Introduction
Irritable bowel syndrome (IBS) is a functional disorder of the gastrointestinal (GI) tract, most commonly associated with symptoms of abdominal pain or discomfort, bloating and a significant change in bowel habits, with no clearly identifiable GI pathology (Longstreth et al. Reference Longstreth, Thompson, Chey, Houghton, Mearin and Spiller2006). IBS affects around 10% of adults in Western countries (Clarke et al. Reference Clarke, Quigley, Cryan and Dinan2009), and is 2–3 times more common in females (Drossman et al. Reference Drossman, Camilleri, Mayer and Whitehead2002). Symptoms of IBS are chronic and in some cases debilitating, considerably reducing the quality of life of IBS sufferers (Wilson et al. Reference Wilson, Longstreth, Knight, Wong, Wade, Chiou, Barghout, Frech and Ofman2004) and accounting for a significant proportion of work absenteeism and presenteeism (Spiegel, Reference Spiegel2009).
The underlying pathophysiology of IBS is still poorly understood. However, it is widely viewed as a disorder caused by a dysregulation of the complex interactions that exist along the brain–gut axis (Grenham et al. Reference Grenham, Clarke, Cryan and Dinan2011). Altered hypothalamic–pituitary–adrenal (HPA) axis function (Mayer, Reference Mayer2000), autonomic dysfunction (Tillisch et al. Reference Tillisch, Mayer, Labus, Stains, Chang and Naliboff2005), immune activation (Ohman & Simren, Reference Ohman and Simren2010) and heightened central pain sensitivity (Mayer et al. Reference Mayer, Aziz, Coen, Kern, Labus, Lane, Kuo, Naliboff and Tracey2009) are considered key pathophysiological features related to abnormal brain–gut interactions in IBS. Observational studies have identified that stressors, such as early life trauma (Chitkara et al. Reference Chitkara, van Tilburg, Blois-Martin and Whitehead2008) or chronic stressful life events experienced in adolescence or adulthood (Blanchard et al. Reference Blanchard, Lackner, Jaccard, Rowell, Carosella, Powell, Sanders, Krasner and Kuhn2008), are major risk factors for IBS. These clinical observations have been substantiated by numerous pre-clinical rodent studies that have shown that chronic stress can induce some of the key clinical features of IBS, such as altered HPA axis function, heightened immune activity and visceral hypersensitivity (Coutinho et al. Reference Coutinho, Plotsky, Sablad, Miller, Zhou, Bayati, McRoberts and Mayer2002; O'Mahony et al. Reference O'Mahony, Marchesi, Scully, Codling, Ceolho, Quigley, Cryan and Dinan2009; Moloney et al. Reference Moloney, O'Leary, Felice, Bettler, Dinan and Cryan2012; Tramullas et al. Reference Tramullas, Dinan and Cryan2012; Wang et al. Reference Wang, Ocampo, Pang, Bota, Bradesi, Mayer and Holschneider2013).
However, attempts to characterize the functioning of the HPA axis and sympathetic nervous system (SNS), the two key stress systems in humans and vital pathways in maintaining normal brain–gut axis interactions (Manabe et al. Reference Manabe, Tanaka, Hata, Kusunoki and Haruma2009), in IBS, have produced inconsistent and conflicting results with regard to basal cortisol levels (Bohmelt et al. Reference Bohmelt, Nater, Franke, Hellhammer and Ehlert2005; McKernan et al. Reference McKernan, Gaszner, Quigley, Cryan and Dinan2011), plasma norepinephrine (Posserud et al. Reference Posserud, Agerforz, Ekman, Bjornsson, Abrahamsson and Simren2004; Manabe et al. Reference Manabe, Tanaka, Hata, Kusunoki and Haruma2009), the HPA axis response to pharmacological challenge (Bohmelt et al. Reference Bohmelt, Nater, Franke, Hellhammer and Ehlert2005; Dinan et al. Reference Dinan, Quigley, Ahmed, Scully, O'Brien, O'Mahony, O'Mahony, Shanahan and Keeling2006), and the HPA axis or SNS response to various physical (Tanaka et al. Reference Tanaka, Manabe, Hata, Kusunoki, Ishii, Sato, Kamada, Shiotani and Haruma2008) and cognitive or psychological type stressors (Murray et al. Reference Murray, Flynn, Ratcliffe, Jacyna, Kamm and Emmanuel2004; Posserud et al. Reference Posserud, Agerforz, Ekman, Bjornsson, Abrahamsson and Simren2004; Elsenbruch et al. Reference Elsenbruch, Lucas, Holtmann, Haag, Gerken, Riemenschneider, Langhorst, Kavelaars, Heijnen and Schedlowski2006). By contrast, a consistent change in GI symptoms, including colonic motility (Fukudo & Suzuki, Reference Fukudo and Suzuki1987) and enhanced perception of visceral pain (Murray et al. Reference Murray, Flynn, Ratcliffe, Jacyna, Kamm and Emmanuel2004), has been found in response to cognitive or physical stressors in IBS.
