are presented for continuous variables, and n (%) are presented for categorical variables.
a Variable infant sleeping location, such as sleeping initially in own cot in separate room before co-sleeping with parents for second half of the night.
b Group differences were compared for percentage above or below 2019 median household income in Australia.
c Self-reported mental health diagnosis.
d Based on structured clinical interview on DSM-5 insomnia disorder without the 3-month duration criteria. All group differences were non-significant, p values ranged 0.06–1.00.
Both interventions were feasible and well-accepted. Drop-out rates were low: one participant (LDT) withdrew and five participants did not respond to contact (four in CBT, one in TAU). Rates of intervention satisfaction were high in both CBT (CSQ M = 86.79, s.d. = 12.62) and LDT (M = 84.38, s.d. = 14.40) groups and did not significantly differ (p = 0.46). Approximately 82.6% and 88.3% of intervention emails were opened by those in the CBT and LDT groups respectively.
Model estimated changes in primary and secondary outcomes for each group and between group comparisons are summarised in Table 2 and displayed in Fig. 3 and online Supplementary Fig. S1. Detailed statistics for each time point, including group differences at each time point, are shown in online Table S1 of the Supplementary.

Fig. 3. Model estimated changes in outcome variables over time. CBT, cognitive behavioural therapy group; LDT, light dark therapy group; TAU, treatment-as-usual control group. Model estimated means and 95% confidence intervals are presented. All models adjusted for baseline levels and strata of the outcome. A reference line is added wherever applicable to facilitate interpretation: Insomnia Severity Index scores at 8 or above indicates subclinical insomnia symptoms; the T-score 50 for PROMIS Sleep Disturbance indicates population mean; an Epworth Sleepiness Scale score above 5 indicates above normal sleepiness.
Table 2. Model estimated changes in primary and secondary outcomes
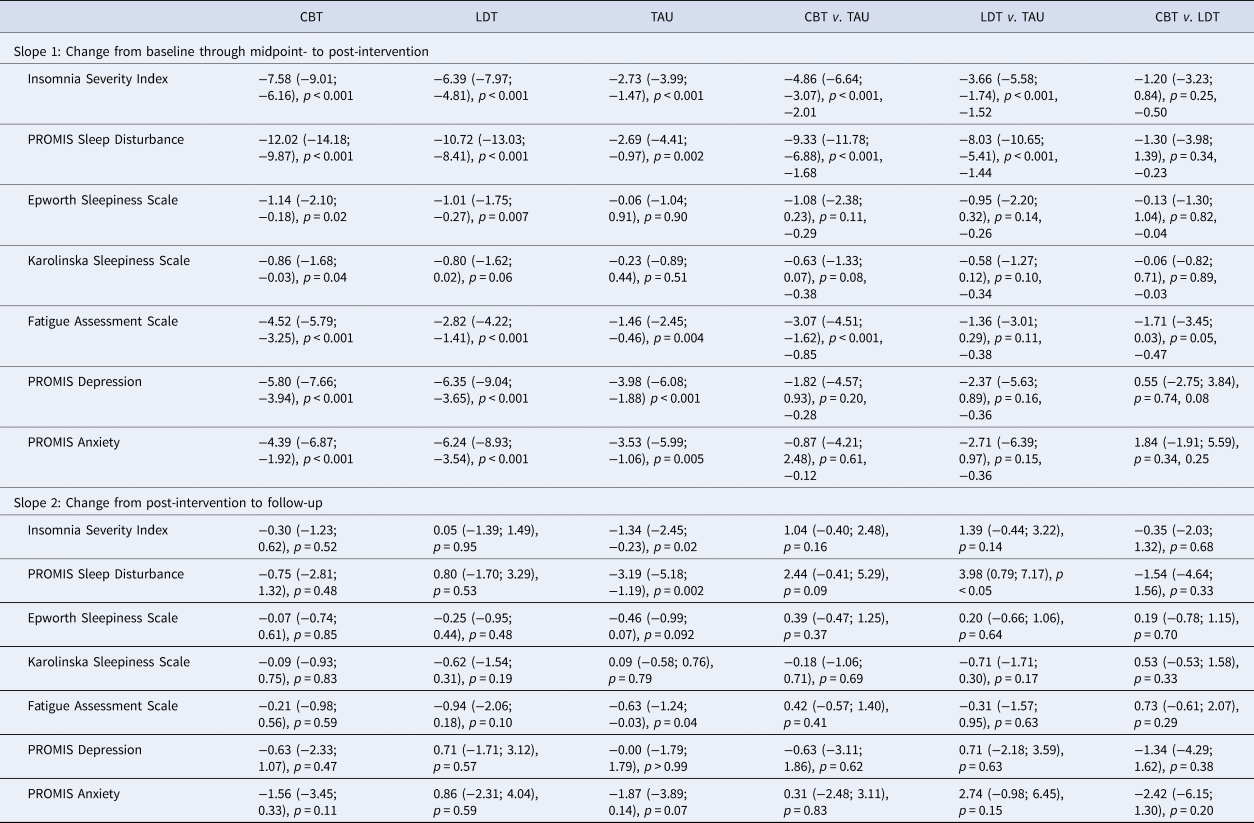
CBT, cognitive behavioural therapy group; LDT, light dark therapy group; TAU, treatment-as-usual control group. Model estimated mean (95% confidence interval), p value adjusting for baseline levels and strata are displayed; for Slope 1, effect sizes were added for between group comparisons based on values at post-intervention. The first three columns show model estimated changes for each group; the remaining columns show group differences in the changes. For model estimated statistics at each time point, please see online Fig. S2 and Table S1 in the Supplementary, which also includes effect sizes.
