Introduction
By late 2015, the number of refugees worldwide reached the highest level ever recorded, at an estimated 22.5 million (United Nations High Commissioner for Refugees, 2016). The often extensive trauma history, both in their countries of origin and during the flight, compounded with post-resettlement stress, makes refugees highly susceptible to developing posttraumatic stress disorder (PTSD; Porter and Haslam, Reference Porter and Haslam2005; Johnson and Thompson, Reference Johnson and Thompson2008). Indeed, an epidemiological meta-analysis of 181 studies estimated that the PTSD prevalence among refugees is 30.6% (Steel et al., Reference Steel2009). The exposure to numerous different types of traumatic experiences, which are often repeated and prolonged in nature might account for the fact that refugees present with a more complex symptomatology than other PTSD groups (Nygaard et al., Reference Nygaard, Sonne and Carlsson2017; Fried et al., Reference Fried2018). This underlines both the special need for mental health support and treatment of this population and the need to study the mechanisms of PTSD as they manifest in this diverse group.
In recent years, PTSD research has seen an increasing effort to elucidate the neural substrates of this disorder. In the field of electroencephalographic (EEG) event-related potentials (ERPs), impairments in mechanisms underlying early information processing such as sensory and sensorimotor gating have been reported in PTSD (Javanbakht et al., Reference Javanbakht2011; Kohl et al., Reference Kohl2013). Sensory gating is an essential feature of the central nervous system reflecting a pre-attentive automatic process, in which responses to irrelevant, repetitive stimuli are filtered, or gated, saving processing resources for perceptually more salient input (Adler et al., Reference Adler1982). An extensively used index of this ability is P50 suppression, assessed in a standardized conditioning-testing paradigm. In healthy subjects, when two identical auditory stimuli are presented with a certain inter-stimulus interval, the response amplitude to the second, testing (T) stimuli is significantly decreased in relation to the response amplitude to the first, conditioning (C) stimuli. This process of suppression is thought to be secondary to inhibitory mechanisms triggered by the C stimulus and is usually expressed as the ratio of an individual's response amplitude to T stimuli and that to C stimuli (Waldo and Freedman, Reference Waldo and Freedman1986). Thus, lower ratios are theorized to reflect better sensory gating (Adler et al., Reference Adler1982; Fuerst et al., Reference Fuerst, Gallinat and Boutros2007). Similarly, prepulse inhibition (PPI) of the acoustic startle reflex is an operational measure of sensorimotor gating. A central measure in this paradigm is the startle response, which is elicited by an intense, sudden-onset stimulus (pulse). PPI refers to the attenuation of the startle response, which normally occurs if the startling stimulus is preceded by a non-startling sensory stimulus (prepulse) (Graham, Reference Graham1975). Habituation denotes another characteristic of the startle response which can be assessed concurrently with the PPI. It describes the gradual decrease of the startle response magnitude upon repeated presentation of the same stimulus and is considered an elementary form of non-associative learning (Christoffersen, Reference Christoffersen1997).
Based on previous studies no firm conclusions can be drawn on early information processing in PTSD. Only one other study has examined P50 suppression and PPI within the same cohort of PTSD patients showing impaired P50 gating in the patient group compared with healthy controls and no difference in PPI (Holstein et al., Reference Holstein2010). Deficient P50 gating has been replicated in a number of PTSD studies (Gillette et al., Reference Gillette1997; Neylan et al., Reference Neylan1999; Skinner et al., Reference Skinner1999; Ghisolfi et al., Reference Ghisolfi2004; Gjini et al., Reference Gjini2013), but reports on PPI are less consistent with some studies indicating a reduction of PPI (Ornitz and Pynoos, Reference Ornitz and Pynoos1989; Grillon et al., Reference Grillon1996; Echiverri-Cohen et al., Reference Echiverri-Cohen2016; Pineles et al., Reference Pineles2016), while the majority do not (Butler et al., Reference Butler1990; Morgan et al., Reference Morgan1997; Grillon et al., Reference Grillon1998; Lipschitz et al., Reference Lipschitz2005; Vrana et al., Reference Vrana2013). The same mixed picture is seen for results on startle magnitude (for reviews, see Orr and Roth, Reference Orr and Roth2000; Pitman et al., Reference Pitman2012). Studies examining habituation (of the eye-blink startle response) more consistently report no difference between patients with PTSD and controls (Orr et al., Reference Orr, Metzger and Pitman2002). Clinical correlates of psychophysiological impairments have been sparse and highly disparate (Javanbakht et al., Reference Javanbakht2011).
While the vast majority of the existing literature make use of a healthy control group from the general population, this study compares trauma-affected refugees with PTSD with another group of refugees without PTSD and matching these two groups not only on gender and age, but also on country of origin, eliminating the potential role of culture, ethnicity and migration.
Thus, this paper presents the results of an investigation of the P50 suppression, PPI, startle reactivity and habituation of the eye-blink startle response in a group of refugee victims with PTSD in comparison to healthy refugee controls. In addition, correlations between these psychophysiological measures and PTSD symptomatology, severity and level of functioning were investigated.
Method
The study was approved by the Ethical Committee of the Capital Region of Copenhagen (H-16019360) and by the Danish Data Protection Agency (2012-58-0004). The study was carried out in accordance with the ethical principles and guidelines for medical research as stated in the Declaration of Helsinki.
Participants and clinical assessment
A total of 25 trauma-affected refugees with PTSD and 20 healthy refugee controls matched on gender, age, and country of origin participated in the present study. Participants’ demographics and characteristics are summarized in Table 1.