It is likely that the heterogeneity of IBS related to different subtypes, symptom fluctuations, psychiatric co-morbidity and indeed underlying pathophysiologies may account for some of the disparity in these findings. In addition, other factors with regard to the participant characteristics that will introduce variability when assessing the HPA axis and SNS response include sex (ratio of male to female participants), age, menstrual status and contraceptive use in females, and several psychological factors (Allen et al. Reference Allen, Kennedy, Cryan, Dinan and Clarke2014). However, it is difficult to determine the nature of this variability because of the lack of a standardized and well-validated stressor or challenge test being used across studies. This methodological drawback has been addressed in two recent studies that have introduced the use of the Trier Social Stress Test (TSST; Kirschbaum et al. Reference Kirschbaum, Pirke and Hellhammer1993), the most widely used and well-validated laboratory-based human psychosocial stress procedure to characterize the stress response in IBS (Suarez-Hitz et al. Reference Suarez-Hitz, Otto, Bidlingmaier, Schwizer, Fried and Ehlert2012; Sugaya et al. Reference Sugaya, Izawa, Kimura, Ogawa, Yamada, Shirotsuki, Mikami, Hirata, Nagano, Nomura and Shimada2012). However, both studies are limited in their scope of assessment as they did not measure any additional physiological parameters that are key to IBS, including SNS activity (Manabe et al. Reference Manabe, Tanaka, Hata, Kusunoki and Haruma2009), immune activity (Dinan et al. Reference Dinan, Quigley, Ahmed, Scully, O'Brien, O'Mahony, O'Mahony, Shanahan and Keeling2006; Liebregts et al. Reference Liebregts, Adam, Bredack, Roth, Heinzel, Lester, Downie-Doyle, Smith, Drew, Talley and Holtmann2007; Clarke et al. Reference Clarke, McKernan, Gaszner, Quigley, Cryan and Dinan2012) or any measure of GI symptomatology.
In the current study, in addition to examining the neuroendocrine response to acute psychosocial stress in IBS, we aimed to characterize the SNS response using skin conductance level (SCL), the immune response by measuring salivary C-reactive protein (CRP), the GI symptom response using visual analogue scales (VAS), and also self-reported stress and mood. We documented sleep quality during the prior month in our study population as sleep disturbances are common in IBS (Jarrett et al. Reference Jarrett, Heitkemper, Cain, Burr and Hertig2000; Vege et al. Reference Vege, Locke, Weaver, Farmer, Melton and NJ2004), and poor sleep quality can blunt HPA axis activity (Wright et al. Reference Wright, Valdimarsdottir, Erblich and Bovbjerg2007). Given the sex bias within IBS, we chose to include only females in our study. We hypothesized that, based on previous investigations, female patients with IBS would exhibit an abnormal HPA axis response and a heightened SNS, CRP and GI symptom response to acute psychosocial stress.
Method
Participants
Study participants were all females between 18 and 40 years of age. IBS participants were recruited by direct telephone contact from a database of patients who had taken part in previous studies at Cork University Hospital, and the remainder were recruited, along with control participants, through advertisement from the staff and student population of University College Cork. Thirteen female IBS participants who met Rome III criteria (Longstreth et al. Reference Longstreth, Thompson, Chey, Houghton, Mearin and Spiller2006) and had undergone previous investigations to exclude the presence of organic GI disease, including inflammatory bowel disease and coeliac disease, and 15 female healthy control participants matched on the basis of age, body mass index (BMI) and average units of alcohol consumed per week, were enrolled in the study. IBS participants presenting with any acute or chronic co-existing illness other than that under study were excluded. Exclusion criteria included: being a current or past habitual smoker; having a BMI ⩾30 kg/m2; formal psychiatric diagnosis of major depression, anxiety disorder, bipolar spectrum disorder, schizophrenia or other DSM-IV Axis I disorder; use of psychoactive medication(s) (anxiolytics, antipsychotics, antidepressants, corticosteroids and opioid pain relievers); regular use of non-steroidal anti-inflammatory medications; antibiotic use in the previous 2 weeks; abnormal menstrual cycle; and if perimenopausal, menopausal or postmenopausal. In addition, healthy control participants were excluded if they reported a history of chronic illness, GI or otherwise.
Study procedures
The study protocol (APC028, March 2011) and all procedures were approved by the University College Cork Clinical Research Ethics Committee of the Cork Teaching Hospitals and conducted in accordance with the International Conference on Harmonisation (ICH) Guidelines on Good Clinical Practice and the Declaration of Helsinki. Participants were screened by telephone to check suitability for study inclusion, and were subsequently scheduled to attend a laboratory visit at the Alimentary Pharmabiotic Centre and Human Nutrition Studies Unit, University College Cork. Study visits began between 1430 and 1500 h for each participant to control for diurnal fluctuations in cortisol levels. All participants provided full written informed consent before any experimental procedures commenced. Participants were instructed to abstain from alcohol and strenuous physical exercise for 24 h prior to their study visit. In addition, they were asked not to consume any caffeine-containing products on the day of their study visit, and to consume only water for 2 h prior to the experimental procedure. Upon arrival to the laboratory, height and weight was measured to determine BMI, clinical history was reviewed and previous illness, surgery or hospital admittance was documented along with currently used medications and family history of GI disease, followed by participants completing the screening assessment questionnaires (detailed in the next section). Equipment for measuring SCL was attached to participants and the first saliva sample for measuring salivary cortisol and CRP was collected. Following a 25-min baseline rest period, participants were given standardized written instructions introducing the TSST, which was carried out as described previously (Kudielka et al. Reference Kudielka, Hellhammer and Kirschbaum2007). In brief, following a baseline rest period of 30 min, participants were led to a separate room equipped with a video camera, a microphone and two desks. After reiterating the task instructions, participants were given a 3-min speech preparation period. Following this, participants were required to perform a 5-min speech outlining their suitability for the ideal job of their choice, followed by a 5-min mental arithmetic task in which they serially subtracted 17 from 2023. Both tasks were performed in front of a committee of judges, consisting of one male and one female wearing white laboratory coats and introduced as being experts in identifying non-verbal aspects of behaviour. In addition, participants were informed that their speech would be both audio and video recorded for later behavioural analysis. At the end of the test period, participants returned to the rest room where further questionnaires were completed and saliva samples were collected at 15-min intervals for a further 60 min. SCL was measured throughout the procedure. Participants were fully debriefed following the last sample collection.
