Introduction
Recent years have seen a growing interest in the long-term prognosis of individuals with autism spectrum disorder (ASD), and in particular their risk of developing non-affective psychotic disorder (NAPD) or bipolar disorder (BD). However, risk estimates vary greatly. Two reviews reported a range of prevalence figures of NAPD in individuals with ASD from 0% to over 34% (Skokauskas and Gallagher, Reference Skokauskas and Gallagher2010; Chisholm et al., Reference Chisholm, Lin, Abu-Akel and Wood2015). Another review reported a prevalence of BD in individuals with ASD of up to 9% (Vannucchi et al., Reference Vannucchi, Masi, Toni, Dell'Osso, Marazziti and Perugi2014), but the authors also noted a general lack of investigations.
Most of these studies examined the co-occurrence of ASD and NAPD or BD, but this can result in diagnostic and/or selection bias. Since some signs typical of ASD, such as social withdrawal, are also common in NAPD and BD, some clinicians might erroneously diagnose ASD based on the assumption that these difficulties were present since early childhood. Selection bias may occur if individuals with previously unrecognized ASD are diagnosed with ASD after they have been diagnosed with NAPD or BD.
These sources of bias are less likely in studies in which young individuals with ASD are followed over time, because NAPD and BD generally do not develop until late adolescence or adulthood. However, earlier studies with such a design had small sample sizes, with few including more than 100 (and no more than 200) individuals with some form of autism (Volkmar and Cohen, Reference Volkmar and Cohen1991; Billstedt et al., Reference Billstedt, Gillberg and Gillberg2005; Cederlund et al., Reference Cederlund, Hagberg, Billstedt, Gillberg and Gillberg2008; Hutton et al., Reference Hutton, Goode, Murphy, Le Couteur and Rutter2008, Mouridsen et al., Reference Mouridsen, Rich and Isager2008). A Swedish population-based study included individuals registered with ASD before 16 years of age over an 11-year period (n = 9062) and calculated their odds of developing NAPD or BD within that period (Selten et al., Reference Selten, Lundberg, Rai and Magnusson2015). Compared with non-ASD peers, individuals with ASD had a higher risk of developing NAPD or BD (adjusted odds ratio 4.6 and 4.3, respectively); however, the study did not take into account the sequence of diagnoses in time. Overall, more large longitudinal studies examining the risk of NAPD and BD are needed, especially studies that consider the sequence of diagnoses in time (ASD preferably before NAPD or BD) and the age at which ASD is diagnosed.
The current study investigated the risk that individuals with ASD aged 16–35 years would develop NAPD or BD, using longitudinal data from Dutch psychiatric case registers (PCRs). Several analyses were conducted to minimize the possible contribution of diagnostic and selection bias. We compared the risk estimates with those for the Dutch population reported in earlier studies. Additionally, we assessed potential risk factors for NAPD or BD in individuals with ASD.
Methods
Psychiatric case registers
Data were retrieved from two PCRs in the Netherlands (PCR Northern-Netherlands and PCR Middle-Netherlands), which received anonymized diagnostic and demographic information on all patients attending inpatient/outpatient facilities for mental healthcare in a specified geographical area. We extracted data collected between 2000 and 2012 by PCR Northern-Netherlands and between 1999 and 2015 by PCR Middle-Netherlands. Data for each patient included information on Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV diagnoses (diagnosis and date of diagnosis), sex, date of birth, country of birth, and country of birth of each parent. Informed consent was not obtained from individual patients, as Dutch privacy law permits the use of anonymous data for medical research.