Table 1. Participants’ demographics and psychometric measures
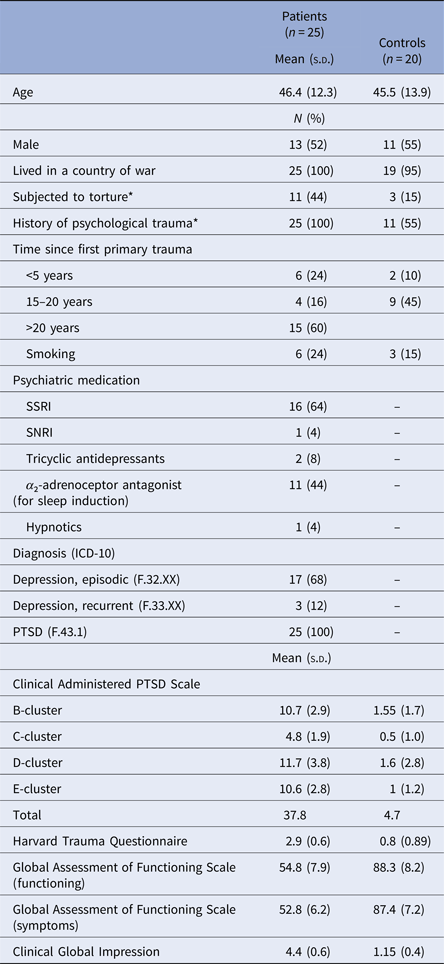
*Group difference significant:
Subjected to torture p = 0.04, History of psychological trauma p < 0.001.
All trauma-affected refugees with PTSD were recruited among patients at the Competence Centre for Transcultural Psychiatry (CTP) in the Capital Region of Denmark. CTP is a specialized mental health out-patient facility and the main target group at CTP consists of trauma-affected refugees with mental health problems (Carlsson et al., Reference Carlsson, Sonne and Silove2014). The healthy refugee controls were recruited from the community by advertisement online and in public areas and were informed to contact the first author (H.M.) if they were interested in participating in the study. The inclusion criteria for the PTSD group were: being a refugee or family-reunified with a refugee, being aged 18 years or older, speaking Danish, English, Arabic, Farsi, or Bosnian, fulfilling the criteria for the PTSD diagnosis according to the ICD-10 and DSM-5 research criteria and being legally competent to provide informed consent. Exclusion criteria were having an ICD-10 diagnosis F2x or F31–32, having a neurological disorder, substance abuse or dependency according to the ICD-10 criteria. Clinicians at CTP referred potential study participants to the researcher (H.M.) carrying out the mental health assessment and the psychophysiology described below. Inclusion criteria for the control group were matching PTSD participants on age (± 5 years), gender and country of origin, speaking Danish, English, Arabic, Farsi, or Bosnian and being legally competent to provide informed consent. Exclusion criteria were the same as for the PTSD group in addition to having any current psychiatric illness confirmed with the Schedule for Clinical Assessment in Neuropsychiatry (SCAN; version 2.1; Wing et al., Reference Wing1990), chapters 1, 10, 14, 16, 17, 18, and 19 (bipolar, schizophrenia spectrum, and other psychotic disorder). PTSD diagnosis was assessed using the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5; Blake et al., Reference Blake1995; Weathers et al., Reference Weathers2015). Symptom severity at the time of electrophysiological testing was further evaluated by administering the Harvard Trauma Questionnaire (HTQ), a validated 16-item self-report rating scale developed for trauma-affected refugees (Mollica et al., Reference Mollica1992). Clinical global impression (CGI) and global assessment of functioning (GAF) were used to assess participants’ overall level of functioning and severity of symptoms.
Psychophysiology
Neither the patients nor the healthy controls had ever participated in psychophysiological research before. All subjects were examined with the Copenhagen Psychophysiology Test Battery (CPTB; Jensen et al., Reference Jensen2008; Wienberg et al., Reference Wienberg2010; Oranje and Glenthøj, Reference Oranje and Glenthøj2012). The CPTB includes PPI, P50 suppression, mismatch negativity, and selective attention paradigms, always administered in this order. Only results of the PPI and P50 suppression paradigms are presented in the present paper. To avoid acute and/or withdrawal effects of nicotine and smoking, all subjects were asked to refrain from smoking 1 h prior and from caffeinated drinks, 2 h prior to test start.
Signal recording
EEG, as well as electromyography (EMG) recordings, were performed with BioSemi® hardware (Amsterdam, The Netherlands), using a cap with 64 active electrodes. The eye-blink component of the acoustic startle response was measured by recording EMG activity from the right m. orbicularis oculi. Two electrodes were placed under the right eye for startle response measurement. The first of these was aligned with the pupil, the other positioned just laterally. BESA software (version 5.2.4, MEGIS Software, Gräfelfing, Germany) was used for further processing of the data.
Paradigms
Both PPI and P50 gating paradigms have been described previously (Jensen et al., Reference Jensen2007; Oranje et al., Reference Oranje2012). Briefly, all auditory stimuli were presented binaurally through stereo insert earphones (Eartone-ABR, C and H Distributors Inc, Milwaukee) by a computer running Presentation (Neurobehavioral Systems, Albany, NY).
PPI paradigm (including habituation and sensitization)
Subjects were seated in a comfortable armchair in a room with a sound level below 40 dB. They were instructed to avoid unnecessary movements, keep their eyes fixed on a spot on the wall directly in front of them, and stay awake. Assessment of PPI and habituation started with five minutes of acclimation to background noise (70 dBa white noise), after which three experimental blocks of stimuli were superimposed on the background noise.