Screening assessments
Anxiety and depression
Current symptoms of anxiety and depression were assessed using the self-reported Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, Reference Zigmond and Snaith1983) and the nine-item Patient Health Questionnaire (PHQ-9; Kroenke et al. Reference Kroenke, Spitzer and Williams2001).
Sleep disturbances
Symptoms of sleep disturbance were assessed using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al. Reference Buysse, Reynolds, Monk, Berman and Kupfer1989), which assesses sleep quality over the previous month. The self-reported 19 items are designed to measure seven key components indicating problematic or non-problematic sleep: sleep latency, sleep duration, sleep efficiency, sleep disturbances, subjective sleep quality, use of sleep medication and daytime dysfunction due to sleep disturbance. Scores on each component are totalled to give a global score, with ⩾5 indicating significant sleep disturbances.
GI symptom severity
GI symptoms were measured using the IBS Symptom Severity Scale (IBS-SSS; Francis et al. Reference Francis, Morris and Whorwell1997). The IBS-SSS is a VAS assessing current abdominal pain, abdominal distension (bloating or swelling), satisfaction with bowel habit and interference of symptoms with daily life. The maximum possible score is 500, with cut-off points determined as: < 75 healthy control; 75–174 mild; 175–300 moderate; and > 300 severe IBS.
Physiological measures
HPA axis: salivary free cortisol sampling and analysis
Saliva samples were obtained using Salivette® devices (Sarstedt, Ireland). At each collection time-point, participants were instructed to roll the synthetic bud around their mouth while chewing lightly for 1.5 min. Samples were kept chilled at ~4°C until the end of the experimental protocol. Salivettes were then centrifuged for 10 min at 1000 g to extract saliva and samples were stored at –80°C until analysis. Cortisol concentrations were determined using the Cortisol Enzyme Immunoassay Kit according to the manufacturer's instruction (Enzo® Life Sciences, UK). The assay detection limit was 0.16 nmol/l. Inter- and intra-assay coefficients of variance (CVs) were 6.6% and 5.4% respectively.
Inflammatory activity: salivary CRP sampling and analysis
Salivary CRP has been shown to correlate moderately well with blood levels of CRP (Browne et al. Reference Browne, Kantarci, Lamonte, Andrews, Hovey, Falkner, Cekici, Stephens, Genco, Scannapieco, Van Dyke and Wactawski-Wende2013) and is being increasingly used as a non-invasive means of measuring systemic inflammatory activity (Pace et al. Reference Pace, Negi, Dodson-Lavelle, Ozawa-de Silva, Reddy, Cole, Danese, Craighead and Raison2013). In addition, salivary CRP has previously been used to assess the psychosocial stress-induced inflammatory response (Campisi et al. Reference Campisi, Bravo, Cole and Gobeil2012). Four saliva samples, collected as outlined earlier, were also analysed for levels of CRP at t0 (immediately pre-TSST), t+20 (immediately post-TSST), t+35 and t+65, using a high-sensitivity commercially available electrochemiluminescence MULTI-SPOT® Meso Scale Discovery kit (MSD, USA). Based on a previous methodology (Browne et al. Reference Browne, Kantarci, Lamonte, Andrews, Hovey, Falkner, Cekici, Stephens, Genco, Scannapieco, Van Dyke and Wactawski-Wende2013), an optimal dilution of the sample with diluent (1:5) was determined prior to completion of sample analysis. Otherwise, the assay was carried out according to the manufacturer's protocol.
SNS activity: SCL
SCL reflects activity in eccrine sweat glands that are directly under SNS control, and as such, measuring SCL is a well-validated and much more sensitive measure of SNS activity than other autonomic measures such as heart rate, blood pressure or respiration (Khalfa et al. Reference Khalfa, Isabelle, Jean-Pierre and Manon2002). SCL was recorded throughout the experimental protocol using a NeXus-4 ambulatory monitoring device (Mind Media BV, The Netherlands). Prior to baseline measurements, disposable electrodes pre-gelled with isotonic gel (BIOPAC, EL507; Linton Instrumentation, UK) were attached to the distal phalanx of the index and middle fingers and the SCL signal was allowed to stabilize. SCL was sampled continuously at 32 Hz and recorded directly onto the built-in flash memory. Data were extracted and pre-analysis processing was performed using Biotrace+ software. SCL data were averaged over 5-min epochs during baseline (5 × 5-min epochs), stress (5 × 5-min epochs) and recovery (12 × 5-min epochs).