Cases
The initial extraction included all individuals registered with a DSM-IV diagnosis of ASD (codes 299.xx) during the above-mentioned periods (n = 39 434). Individuals with ASD were excluded if information on their date of birth, date of first PCR registration, date of ASD diagnosis, or date of NAPD or BD diagnosis (if made) was missing (n = 195), or if the date of the ASD, NAPD, or BD diagnosis had been registered retroactively (n = 4855), that is, if the diagnosis had been made before 2000 (PCR Northern-Netherlands), before 1999 (PCR Middle-Netherlands), or before the first PCR registration. Furthermore, as we were interested in the risk of NAPD and BD in individuals aged 16–35 years, we excluded individuals diagnosed with NAPD or BD at or before 16 years of age (n = 129), who were younger than 16 years when the PCR closed (n = 12 785), or who had been diagnosed with ASD when aged 36 or older (n = 4236). In total, 17 234 individuals with ASD were included in the analyses.
Outcomes
The outcome of interest was a diagnosis of NAPD or BD made between 16 and 35 years of age. NAPD was defined as a DSM-IV diagnosis of schizophrenia, schizophreniform disorder, or schizoaffective disorder (codes 295.x), delusional disorder (code 297.1), or psychotic disorder not otherwise specified (NOS) or brief psychotic disorder (codes 298.x). BD was defined as a DSM-IV diagnosis of bipolar I disorder (codes 296.0x, 296.4x, 296.5x, 296.6x, or 296.7), bipolar II disorder (code 296.89), or BD NOS (296.80). If individuals were diagnosed with both NAPD and BD (n = 42), we assumed that the last diagnosis reflected a valid revision of the previous one. In these cases, only the last diagnosis was used in the analyses but the date of the previous diagnosis was used as an estimate of the age at onset. Two individuals were simultaneously diagnosed with NAPD and BD and were only included in the NAPD analyses.
General population estimates of the cumulative incidence of NAPD were obtained from a 2-year first-contact study conducted in The Hague, the Netherlands, in the period 1997–1999 (Selten et al., Reference Selten, Veen, Feller, Blom, Schols, Camoenie, Oolders, van der Velden, Hoek, Rivero, van der Graaf and Kahn2001). General population estimates of the cumulative incidence of BD were obtained from a population-based cohort study conducted in the Netherlands in the period 1996–2007 (Kroon et al., Reference Kroon, Wohlfarth, Dieleman, Sutterland, Storosum, Denys, de Haan and Sturkenboom2013). Incidence rates for different age groups (for NAPD 15–19, 20–24, 25–29, 30–34 years; for BD 15–24, 25–34 years) by sex were provided by the researchers involved in these studies and were used to estimate the cumulative incidence.
Statistical analyses
Risk of NAPD or BD
The risk of NAPD among individuals with ASD was calculated using Kaplan–Meier survival estimates, which reflect the proportion of individuals with ASD who had not been diagnosed with NAPD at a certain age. Individuals were followed up until they were 36 years old, until NAPD or BD was diagnosed, or until the PCR closed, whichever came first. Kaplan–Meier estimates for men and women separately, and their comparison with general population estimates, are reported in online Supplementary Table S1.
In the first analysis, we estimated the risk of a first diagnosis of NAPD between ages 16 and 36 among all individuals diagnosed with ASD before 36 years of age. Here we did not consider the sequence in which diagnoses were made or the age at which ASD was diagnosed. Consequently, we expected a greater likelihood of diagnostic bias (i.e. signs of NAPD being mistakenly diagnosed as ASD) and selection bias (i.e. individuals being recognized and/or registered with ASD due to the development of NAPD). The follow-up started at the age of first contact with mental healthcare services for any DSM-IV diagnosis or at age 16, whichever came later.
In the second analysis, we excluded individuals who were diagnosed with ASD after they had been diagnosed with NAPD, or who were diagnosed with ASD and NAPD simultaneously. By restricting the analysis to individuals still at risk of being diagnosed with NAPD at the time they were diagnosed with ASD, we expected that the influence of diagnostic and selection bias would be reduced. Follow-up started at age 16 or at the age of ASD diagnosis, whichever came later.
In the third analysis, we further restricted the analysis to individuals diagnosed with ASD before 16 years of age. We expected selection or diagnostic bias to play a minor role if ASD was diagnosed at an early age. Of note, individuals diagnosed with NAPD or BD before 16 years of age had already been excluded.