Blocks 1 and 3 were used to assess habituation of the acoustic startle reflex. The two blocks were identical and consisted of eight pulse-alone trials (white noise with an intensity of 115 dBa, and duration of 20 ms, instant rise, and fall) with randomized intertrial intervals between 10 and 20 s. Block 2 consisted of 50 trials presented in a pseudo-randomized order to assess PPI. Because prepulse intensity and interstimulus intervals (ISI) can affect levels of PPI (Braff et al., Reference Braff, Geyer and Swerdlow2001), our paradigm contained two levels of each; prepulse intensities of 6 and 15 dB (white noise, 20 ms in duration) above background and stimulus onset asynchronies (SOA) of 60 and 120 ms. The intertrial intervals were randomized between 10 and 20s. Randomized across the session, 10 pulse alone and 10 of each prepulse–pulse combination (60 ms/76 dBa, 60 ms/85 dBa, 120 ms/76 dBa, and 120 ms/85 dBa) were presented.
Following offline filtering of the data between 25 and 250 Hz, startle amplitude was scored as the highest absolute amplitude in the time interval 20–120 ms after the startle-eliciting pulse, while PPI was expressed as: [(1 − (PP/PA)) × 100%]; where PP: average startle amplitude to prepulse–pulse trials and PA: average amplitude to pulse alone trials.
Sensitization was defined as the increase in startle amplitude from the first to the second trial in the first habituation block. Habituation was calculated as the percentage decrease in startle amplitude from habituation trial 3 (to avoid effects of sensitization) through 16, compared with habituation trial 1 according to the formula: [((trial(n)/trial 1) − 1) × 100%]. Habituation was then defined as the beta coefficient of the best linear fit through these percentages per individual.
P50 suppression
P50 gating was assessed in three identical experimental blocks, each consisting of 40 pairs bursts of white noise (1.5 ms and 80 dB), with an instantaneous rise time, an ISI of 500 ms and a fixed intertrial interval of 10 s. Processing of the data started with correction for eye-movement by applying the surrogate model of BESA. Correction of movement and other non-paradigm-related artifacts were subsequently performed by removing those epochs from the database in which maximum and minimum amplitude differences exceeded 150 µV in the relevant scoring window. Averaged epochs were then filtered between 1 and 70 Hz. P50 amplitudes were scored from the electrode where the highest amplitude was reached (Cz) with average reference, and were defined as the largest trough-to-peak amplitude within an interval of 40–90 ms following the first (conditioning, or ‘C’) stimulus in each paired-click. The P50 amplitude following the second (testing or ‘T’) stimulus was identified as the largest trough to peak amplitude within an interval of ± 10 ms of the latency of the maximum P50 amplitude to the C-stimulus. In addition, N100, and P200 amplitudes to C- and T-stimuli were scored in time windows between 60–140 and 130–250 ms, respectively. P50, N100, and P200 suppression were expressed as the ratio ‘T/C’.
Statistical analyses
All analyses were performed with SPSS version 21.00 (SPSS. Inc., USA). Results were considered to be statistically significant at an alpha level of 0.05.
Although raw startle amplitude and sensitization data were normally distributed, this was not the case for habituation (β-coefficients, see above) nor for all PPI data. Therefore, only PPI tests for which no suitable non-parametric alternative was available were performed parametrically. All others were performed non-parametrically. Raw startle amplitude was analyzed with repeated measures ANOVA with ‘group’ as a between-subjects variable (controls v. patients), and ‘stimulus’ as a within-subjects variable (pulse alone and the four prepulse–pulse combinations). PPI was analyzed similarly using ‘group’ as between-subjects variable and ‘prepulse intensity’ and ‘SOA’ as within-subjects variables. A possible group effect on habituation was analyzed with a Mann–Whitney test, while habituation within groups was analyzed by one sample Wilcoxon tests. Sensitization was analyzed with repeated measures ANOVA with ‘group’ as between-subjects variable and ‘trial’ as a within-subjects variable (amplitude trial 1 v. amplitude trial 2 of block 1).
Raw data from the P50 suppression paradigm (P50, N100, and P200 amplitudes) were analyzed by repeated measures ANOVA with ‘group’ as between-subjects variable and ‘stimulus’ as a within-subjects variable (average amplitude to C v. T stimuli). Possible group effects on suppression (T/C) of the three ERPs were analyzed by Mann–Whitney tests.
The relation between the EEG/EMG measures (data from PPI and P50 suppression paradigms) and symptom/function scales (GAF-f, GAF-S, CGI, HTQ, CAPS-total, and subscores) were investigated with either Pearson's or Spearman's correlation tests, depending on the distribution of the data.
Results
Age, gender, and smoking did not display statistically significant covariance in any of our tests likely reflecting our matching procedures. Table 1 gives the sociodemographic characteristics, trauma history, psychiatric medication, diagnoses, and level of symptom severity.
PPI paradigm (Table 2, Figs 1 and 2)
Analysis of the raw startle amplitude data in the PPI block showed a significant effect of trial type [F (4,40) = 7.07, p < 0.001, η 2 = 0.41], indicating significantly reduced amplitudes to prepulse–pulse trials compared to pulse-alone trials. The average startle amplitude in the PPI block was significantly higher in patients than in controls (Z = 2.17, p = 0.030, d = 0.42). We found no further significant group effects in the raw amplitude data (p > 0.109, η 2<0.091).