Self-report measures
GI symptoms
At four time-points (t − 30, t0, t + 20 and t + 80) during the TSST protocol, participants completed self-report measures. GI symptoms were assessed using subscales of the IBS-SSS (Francis et al. Reference Francis, Morris and Whorwell1997) that measure the specific symptoms of 'abdominal pain’ and 'abdominal fullness, bloating or swelling’, using a VAS ranging from 0 (no pain/abdominal fullness, bloating or swelling) to 100 (very severe pain/abdominal fullness, bloating or swelling). In addition, 'urge to have a bowel movement’ was rated on a VAS ranging from 0 (not at all) to 100 (completely).
Self-reported stress and mood
Mood was assessed using the Positive and Negative Affect Schedule (PANAS; Watson et al. Reference Watson, Clark and Tellegen1988) and self-reported stress was measured using a VAS, ranging from 0 (not stressed at all) to 100 (as stressed as I could possibly imagine).
Statistical analysis
Independent-sample t tests were used to explore differences in group characteristics (age, BMI, units of alcohol per week), measures of anxiety and depression [HADS anxiety (HADS-A), HADS depression (HADS-D), PHQ-9], sleep disturbance (PSQI global scores) and GI symptom severity (IBS-SSS total scores). χ 2 was used to determine whether stage of menstrual cycle and contraceptive use differed between IBS patients and healthy controls. Cortisol data were not normally distributed and were therefore transformed at each time-point using a natural log transformation (ln) before analysis. Individual baseline cortisol, CRP and SCL values were compared between groups with independent-sample t tests to ensure that groups did not differ on these values at the beginning of the stress procedure. Repeated-measures analysis of variance (ANOVA) was used to determine group differences and the main effect of stress on cortisol levels, SCL, CRP levels, GI symptoms, self-reported stress and positive and negative affect (PANAS), followed by post-hoc inspection of pairwise comparisons using a Bonferroni correction for multiple comparisons as appropriate. The total GI symptom response was further investigated within the IBS group alone, with planned comparisons of pairwise contrasts between t − 30 and t0, t0 and t + 20 and t + 20 and t + 80. As the GI symptom response to the TSST has not previously been reported, this analysis was exploratory and we did not correct for multiple comparisons (Bender & Lange, Reference Bender and Lange2001; Feise, Reference Feise2002). Where Mauchly's test of sphericity was significant, the Greenhouse–Geisser or Huynh–Feldt correction was applied. An area under the curve with respect to ground (AUCG), area under the curve with respect to increase (AUCi; Pruessner et al. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003) and delta cortisol calculation was performed, and a mean change from baseline on SCL data was determined, followed by independent-sample t tests to determine group differences on each measure. Self-report measures of stress, negative affect and positive affect were further analysed by determining an AUGG and delta response in each group, followed by independent-sample t tests to determine group differences. The AUCG cortisol outcome measure was selected a priori as our primary end-point. Based on previous investigations in other pathological conditions with sample sizes of 10–14 participants per group (Chopra et al. Reference Chopra, Ravindran, Kennedy, Mackenzie, Matthews, Anisman, Bagby, Farvolden and Levitan2009; Monteleone et al. Reference Monteleone, Scognamiglio, Canestrelli, Serino, Monteleone and Maj2011), our study was powered to detect a 1.5-fold group difference on AUCG cortisol levels with an expected large effect size d = 0.80 (Cohen, Reference Cohen1988), with ⩾0.80 power and α = 0.05. Data are presented as mean ± standard error of the mean (s.e.m.). Effect sizes are reported as partial eta squared (η p 2) with a small effect approximately η p 2 = 0.01; medium effect, η p 2 = 0.06; large effect, η p 2 = 0.14 (Cohen, Reference Cohen1988). All statistical procedures were carried out using the IBM SPSS Statistics 20.0 for Windows software package.
Results
Sample characteristics
Group characteristics for healthy control participants and patients with IBS are presented in Table 1. The groups did not differ significantly in age, BMI or number of units of alcohol consumed per week. According to Rome III criteria (Longstreth et al. Reference Longstreth, Thompson, Chey, Houghton, Mearin and Spiller2006), two patients with IBS were diarrhoea predominant (IBS-D), three were constipation predominant (IBS-C) and eight were mixed (IBS-M). Patients with IBS scored higher on the PHQ-9 (p = 0.005), suggesting mild levels of possible depression. However, the groups did not differ on levels of anxiety or depression as measured by the HADS. Patients with IBS and healthy controls exhibited sleep disturbance in the previous month (PSQI⩾5). However, global sleep quality scores were not significantly different between the groups. IBS patients were in the moderate symptom severity range as measured by the IBS-SSS (175–300), and healthy controls exhibited no problematic GI symptoms (IBS v. control, p < 0.001). The groups did not differ significantly on stage of menstrual cycle or oral contraceptive use.