Compared to TAU, there was a significantly greater reduction in ISI scores from baseline to post-intervention in the CBT group (p < 0.001; effect size −2.01 at post-intervention). Comparable effects relative to TAU were found for the LDT group (p < 0.001; effect size of −1.52 at post-intervention). Scores on the ISI did not change significantly from post-intervention to one-month follow-up in either CBT or LDT groups (p = 0.52 and 0.95 respectively), indicating improvements were maintained. These changes in the primary outcome are consistent with a significantly greater reduction in the percentage of DSM-5 insomnia disorder diagnosis (without the duration criteria) in both treatment groups compared to TAU: at post-intervention, 3 (8.6%), 3 (8.3%), and 13 (36.1%) participants in the CBT, LDT, and TAU groups met criteria respectively (p values < 0.01 based on chi-square analyses; see baseline rates in Table 1).
There were significantly greater reductions in PROMIS Sleep Disturbance in the CBT and LDT groups (p values < 0.001; effect sizes −1.68 and −1.44 at post-intervention respectively) compared to TAU from baseline to post-intervention; these improvements were maintained from post-intervention to follow-up (p values > 0.48). These are consistent with changes in total sleep time and sleep efficiency (see online Table S1 of Supplementary). Compared to TAU, the CBT (p < 0.001, effect size −0.85 at post-intervention) but not the LDT group (p = 0.11) had significantly greater reduction in fatigue symptoms on FAS from baseline to post-intervention, and improvements were maintained at follow-up (p = 0.59). Group differences in the changes of other outcomes, including trait and state sleepiness, and symptoms of depression and anxiety, were not statistically significant (all p values > 0.05).
Exploratory analyses showed that CBT and LDT groups did not differ significantly on the changes in all primary (mean difference on ISI was −1.20) and secondary outcome measures (all p values > 0.05). There was a trend for the CBT v. LDT group to have greater reduction in fatigue symptoms from baseline to post-intervention (p = 0.054, effect size −0.47).
Side-effects were reported by four participants, all in the LDT group. Participants reported headache (n = 1) and dizziness (n = 1) within the first three days, and headaches, dizziness, and nausea 9 days after commencing intervention (n = 1). These participants were instructed to replace light therapy glasses with natural light sources, and symptoms subsided. A fourth participant reported occasional headaches after light therapy glasses usage in the post-intervention questionnaire. One LDT participant was not offered light therapy glasses due to a history of bipolar disorder but received all other LDT strategies. Participants were not lost due to adverse events.
Of the 66 participants who completed COVID-19 impact questions, 74.2% reported their participation not at all affected by the pandemic, 24.2% reported part of the participation was affected, and 1.5% (n = 1) reported the whole participation was affected. No participants were diagnosed with COVID-19.