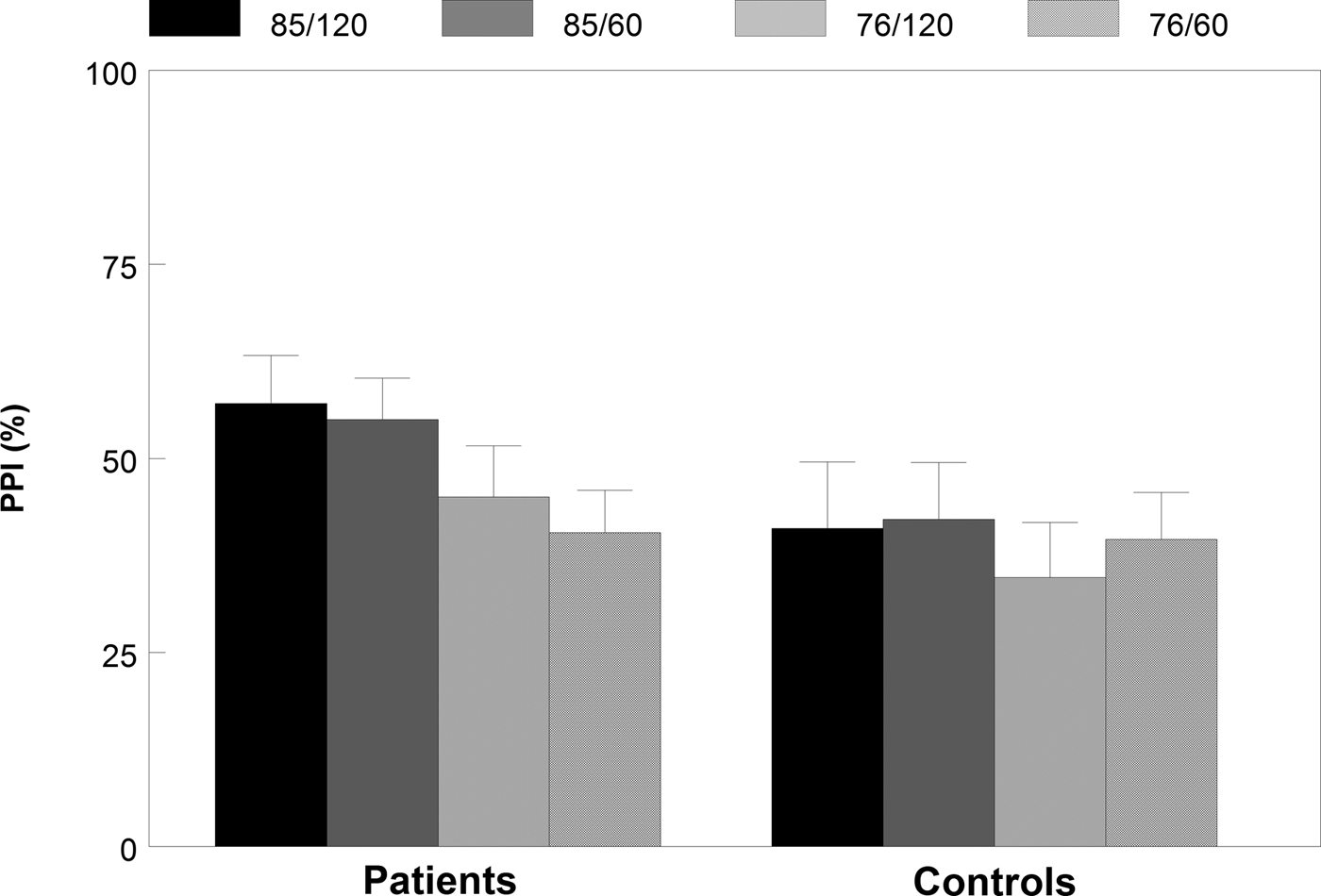
Fig. 1. Percentage PPI (s.e.m.) for all four different prepulse-pulse trials in patients and matched controls.
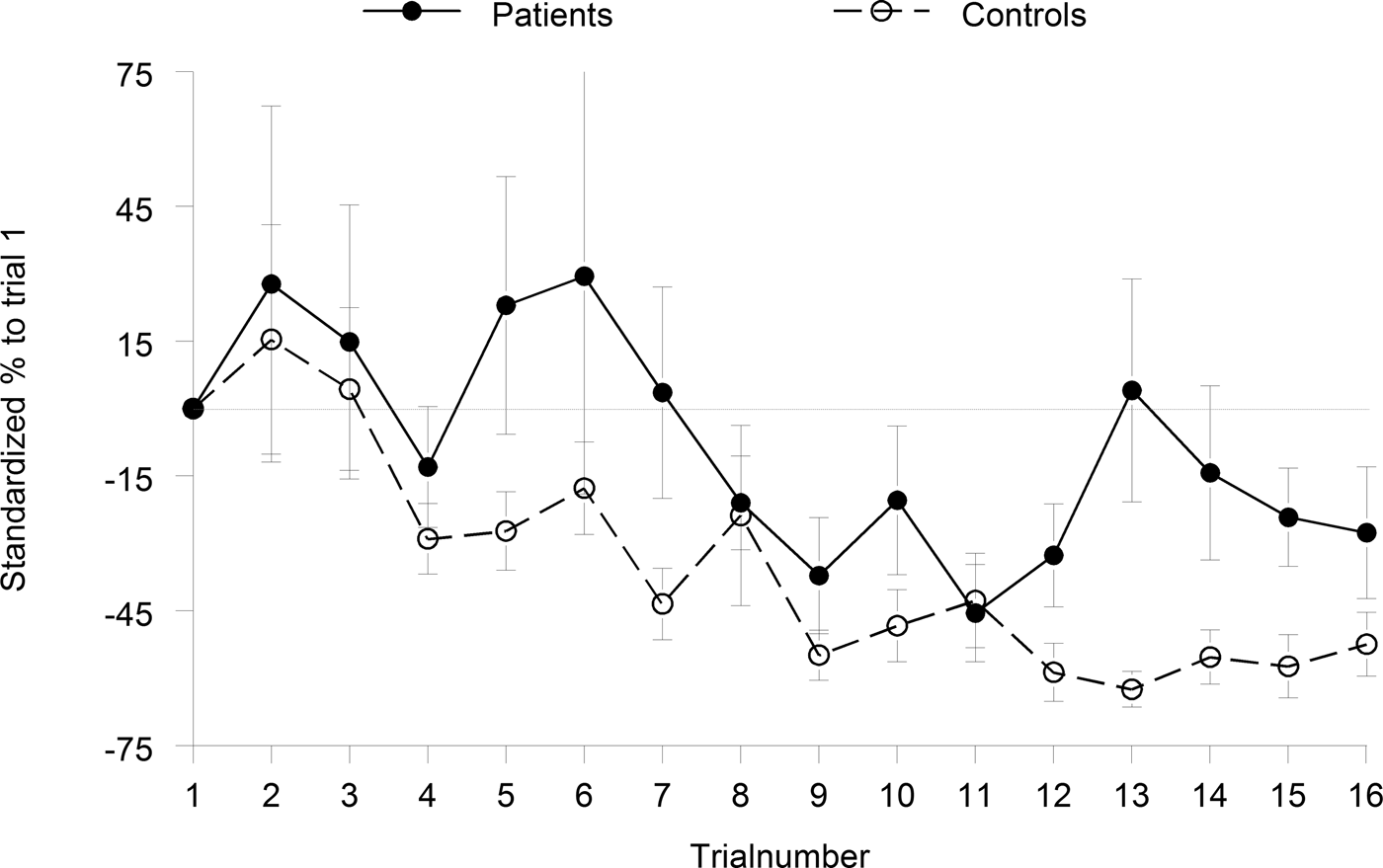
Fig. 2. Habituation and sensitization responses to the pulse-alone trials of blocks 1 and 3. Habituation was calculated as the percentage decrease in startle amplitude from trial 3 through 16.
Table 2. Electrophysiological measures

C, T, and PA values are in μV (s.e.m.).
C, response to conditioning stimulus; T, response to testing stimulus; PA, pulse alone.
*p < 0.05 (difference between patients and controls).
In the percentage PPI data, we found a significant main effect of prepulse intensity, [F (1,43) = 17.73, p < 0.001, η 2 = 0.19], indicating higher PPI in the high intensity (85 dB) prepulse–pulse trials than in the low intensity (76 dB) prepulse–pulse trials. In addition, a significant prepulse intensity × group interaction effect was found, indicating that patients had significantly higher levels of PPI to the high-intensity prepulses than to the low-intensity prepulses compared with controls [F (1,43) = 4.41, p = 0.042, η 2 = 0.093]. However, no further significant group effects were found in the percentage PPI data (t < 1.56, p > 0.13, d < 0.46).