Table 1. Comparison of group demographics and clinical characteristics
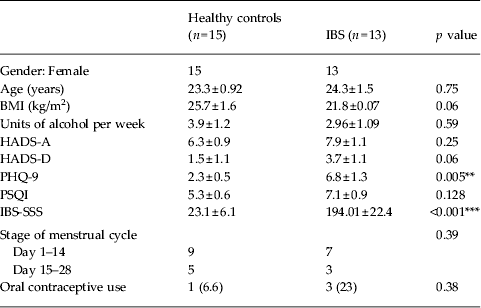
IBS, Irritable bowel syndrome; BMI, body mass index; HADS-A/D, Hospital Anxiety and Depression Scale – Anxiety/Depression; PHQ-9, nine-item Patient Health Questionnaire; PSQI, Pittsburgh Sleep Quality Index; IBS-SSS, IBS Symptom Severity Scale.
Data given as mean ± standard error of the mean (s.e.m.) or number (percentage).
** p < 0.01, *** p < 0.001.
HPA axis response
The groups did not differ on baseline cortisol sample collected at t–30 (t 26 = 0.383, p = 0.23). Repeated-measures ANOVA across all sample collection time-points revealed a significant main effect of the TSST on salivary cortisol levels (F 4.16,108.11 = 8.34, p < 0.001, η p 2 = 0.243) and a significant main effect of group (F 1,26 = 4.96, p = 0.035, η p 2 = 0.16; Fig. 1 a) but no group × stress interaction (F 4.16,108.11 = 1.19, p = 0.32, η p 2 = 0.044). The total overall output of cortisol throughout the stress procedure, as examined by AUCG analysis, showed that patients with IBS exhibited a greater total cortisol output following exposure to the TSST (t 26 = 2.116, p = 0.044, see Fig. 1 b), but no difference in the delta cortisol response (t 26 = 0.16, p = 0.87, see Fig. 1 c) or in the AUCI analysis (t 26 = 0.57, p = 0.57; data not shown) was found between patients and healthy control participants. Finally, analysis of cortisol levels at t+80 alone indicated a failure to shut off the HPA axis response in IBS, as cortisol levels remained elevated in comparison to healthy controls at the end of the recovery period (t 26 = 2.486, p = 0.02).

Fig. 1. Group comparisons of the salivary cortisol response to the Trier Social Stress Test (TSST) in patients with irritable bowel syndrome (IBS) (n = 13) and healthy control participants (n = 15). (a) Cortisol values collected at each time-point throughout the TSST protocol (ANOVA group effect: IBS versus control with Bonferroni correction, p < 0.05). (b) Area under the curve with respect to ground (AUCG) analysis on salivary cortisol samples at each measurement time-point (* p < 0.05, IBS versus control). (c) Delta cortisol response to the TSST. Data are presented as mean ± standard error of the mean (s.e.m.).
Salivary CRP response
The groups did not differ relative to the baseline CRP sample collected at t0 (t 26 = 0.854, p = 0.401). Repeated-measures analysis showed a significant main effect of stress on salivary CRP levels (F 3,78 = 6.63, p < 0.001, η p 2 = 0.203) but no effect of group (F 1,26 = 0.32, p = 0.58, η p 2 = 0.012) or stress × group interaction (F 3,78 = 1.052, p = 0.37, η p 2 = 0.039; see Fig. 2 a).

Fig. 2. Group comparisons of (a) salivary C-reactive protein (CRP) and (b) sympathetic nervous system (SNS) skin conductance level (SCL) response to the Trier Social Stress Test (TSST) in patients with irritable bowel syndrome (IBS) and healthy control participants. Data are presented as mean ± standard error of the mean (s.e.m.).
SNS response: SCL
The average baseline SCL (epochs 1–5) did not differ between groups (t 21 = 0.73, p = 0.47). SCL averaged over 5-min epochs showed a significant increase due to stress (F 2.12,44.63 = 7.73, p = 0.001, η p 2 = 0.27) but there was no group effect (F 1,21 = 0.51, p = 0.48, η p 2 = 0.024) or group × stress interaction (F 2.12,44.63 = 0.233, p = 0.81, η p 2 = 0.011; see Fig. 2 b). In concordance, there was no difference between patients with IBS and healthy controls on the mean change in SCL from baseline (t 21 = 0.19, p = 0.85; data not shown).
GI symptom response
As expected, there was a main effect of group on total GI symptom severity throughout the experimental stress protocol. Patients with IBS reported greater symptoms than healthy control participants (F 1,25 = 20.765, p < 0.001, η p 2 = 0.454). However, there was no main effect of stress (F 3,75 = 2.209, p = 0.094, η p 2 = 0.081) or stress × group interaction (F 3,75 = 2.036, p = 0.116, η p 2 = 0.075). To investigate the GI symptom response to stress within the IBS group alone, paired-samples t tests were carried out between each time-point and revealed a significant increase in total symptom severity from t+20 to t+80 (t 12 = 2.235, p = 0.045; see Fig. 3), but no significant change from t–30 to t0 (t 12 = 0.811, p = 0.42) or from t0 to t20 (t 12 = 1.021, p = 0.33). There was a trend towards a decrease in abdominal pain from t−30 to t+20 (t 12 = 2.01, p = 0.067), but there were no other significant changes on individual GI symptom measures of 'abdominal pain’ and 'abdominal fullness, bloating or swelling’ or 'urge to have a bowel movement’ (data not shown).