Discussion
In this RCT, both therapist-assisted CBT and LDT delivered over six weeks were safe, feasible, and efficacious (compared to a TAU control) in reducing postpartum insomnia symptoms presented between 4 and 12 months postpartum. Further, reductions in insomnia symptoms and sleep disturbance were maintained at one-month follow-up for both intervention groups. The CBT group also demonstrated significantly greater reductions in fatigue compared to the control, which were maintained at follow-up. Despite notable improvements in sleep outcomes, intervention groups did not differ significantly compared to TAU in changes of sleepiness and symptoms of depression and anxiety.
Both CBT and LDT were associated with very large reductions in symptoms of insomnia and sleep disturbance. From baseline to post-intervention, in both intervention groups: (a) mean ISI scores reduced from >13 [just under ‘clinical insomnia (moderate severity)’] to <8 (‘no clinically significant insomnia’); (b) mean PROMIS Sleep Disturbance reduced from the ~75th to the ~34th percentile (i.e. about half a standard deviation lower) compared to the general population; (c) self-reported total sleep time increased from just under six hours to about seven hours at night. In contrast, at post-intervention, the TAU group continued to report an average elevated ISI score (10.74) and 69th population percentile of sleep disturbance.
These findings support the existing body of literature on the efficacy of both CBT (Seyffert et al., Reference Seyffert, Lagisetty, Landgraf, Chopra, Pfeiffer, Conte and Rogers2016) and LDT (van Maanen et al., Reference van Maanen, Meijer, van der Heijden and Oort2016) for improving sleep among diverse populations, and extend the evidence base to insomnia (Manber et al., Reference Manber, Bei, Simpson, Asarnow, Rangel, Sit and Lyell2019) in the latter postpartum. While investigation of potential mechanisms lay outside the scope of the current paper, given the different focus and comprising strategies of the interventions, it is possible that improvements in insomnia symptoms were caused by different mechanisms (e.g. changes in sleep-related cognitions and behaviours in the CBT group, changes to circadian phase and/or amplitude in the LDT group). Future studies are needed to determine possible mechanisms.
The CBT group demonstrated significantly greater improvements in symptoms of fatigue compared to the control. It is likely that the inclusion of CBT-based fatigue strategies (e.g. planning and pacing of daily activities) contributed to these findings. While there was a modest improvement in fatigue symptoms for the LDT group, these changes were not significantly different from the control group. Compared to light therapy trials that found significant reduction in fatigue symptoms [e.g. fatigue following traumatic brain injury (Sinclair, Ponsford, Taffe, Lockley, & Rajaratnam, Reference Sinclair, Ponsford, Taffe, Lockley and Rajaratnam2013)], fatigue experienced in the postpartum period may strongly influenced by psychosocial causes (e.g. family, social demands) that may particularly benefit from CBT-based strategies.
Neither intervention significantly improved sleepiness, a direct consequence of sleep deprivation/disruption. This needs to be interpreted in the context of unique postpartum circumstances, such as frequent overnight awakenings for infant care, which contribute to insufficient sleep (Quin et al., Reference Quin, Lee, Pinnington, Newman, Manber and Bei2022) in new parents. Although sleep improved significantly in both CBT and LDT groups, average sleep efficiency remained low (<80%) at post-intervention, below the >85% that is commonly considered ‘good’. It is possible that sleep disruption may continue to cause sleep-related daytime consequences beyond the subsiding of insomnia symptoms.
Neither intervention produced significant changes in symptoms of depression and anxiety compared to the control. These results are consistent with previous findings (Bei et al., Reference Bei, Pinnington, Quin, Shen, Blumfield, Wiley and Manber2021; Kempler, Sharpe, Marshall, & Bartlett, Reference Kempler, Sharpe, Marshall and Bartlett2020) including two recent RCTs of CBT for prenatal insomnia that found small (Manber et al., Reference Manber, Bei, Simpson, Asarnow, Rangel, Sit and Lyell2019) or non-significant (Kalmbach et al., Reference Kalmbach, Cheng, O'Brien, Swanson, Sangha, Sen and Drake2020) effects on maternal depressive symptoms. Postpartum depression and anxiety are multifactorial, with poor sleep being one of the many contributing factors (Lawson et al., Reference Lawson, Murphy, Sloan, Uleryk and Dalfen2015); numerous psychosocial factors were not addressed in either intervention. Further, studies that found light therapy to be effective in reducing postpartum depression targeted those with depressive disorders (Crowley & Youngstedt, Reference Crowley and Youngstedt2012); participants in this study reported mildly elevated, but not significant symptoms of depression and anxiety.