In the habituation blocks, we found no significant increase of startle amplitude from the first to the second trial, indicating no significant sensitization in either group (p > 0.46, d < 0.14). Although the group difference in habituation (=β coefficients) reached only trend level of significance albeit with a large effect size (Z = 1.91, p = 0.056, d = 0.63), both groups expressed significant habituation, i.e. the median β-coefficients differed from 0 (controls: Z = 3.82, p < 0.0001, r = 0.85; patients: Z = 2.37, p = 0.018, r = 0.48).
P50 suppression paradigm (Table 2, Fig. 3)
The analysis of the raw P50 amplitude data showed a significant main effect of stimulus [F (1,42) = 56.67, p < 0.001, η 2 = 0.57], but no significant effects of group (p > 0.14, η 2<0.052). The raw N100 data showed main effects of stimulus type [F (1,43) = 40.01, p < 0.001, η 2 = 0.48] and group [F (1,43) = 4.98, p = 0.031 η 2 = 0.104], indicating decreased amplitudes to T-stimuli compared with C-stimuli regardless of group, yet increased N100 amplitudes to C- and T-stimuli in patients compared with controls. No group × stimulus interaction [F (1,43) = 1.36, p = 0.25, η 2 = 0.031] was found. Last, the raw P200 data showed main effects of stimulus type [F (1,43) = 47.78, p < 0.001, η 2 = 0.526] and group [F (1,43) = 7.13, p = 0.011, η 2 = 0.142], as well as a group × stimulus interaction [F (1,43) = 4.80, p = 0.034, η 2 = 0.010]; indicating higher amplitudes to C-stimuli than to T-stimuli regardless of group, with higher amplitudes to both types of stimuli in patients as well as a higher decrease in amplitude from C- to T-stimuli in patients compared with controls.