In the fourth analysis, we restricted the analysis to individuals diagnosed with ASD after (or at) 16 years of age. In these cases, a diagnosis of NAPD shortly after ASD was diagnosed may indicate that ASD was only diagnosed because of the development of NAPD. Therefore, we also conducted a fifth analysis from which we excluded individuals diagnosed with NAPD within 1 year of the ASD diagnosis.
The risk of BD among individuals with ASD was calculated using the same analyses described for NAPD, but with a diagnosis of BD as the outcome of interest.
For general population risk estimates, we calculated the cumulative incidence of NAPD or BD by subtracting the survivor function S(t) = {exp[−H(t)]} from 1, in which S denotes the probability that a member of the general population did not receive a diagnosis of NAPD or BD up to t years, and H represents the cumulative hazard function (Cleves et al., Reference Cleves, Gutierrez, Gould and Marchenko2010). This formula was equated to S(t) = exp{−∑(incidence(t)·Δt)}, in which we implemented the NAPD and BD incidence rates for different age groups, as provided by Selten et al. (Reference Selten, Veen, Feller, Blom, Schols, Camoenie, Oolders, van der Velden, Hoek, Rivero, van der Graaf and Kahn2001) and Kroon et al. (Reference Kroon, Wohlfarth, Dieleman, Sutterland, Storosum, Denys, de Haan and Sturkenboom2013). The 95% confidence interval (CI) of the cumulative incidence for the general population was compared with the 95% CIs of the cumulative incidence for individuals with ASD (1 minus Kaplan–Meier estimate) to examine whether they significantly differed. For the general population estimates, CIs were calculated assuming a Poisson distribution of the number of NAPD/BD cases (Rothman, Reference Rothman2015). For the comparison of the cumulative incidence, relative risks (RRs) are also provided.
Risk factors for NAPD or BD
Cox regression analyses were used to identify potential risk factors for the development of NAPD or BD. For these analyses, we used information from individuals diagnosed with ASD before age 16 years and from those diagnosed with ASD after (or at) this age, provided that NAPD or BD was diagnosed at least 1 year after ASD was diagnosed. We expected that the contribution of diagnostic or selection bias to the risk estimates would be small in these individuals.
The following potential risk factors were evaluated: sex, intellectual disability (ID; DSM-IV codes 317, 318.x, 319) present before or at the moment of the ASD diagnosis, migrant status based on parental country of birth (non-migrant if both parents were Dutch, migrant if at least one of the parents had been born abroad, or other if this information was unknown), ASD subtype [autistic disorder v. pervasive developmental disorder-not otherwise specified (PDD-NOS) or Asperger's syndrome], and age at ASD diagnosis (four age groups: 0–7, 8–15, 16–25, 26–35 years). Owing to the limited data available for the Cox regression analyses for BD, migrant status was not included, and the age at ASD diagnosis was split into three groups (0–15, 16–25, 26–35 years). An alpha of 0.05 was used to examine whether a variable violated the proportional hazard assumption and to evaluate whether a variable was significantly related to an increased or decreased risk of NAPD or BD.
Missing data
Information about sex was missing for six individuals with ASD (0.03%). Data for these individuals were omitted from Kaplan–Meier estimates by sex and from the Cox regression analyses. Migrant status could not be ascertained in 3019 individuals (17.5%). As described above, in the Cox regression analyses the migrant status of these individuals was coded as ‘other’.
Software
Data were prepared with SPSS version 22.0 (IBM). STATA version 13.1 (StataCorp) was used to calculate Kaplan–Meier survival estimates (using the sts list and sts graph commands) and for Cox regression analyses and corresponding proportional hazards tests (using the stcox and estat phtest commands).
Results
Risk of NAPD or BD
Table 1 gives an overview of the characteristics of the individuals with ASD included in each analysis. Figure 1 shows the estimated risk of NAPD or BD in each analysis, which are compared with general population estimates in Table 2.