Fig. 3. Group comparison of the total gastrointestinal (GI) symptom response to the Trier Social Stress Test (TSST) [* p < 0.05; change in GI symptoms from t + 20 to t + 80 within the irritable bowel syndrome (IBS) group]. Data are presented as mean ± standard error of the mean (s.e.m.).
Self-reported stress and mood response
The TSST significantly increased self-reported stress (F 1.59,38.32 = 11.91, p < 0.001, η p 2 = 0.32) and reduced positive affect (F 2,52 = 9.05, p < 0.001, η p 2 = 0.258) and increased negative affect (F 2,52 = 21.42, p < 0.001, η p 2 = 0.45), but there was no differential effect on the mood and subjective stress response to the TSST between patients with IBS and healthy control participants (all p > 0.05; see Fig. 4). In concordance, the AUCG analysis of self-reported stress (t 20 = 0.456, p = 0.654) and positive affect (t 26 = 1.115, p = 0.275) and negative affect (t 26 = 0.858, p = 0.399) showed no significant group differences. Finally, the delta response for self-reported stress showed a more pronounced increase from baseline in healthy control participants (t 20 = 2.28, p = 0.034), but no group differences were found on the delta response for positive affect (t 26 = 0.307, p = 0.761) and negative affect (t 26 = 1.087, p = 0.287).

Fig. 4. Group comparisons throughout the Trier Social Stress Test (TSST) protocol on (a) self-reported stress, (b) positive affect and (c) negative affect in patients with irritable bowel syndrome (IBS) and healthy control participants. PANAS, Positive and Negative Affect Schedule. Data are presented as mean ± standard error of the mean (s.e.m.).
Discussion
In this study we aimed to characterize the physiological and psychological responses to acute psychosocial stress in IBS. We used the TSST, the most widely used and well-validated, standardized laboratory-based psychosocial stress task (Kudielka et al. Reference Kudielka, Hellhammer and Kirschbaum2007), to examine the stress responsiveness of several physiological parameters including the HPA axis, SNS and immune system, in addition to self-reported GI symptoms, stress and mood, in female patients with IBS. This is, to our knowledge, the first time the effects of acute psychosocial stress on a broad range of physiological and psychological parameters have been examined in IBS.
Our key finding is that, following acute psychosocial stress, patients with IBS exhibit a more sustained HPA axis response, as shown by a greater total salivary cortisol output throughout the TSST procedure, when compared to healthy control participants. Furthermore, when cortisol levels were examined at the end of the recovery period, we found that levels were significantly elevated in IBS in comparison to healthy controls. It should be noted, however, that we did not identify a group difference on the AUCi cortisol levels (an index of system sensitivity) throughout the TSST (Pruessner et al. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003) or in the overall magnitude of response from baseline to peak output. Taken together, this indicates a total greater cortisol output in response to acute psychosocial stress in IBS that is largely reflective of both a failure to adequately shut off the HPA axis following stress and a generalized elevation in HPA axis activity in IBS, rather than a difference in the intensity of the response. Despite the well-recognized role of HPA axis dysfunction in the pathophysiology of IBS (Mayer, Reference Mayer2000), results of HPA challenge tests have been inconsistent between studies, with many reports that patients with IBS do not differ in the neuroendocrine response to various stressors including a dichotomous listening task (Murray et al. Reference Murray, Flynn, Ratcliffe, Jacyna, Kamm and Emmanuel2004), the Stroop task (Posserud et al. Reference Posserud, Agerforz, Ekman, Bjornsson, Abrahamsson and Simren2004) and a short (3-min) unstandardized public-speaking task (Elsenbruch et al. Reference Elsenbruch, Lucas, Holtmann, Haag, Gerken, Riemenschneider, Langhorst, Kavelaars, Heijnen and Schedlowski2006). However, in accordance with our findings, it has previously been shown that pharmacological challenge of the HPA axis using a corticotrophin-releasing factor (CRF) infusion, in a large well-characterized and carefully phenotyped population of patients with IBS, caused an exaggerated release of serum adrenocorticotrophic-releasing hormone (ACTH) and cortisol, with a normal response to the dexamethasone (DEX) suppression test (Dinan et al. Reference Dinan, Quigley, Ahmed, Scully, O'Brien, O'Mahony, O'Mahony, Shanahan and Keeling2006).