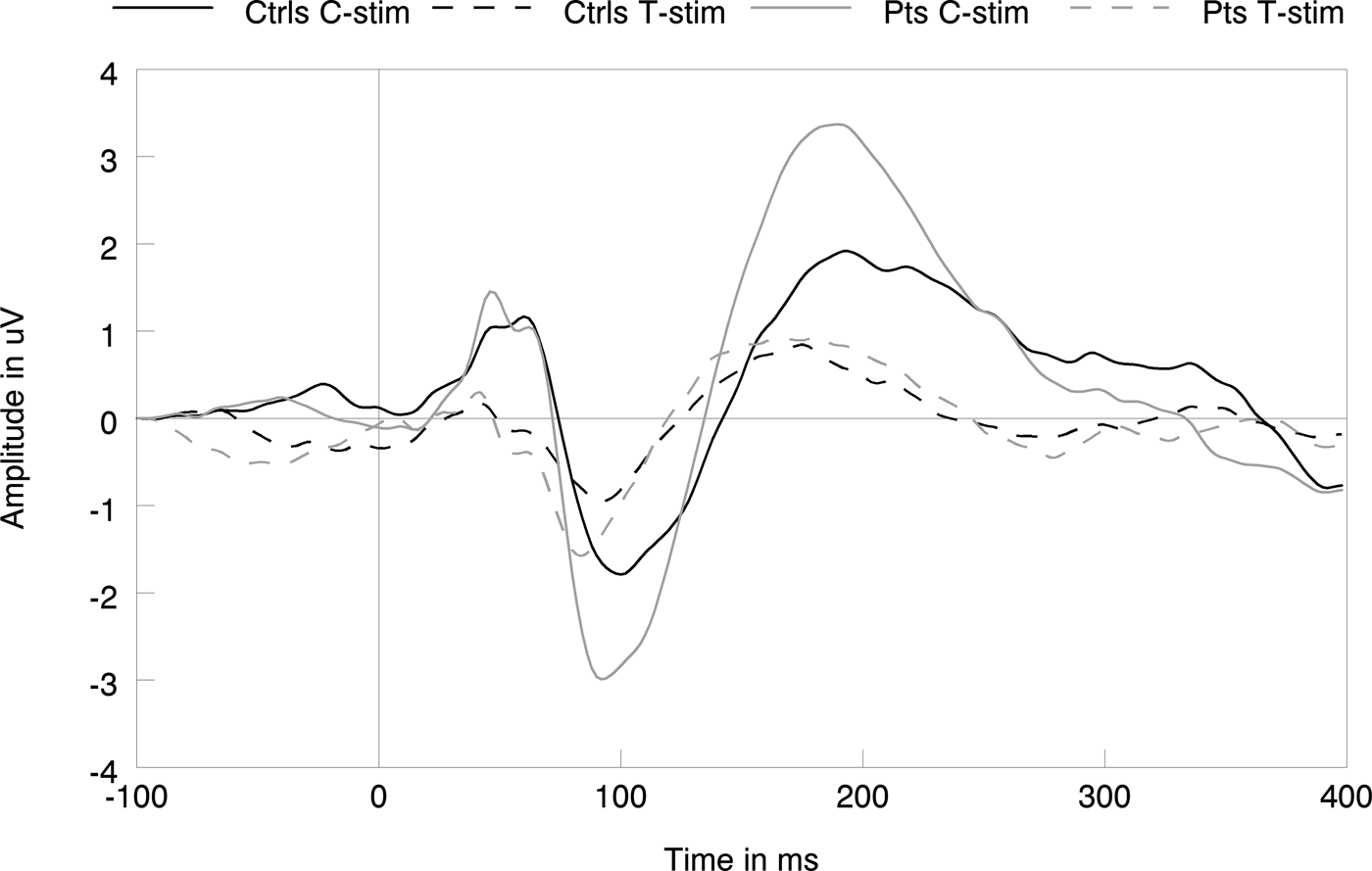
Fig. 3. Grand average data showing the subjects responses to conditioning (C-stim) and testing (T-stim) stimuli for patients and controls, indicating no group differences.
Analysis of the suppression data (T/C ratio) showed no effects of group in any of the ERPs (Z < 8.22, p > 0.41, d < 0.36).
Correlations (Table 3)
In the overall data, we found a number of interesting correlations between parameters of the PPI paradigm (i.e. %PPI and amplitude of pulse alone trials) and psychometric measures (GAF-F GAF-S, CGI, CAPS-total, and subscores), in the patient group. These correlations were not found in controls. Except for a correlation between the P50-response to T-stimuli and the CAPS-c scale, no correlations between P50 ERPs and psychometric scales were found. Similarly, only one psychometric measure (GAF-f) correlated with sensitization, while none of the psychometric measures at all correlated with habituation. In contrast, several psychometric measures were found to correlate significantly with either the raw amplitudes or T/C ratio of the N100 and P200 ERPs in the overall population and in controls, yet not in patients.
Table 3. Correlations EEG and psychometric measures
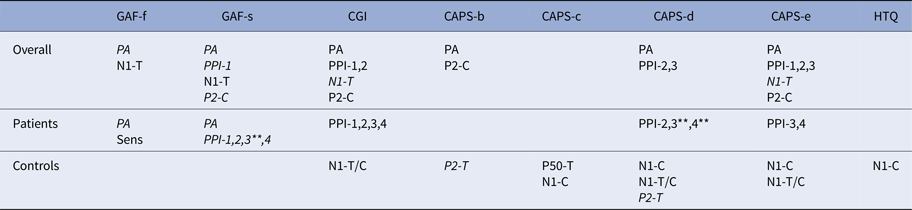
The table displays all significant correlations (p < 0.05, except when indicated differently) between EEG and psychometric measures. As can be seen in this table, except for habituation, N1-T and P2-T/C all other EEG and psychometric measures correlated significantly with at least one other measure.
Normal font: positive correlation; Italic font: negative correlation.
PA, Pulse alone; PPI1, PPI85120; PPI2, PPI8560; PPI3, PPI76120; PPI4, PPI7660; N1, N100; P2, P200; Sens, Sensitization; T, amplitude to T-stimulus; C, amplitude to C-stimulus; T/C, ratio T over C.