Findings need to be interpreted in light of limitations. (1) Participants were mostly white, partnered, educated at the tertiary-level, and lived in a developed country (Australia); further, a third of those registered interest did not proceed to complete initial questionnaire; these limit generalisability of findings. Emerging evidence showed that CBT for prenatal insomnia was well-received by an ethnically diverse sample (38% Hispanic; Manber et al., Reference Manber, Bei, Simpson, Asarnow, Rangel, Sit and Lyell2019), and future research is much needed among culturally, linguistically, and socioeconomically diverse communities. (2) This trial focused on first-time parents to reduce confounds of having older children on sleep; adaptations are needed if future research applies these interventions to parents with more than one child. (3) Both interventions focused on maternal insomnia, and do not address sleep disruption caused by infant awakenings. (4) Partner behaviours/sleep was not assessed; future intervention could benefit from further involvement of the partner. (5) Findings cannot generalise to those with severe mental health conditions as these participants were excluded. Further, among individuals who met questionnaire-based criteria, those who attended further telephone screening reported lower anxiety (M = 54.25, s.d. = 7.58) and depressive symptom (M = 53.18, s.d. = 6.84) compared to those who did not proceed with further screening (anxiety M = 56.97, s.d. = 7.77; p = .007, d = 0.36; depression M = 55.37, s.d. = 7.39; p = .02, d = 0.31; p > 0.064 for other demographics, sleep, and mental health measures), suggesting further limitations to the generalisability of current findings. (6) Due to resource constraints, objective assessments of sleep and circadian phase were not taken, and longer-term follow-up, beyond one month, was not conducted.
Nevertheless, this is an adequately powered RCT of non-pharmacological interventions to specifically target postpartum insomnia. Perinatal services typically diminish after the initial postpartum months, and insomnia that emerges later in the postpartum period often remains unaddressed. This trial simultaneously tested two promising interventions to expedite intervention development. The non-pharmacological nature of these interventions is particularly relevant for those who breastfeed. Further, the interventions combined low-cost remote digital intervention delivery with brief therapist assistance to allow for personalisation. The scalable nature of both CBT and LDT, both shown efficacious for postpartum insomnia in this trial, holds promise for reaching large number of people in the community. Future research is needed to not only better understand the differential mechanisms of these interventions, but also, who may be better suited for which intervention, including investigation of whether interventions may be more efficacious depending on certain characteristics, such as chronotype and levels of insomnia symptoms. Larger trials evaluating the effectiveness, cost-effectiveness, and implementation potentials of these interventions in diverse communities are needed to further inform possible translation of these interventions into real-world practice.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722002616.
Acknowledgements
We thank all participants for their time and commitment. We are grateful for input from: Tracey Sletten, Elise McGlashan, Katherine Lawrence, Darren Mansfield, and Sean Cain. We would like to acknowledge Smiling Mind for use of audio materials. We acknowledge the Aboriginal and Torres Strait Islander peoples as the traditional custodians of the land upon which this trial was carried out and pay respects to Elders past, present and emerging.
Financial support
Official Trial Sponsor: Monash University
Verma and Quin are recipients of Australian Government Research Training Program Scholarship. Bei (APP1140299) and Wiley (APP1178487) are supported by National Health and Medical Research Council Fellowships. The funding sources had no role in design or undertaking of the current trial, including data analyses, interpretation, or decision to submit the manuscript for publication.
Conflict of interest
Rajaratnam is the Chair of the Sleep Health Foundation (Australia) and separately received a payment from the Restorative Sleep Expert Panel (USD1000 paid to the University). Lucimed SA provided light therapy glasses free-of-cost for the duration of the trial; Lucimed SA had no input to the design and execution of the trial.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Ethics approval was obtained from the Monash University Human Research Ethics Committee (9780) and the Department of Education and Training Victoria (2018_003774). The trial was registered prospectively with Australia and New Zealand Clinical Trials Registry (ACTRN12618000842268). Informed consent was obtained from all participants. All participants were screened via telephone interview for risk of harm to themselves and/or others. Those deemed to be of high risk were referred to appropriate services as per the research protocol.