Unlike CRF infusion or the DEX suppression test, which activate and suppress respectively the HPA axis using synthetic pharmacological compounds, the TSST creates an environment where the individual experiences psychosocial stress induced by the threat of negative social evaluation, and a sense of uncontrollability (Dickerson & Kemeny, Reference Dickerson and Kemeny2004). As such, higher cortical and subcortical brain regions play a predominant role in mediating the subsequent activation of the HPA axis in response to this type of stress. A study using the Montreal Imaging Stress Task (MIST), a neuroimaging adaptation of the TSST, has highlighted that deactivation of the hippocampus following exposure to psychosocial stress may play a significant role in mediating the extent of HPA axis activation (Pruessner et al. Reference Pruessner, Dedovic, Khalili-Mahani, Engert, Pruessner, Buss, Renwick, Dagher, Meaney and Lupien2008). The role of these brain regions in mediating the HPA axis response to acute psychosocial stress in IBS requires further investigation at a functional brain imaging level. However, it is well established that altered central processing of visceral pain plays a key role in IBS (Mayer et al. Reference Mayer, Aziz, Coen, Kern, Labus, Lane, Kuo, Naliboff and Tracey2009), and we have recently provided evidence that IBS is associated with a deficit in hippocampal-mediated cognitive function (Kennedy et al. Reference Kennedy, Clarke, O'Neill, Groeger, Quigley, Shanahan, Cryan and Dinan2013). Thus, when considering our results along with previous pharmacological challenge tests (Dinan et al. Reference Dinan, Quigley, Ahmed, Scully, O'Brien, O'Mahony, O'Mahony, Shanahan and Keeling2006) and a recent study describing elevated cortisol levels in anticipation of a public-speaking task (Heitkemper et al. Reference Heitkemper, Cain, Deechakawan, Poppe, Jun, Burr and Jarrett2012), it is likely that HPA axis dysfunction in IBS is best characterized as an exaggerated response, or sustained activation following acute stress, which may, in part, be mediated by altered central processing of the stressful environment.
In concordance with previous reports (Fukudo & Suzuki, Reference Fukudo and Suzuki1987; Murray et al. Reference Murray, Flynn, Ratcliffe, Jacyna, Kamm and Emmanuel2004), we found that GI symptoms in IBS increased significantly during the recovery period after stress, with no change in healthy control participants. Although not reaching statistical significance, it is noteworthy that stress seemed to have differential effects on mean scores of individual GI symptoms with very little change in abdominal fullness, bloating and swelling throughout the procedure, whereas abdominal pain and urge for a bowel movement seemed to reduce in severity from pre- to post-assessment, and subsequently increase throughout the recovery period. Nonetheless, given that GI symptoms in general are often exacerbated during periods of stress in IBS, due to environmental factors experienced in everyday life (Blanchard et al. Reference Blanchard, Lackner, Jaccard, Rowell, Carosella, Powell, Sanders, Krasner and Kuhn2008), modelling this response in a fully standardized laboratory stress task paves the way for future intervention studies to test the efficacy of novel treatments that may reduce the impact of psychosocial stress on symptoms in IBS.
Two recent studies have used the TSST to examine the stress responsiveness of various parameters in IBS (Suarez-Hitz et al. Reference Suarez-Hitz, Otto, Bidlingmaier, Schwizer, Fried and Ehlert2012; Sugaya et al. Reference Sugaya, Izawa, Kimura, Ogawa, Yamada, Shirotsuki, Mikami, Hirata, Nagano, Nomura and Shimada2012). Sugaya et al. (Reference Sugaya, Izawa, Kimura, Ogawa, Yamada, Shirotsuki, Mikami, Hirata, Nagano, Nomura and Shimada2012) found that, in response to the TSST, the cortisol to dehydroepiandrosterone (DHEA) ratio was higher in IBS than in healthy controls, which is in line with our findings. By contrast, Suarez-Hitz et al. (Reference Suarez-Hitz, Otto, Bidlingmaier, Schwizer, Fried and Ehlert2012) found only a trend towards a blunted HPA axis response to the TSST in female patients with IBS and without psychiatric co-morbidity. These conflicting results may be due to several factors, such as differences in the stress responsivity of IBS subtypes or symptom fluctuations, and probably reflect the well-acknowledged heterogeneity of IBS. However, when compared to our study population, a greater proportion of participants in the study by Suarez-Hitz et al. (Reference Suarez-Hitz, Otto, Bidlingmaier, Schwizer, Fried and Ehlert2012) were using an oral contraceptive (~50%). Given that oral contraceptives have been shown to blunt the salivary free cortisol response to the TSST in healthy females (Kudielka et al. Reference Kudielka, Hellhammer and Kirschbaum2007), it is possible that contraceptive use led to an overall blunting of the HPA axis response in both groups, and may have masked the effects we report here in IBS. However, this is clearly an issue that needs further investigation, and future studies that are powered to detect differences in the HPA axis response to the TSST due to hormonal contraceptive use in patients with IBS are needed.
Patients with IBS did not exhibit an exaggerated SNS response to the TSST as measured by SCL. Although autonomic pathways are considered key in maintaining normal brain–gut interactions (Manabe et al. Reference Manabe, Tanaka, Hata, Kusunoki and Haruma2009), our results suggest that, in response to acute psychosocial stress, the sympathetic branch of the autonomic nervous system is functioning normally in IBS. Furthermore, when comparing the methodological approach between studies, it seems that the SNS response in IBS is dependent on the type of stressor used. For example, studies using either cold pain (Tanaka et al. Reference Tanaka, Manabe, Hata, Kusunoki, Ishii, Sato, Kamada, Shiotani and Haruma2008) or rectal distension (Walter et al. Reference Walter, Bodemar, Hallböök and Thorell2008) have reported blunted fingertip blood flow using Doppler flowmetry and elevated SCL respectively in IBS. By contrast, when a cognitive or psychological type stressor is used, no difference in the noradrenaline or adrenaline response was found in IBS in comparison to healthy control participants (Fukudo & Suzuki, Reference Fukudo and Suzuki1987; Murray et al. Reference Murray, Flynn, Ratcliffe, Jacyna, Kamm and Emmanuel2004; Posserud et al. Reference Posserud, Agerforz, Ekman, Bjornsson, Abrahamsson and Simren2004). Thus, our results are consistent with a growing body of evidence that physical but not psychological stressors can induce an exaggerated SNS response in IBS.