**Significant at p < 0.01.
Discussion
The aim of this present study was to investigate psychophysiological characteristics in trauma-affected refugees with PTSD and the possible association with clinical measures. Overall, results revealed elevated stimuli responses in the PTSD sample, while the primary outcome of each paradigm, i.e. PPI and P50 suppression, did not differ from healthy controls.
Across the two distinct paradigms, we found significantly increased amplitude responses in PTSD patients compared to healthy refugee controls. In the PPI paradigm, the increase was reflected in the eye-blink startle response which was on average higher in the patient group, although this reached only statistical significance in the magnitude of the startle response to pulse alone trials in the PPI block. This elevation can be attributed to the relatively slower process of habituation of the eye-blink startle response in the patient group compared with the control group, although this reached only trend level of significance. While the evidence of exaggerated startle response in PTSD is mixed (Orr et al., Reference Orr, Metzger and Pitman2002), reduced habituation of the eye-blink startle in PTSD represents a rare finding, as a long line of studies report no difference between patients and controls (Orr et al., Reference Orr, Pitman and Shalev1995; Shalev et al., Reference Shalev1997; Metzger et al., Reference Metzger1999; Carson et al., Reference Carson2007; Holstein et al., Reference Holstein2010). As such, our finding of only a trend level difference in habituation between patients and controls is no exception to this rule. Differences in methodology might account for some of the divergent results (e.g. stimulus intensity, number of different intensities), and it has been suggested that elevated startle responses are primarily restricted to stressful conditions, since studies more consistently report higher startle responses in patients when tested in threatening contexts, e.g. fear-potentiated startle (Pole, Reference Pole2007). Considering this along with our findings of increased startle and trend-level reduced habituation to intense, but neutral stimuli in PTSD patients, indicates an overall neural hypersensitivity, rather than simply increased stress responding (Orr et al., Reference Orr, Metzger and Pitman2002). Our correlational data revealed several significant findings; however, these were exploratory tests and should be viewed accordingly. The most consistent finding was that of pulse-alone, i.e. eye-blink startle responses, which was associated with higher overall illness severity on two different measures (GAF-S/CGI), lower levels of functioning (GAF-F) as well as CAPS-b, d, and e, thus possibly pointing to detrimental and broad-reaching implications of this component.
In the P50 paradigm, patients responded with higher N100 and P200 amplitudes both to T- and C-stimuli. The early attentive N100 and P200 components have been linked to arousal, e.g. with studies showing correlations with electrodermal activity (Clearwater, Reference Clearwater2008). Previous studies of the N100 and P200 components in PTSD patients have typically applied paradigms relying more on attentional processes, e.g. the oddball paradigm, relative to the double-click paradigm of this study, and report mixed results of increased and reduced responses (Javanbakht et al., Reference Javanbakht2011). Our study did not detect a difference in either PPI or P50 suppression between the two groups. While the P50 suppression and PPI both are used to operationally quantify gating of incoming stimuli, evidence indicates that the two paradigms are not correlated in either healthy subjects or patients with schizophrenia (Schwarzkopf et al., Reference Schwarzkopf, Lamberti and Smith1993; Oranje et al., Reference Oranje1999, Reference Oranje2006; Braff et al., Reference Braff, Light and Swerdlow2007) and might represent fundamentally different processes.
Thus, while deficits in psychophysiological measures were observed in the patient group, these did not seem to manifest in either one of the basic filtering mechanisms, as initially hypothesized. The patterns of deficits could therefore be viewed as more top-down in nature and, to a degree, less pre-attentive. PPI and P50 suppression are substantially bottom-up processing of stimuli (Javanbakht et al., Reference Javanbakht2011), i.e. more automatic and reflexive, while later components, and habituation in particular, to a larger extent are dependent on cognitive involvement. Possible clinical implications could be that an exaggerated responsiveness to the majority of incoming stimuli leads to patients being easily overwhelmed and other downstream effects, e.g. difficulty concentrating. The postulated clinical manifestations, however, did not appear to be based on a deficient fundamental gating mechanism, as initially hypothesized, but rather founded in a different form of sensory overload, a general sensitization of the nervous system and disrupted the ability to mobilize appropriate levels of arousal (van der Kolk, Reference van der Kolk2000). However, the relation to symptomatology remains undetermined, as no clear pattern or highly significant correlations was found, and thus might indicate a more complex relation. The fact that we did not find any gender effect in our data indicates that in spite of the differences in experienced trauma that one could expect between males and females, this did not have a major impact on our findings.
Our findings add to a growing number of studies where PPI did not differ between PTSD patients and controls (Butler et al., Reference Butler1990; Morgan et al., Reference Morgan1997; Grillon et al., Reference Grillon1998; Lipschitz et al., Reference Lipschitz2005; Holstein et al., Reference Holstein2010; Vrana et al., Reference Vrana2013), but are incongruent with existing P50 suppression studies in the PTSD literature (Lobo et al., Reference Lobo2015). However, it should be noted that literature on psychophysiology in PTSD has mainly been based on victims of single and, for the most part, recent trauma events in Western populations. Besides an extensive and prolonged trauma history, refugees with PTSD differ from these samples as well as victims of multiple trauma, such as war veterans, by the immense psychosocial consequences of the migration and resettlement (Carlsson et al., Reference Carlsson, Sonne and Silove2014). Although the patient sample included in this present study constitutes a less severely affected subgroup of the general patient population in treatment at CTP, as indicated by a higher mean GAF score and a lower mean HTQ score (Sonne et al., Reference Sonne2016), these patients have been in treatment for years and indeed present a highly chronic form of the disorder. Trauma-affected refugees have been described to present with a complex symptom pattern that is not fully captured by the ICD-10 and DSM-5 diagnostic criteria for PTSD (Nygaard et al., Reference Nygaard, Sonne and Carlsson2017; Fried et al., Reference Fried2018). Complex PTSD has been proposed as a diagnostic category for ICD-11, which we expect a large part of our study population would meet the criteria for, as it is considered to occur following exposure to repeated, prolonged, interpersonal trauma (Nickerson et al., Reference Nickerson2016), and describes a symptom profile which in addition to the three core features of PTSD includes disturbances in the domains of affect regulation, self-concept, and interpersonal relations (Maercker et al., Reference Maercker2013). Furthermore, evidence of biological differences in the field of psychiatric pharmacogenomics between patients from the Middle East and Asia and patients from Western countries has been found (Noerregaard, Reference Noerregaard2012; Sonne et al., Reference Sonne2017).