In support of previous findings (Kimura et al. Reference Kimura, Izawa, Sugaya, Ogawa, Yamada, Shirotsuki, Mikami, Hirata, Nagano and Hasegawa2013), we found a significant main effect of stress on salivary CRP levels but no difference between patients with IBS and healthy control participants. Salivary CRP as a non-invasive means of measuring systemic immune activity has become increasingly utilized (Pace et al. Reference Pace, Negi, Dodson-Lavelle, Ozawa-de Silva, Reddy, Cole, Danese, Craighead and Raison2013), given that salivary and blood levels of CRP are moderately well correlated (Browne et al. Reference Browne, Kantarci, Lamonte, Andrews, Hovey, Falkner, Cekici, Stephens, Genco, Scannapieco, Van Dyke and Wactawski-Wende2013). However, and in contrast to cortisol, it is yet to be established if salivary-based inflammatory markers track circulating concentrations in response to acute stress. As such, given that immune dysfunction is a key feature of IBS (Ohman & Simren, Reference Ohman and Simren2010; McKernan et al. Reference McKernan, Gaszner, Quigley, Cryan and Dinan2011), caution is warranted in concluding that the inflammatory response to stress is normal in IBS, and future studies are needed in which both blood-based and salivary inflammatory markers are recorded following the TSST.
A limitation of our study is that we examined the stress response only in female patients with IBS. We chose this approach as IBS is much more prevalent in the female population (Drossman et al. Reference Drossman, Camilleri, Mayer and Whitehead2002), and hence focusing on females alone means that our results are more widely applicable to the general IBS population. Nonetheless, future studies adequately powered to detect differences in the neuroendocrine and GI response to the TSST between male and female patients, in addition to examining the influence of menstrual status, both factors that are known to mediate the HPA axis response in healthy populations (Kudielka et al. Reference Kudielka, Hellhammer and Kirschbaum2007), are needed. It is also worth noting that our sample consisted of a relatively young population of female patients and healthy controls, and therefore it will be important in future studies to determine the impact of age in the neuroendocrine and GI symptom response to acute psychosocial stress in IBS.
Finally, we aimed to exclude participants who had a formally diagnosed mood or anxiety disorder to avoid confounding our results with the presence of psychiatric co-morbidity. Nevertheless, given the high rates of mood and anxiety disorders commonly found in IBS, it is important that future studies include patients with psychiatric co-morbidity when determining the physiological and psychological response to stress in IBS. Such patients may potentially exhibit a different response to the TSST than the one we found. For example, patients with panic disorder have been shown to exhibit a blunted HPA axis response whereas patients with generalized anxiety disorder seem to show a normal HPA axis response but an exaggerated response to the TSST in other parameters such as noradrenaline (Allen et al. Reference Allen, Kennedy, Cryan, Dinan and Clarke2014). Thus, determining the influence of psychiatric co-morbidity on the stress response in IBS will require precise identification of the mood or anxiety disorder the patient may suffer from, and appropriate stratification on this basis.
In conclusion, patients with IBS exhibited a sustained HPA axis response to acute psychosocial stress, in addition to an increase in self-reported GI symptomatology. Given that psychosocial stress is considered a key factor in both the onset and exacerbation of symptoms in IBS (Blanchard et al. Reference Blanchard, Lackner, Jaccard, Rowell, Carosella, Powell, Sanders, Krasner and Kuhn2008), the results of the current study suggest that interventional studies aimed at modulating the HPA axis and GI response to the TSST may help to identify novel therapeutic approaches in IBS. The impact of an overactive HPA axis in daily life is clearly problematic and can impact negatively on numerous physiological processes and possibly on cognition, as has recently been reported in IBS (Kennedy et al. Reference Kennedy, Clarke, O'Neill, Groeger, Quigley, Shanahan, Cryan and Dinan2013). Furthermore, as altered immune function and response to stress are associated with numerous other health problems such as increased susceptibility to heart disease (Kiecolt-Glaser et al. Reference Kiecolt-Glaser, McGuire, Robles and Glaser2002), it is important to fully characterize the inflammatory response to stress in IBS. It has become increasingly clear that the impact of IBS reaches beyond GI problems, and therefore future studies addressing the underlying neurobiological mechanisms mediating altered stress responsiveness in IBS may be beneficial in generating new approaches to treatment.
Acknowledgements
We thank Dr R. Moloney, Dr A. Allen, K. Lomasney, Dr F. Sweeney, Dr R. O'Connor, Dr S. Heuston, Dr J. Dollard and Dr K. Davey for their assistance in data collection.
The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. This study was supported by SFI (grant nos SFI/12/RC/2273, 02/CE/B124 and 07/CE/B1368) and by the Health Research Board (HRB) through Health Research Awards (grant no. HRA_POR/2011/23; T.G.D., J.F.C. and G.C.).
Declaration of Interest
None.