This suggests that genetic variation may explain different responses among ethnic groups to psychotropic medication. Given these biological, cultural and psychosocial differences, it is likely that trauma-affected refugees develop a distinctive form of PTSD with unique neurophysiological alterations. Something similar along this line of reasoning could be expected for the healthy controls that were recruited for the present study since they were matched to our patient group not only on age and gender but also to the country of origin. These controls therefore also share with our patients that they left their country of origin to settle in a totally different culture that they need to adapt to, which might have had some influence on psychophysiology as well. Therefore, we compared the data of the current controls with that of controls native to Denmark out of our database, however, given that we did not have enough data on females with matching age we could do this for males only. We found no differences in levels of P50 suppression or PPI to the higher intensity prepulses, but did observe a slightly increased level of PPI to the lower intensity prepulses in our current controls (data not shown here). Given that our study was not designed to investigate this properly, we cannot make firm conclusions on this observed difference.
Diminished habituation has also been reported in a subgroup of patients with schizophrenia (Williams et al., Reference Williams2013), and as proposed by Acheson et al. (Reference Acheson, Geyer and Risbrough2012), this abnormality may even characterize a specific phenotype across psychiatric disorders. The finding that this diminished habituation only reached trend level of significance in the patients indicates a certain degree of heterogeneity in our patient group. Applying a cross-diagnostic view and identifying subtypes with distinct patterns of neurobiological/psychophysiological metrics, would be in accordance with the concept of the Research Domain Criteria (RDoC) framework (Simmons and Quinn, Reference Simmons and Quinn2014), a plea for leaving the traditional diagnostic systems in psychiatric research. PTSD and schizophrenia have several other similarities, ranging from phenomenological manifestations (hallucinations and other psychotic symptoms) to overlaps in cognitive dysfunctions and pathophysiological mechanisms, e.g. prefrontal dysfunction (Galletly et al., Reference Galletly, McFarlane and Clark2008; Dichter et al., Reference Dichter, Damiano and Allen2012). Furthermore, a recent genome-wide association study of PTSD has revealed large genetic overlap with schizophrenia (Duncan et al., Reference Duncan2017). Thus, this approach might be worth pursuing in the future, as it has the potential to take us a step further in understanding the immense heterogeneity within specific psychiatric disorders.
A main strength of the current study has been a combination of the inclusion of trauma-affected refugees with PTSD and the use of an extensive psychophysiological test battery, extending the field of psychophysiology and information processing to a psychiatric population with little or no scientific attention, despite large technical advancements in this area. In addition, matching the patient sample to healthy refugees not only on age and gender but also a country of origin allowed us to isolate the potential role of culture, ethnicity, and migration.
Among limitations of this study was the small sample, current medication use in the patient group and the use of a mixed control group of trauma and non-trauma exposed individuals, which limits inferences regarding the causal relationship between traumas and psychophysiological measures in PTSD. Finally, a confounding factor which is known to affect PPI, menstrual cycle, was not controlled for. In summary, to the best of our knowledge, the present study is the first to report findings of sensory and sensorimotor gating, startle reactivity and habituation within the same group of trauma-affected refugees with PTSD. Fundamental gating mechanisms, as defined in the PPI and P50 paradigms, appeared intact, while PTSD patients demonstrated significantly elevated stimuli responses across two paradigms, reflected in both increased amplitude of the eye-blink startle response and increased N100 and P200 amplitudes relative to healthy refugee controls. We found a trend toward reduced habituation in the patients, which does indicate a certain degree of heterogeneity in the PTSD patients, and thus is suggestive of distinct phenotypic subcategories within this disorder.
Taken together, the patterns of deficits point toward an overall neural hypersensitivity and disrupted the ability to down-regulate stimuli responses. This study, thus, represents an initial step toward elucidating sensory processing deficits in PTSD in a PTSD subgroup. However, much remains unknown and efforts to address controversies in this field will benefit from replication in larger-scale studies. One of the major challenges will be to identify subtypes of patients across disorders based on deficits in neurobiological mechanisms as well as to determine how these translate or contribute to clinical manifestations. Furthermore, the issue of whether these disturbances represent pre-existing traits or are acquired, will have to be addressed in longitudinal follow-up studies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171800123X
Acknowledgements
The authors would like to thank the staff of CTP for their support throughout the implementation of the study and all of the participants that made this study possible. Furthermore, thanks to research assistant Mikkel Erlang Sørensen (M.Sc.) from CNSR for technical support in psychophysiological assessments.
Financial support
H.M. was supported by a grant from The Lundbeck Foundation (F-61171-19-27) and Psykiatrisk Forskningsfond of 1967. The funders had no role in the design and conduct of the study, drafting of the manuscript, or decision to publish.
Conflict of interest
None.