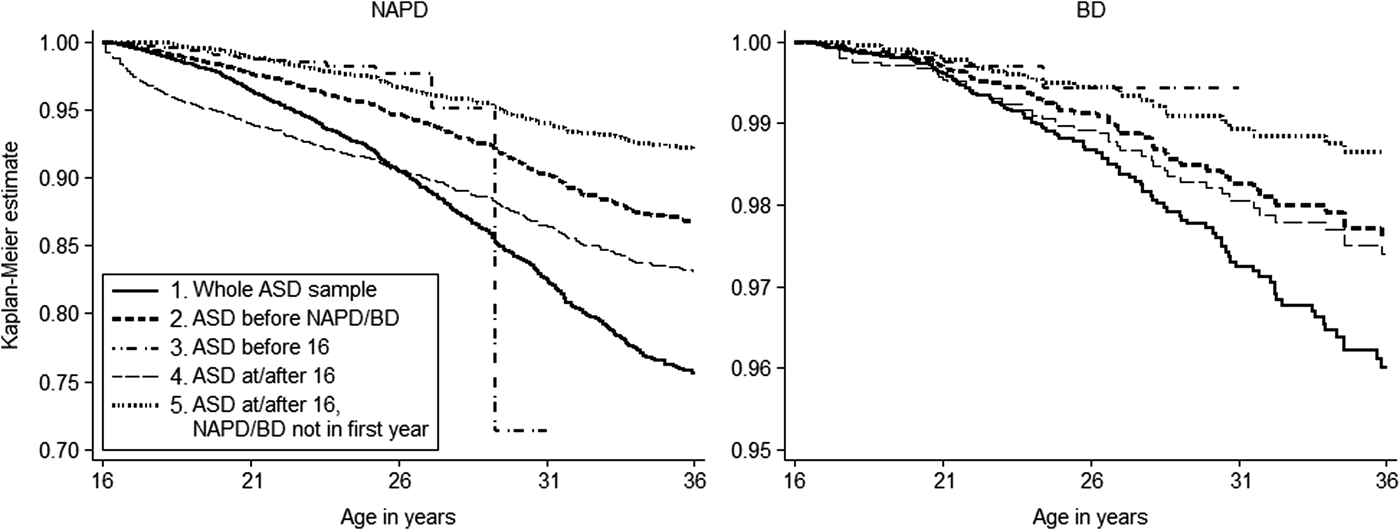
Fig. 1. The Kaplan–Meier survival estimates per analysis showing the estimated proportion of individuals with ASD registered in Dutch PCRs remaining without a diagnosis of NAPD (left panel) or BD (right panel) with increasing age. Note that the scale on the y-axis differs for the two figures. The large increase in risk of NAPD after age 26 years in individuals who were diagnosed with ASD before age 16 is the result of a small number of remaining cases.
Table 1. Characteristics of individuals with ASD registered in Dutch PCRs

ASD, autism spectrum disorder; NAPD, non-affective psychotic disorder; BD, bipolar disorder; ID, intellectual disability; s.d., standard deviation.
a No restrictions with regard to age or sequence in time of diagnosis (ASD before or after NAPD/BD).
b Restricted to ASD cases still at risk of a first diagnosis of NAPD or BD at the time of the ASD diagnosis.
c Restricted to cases diagnosed with ASD before age 16.
d Restricted to cases diagnosed with ASD after (or at) age 16.
e Restricted to cases diagnosed with ASD after (or at) age 16, in which an NAPD or BD diagnosis was not made within the first year after the ASD diagnosis.
f Calculated over total number of cases. Information about sex was missing for n = 6.
g Diagnosed before or simultaneously with ASD.
Table 2. Estimated cumulative incidence (%) and associated 95% CIs (in brackets) of NAPD and BD as reported for the Dutch general population and for individuals with ASD registered in Dutch PCRs
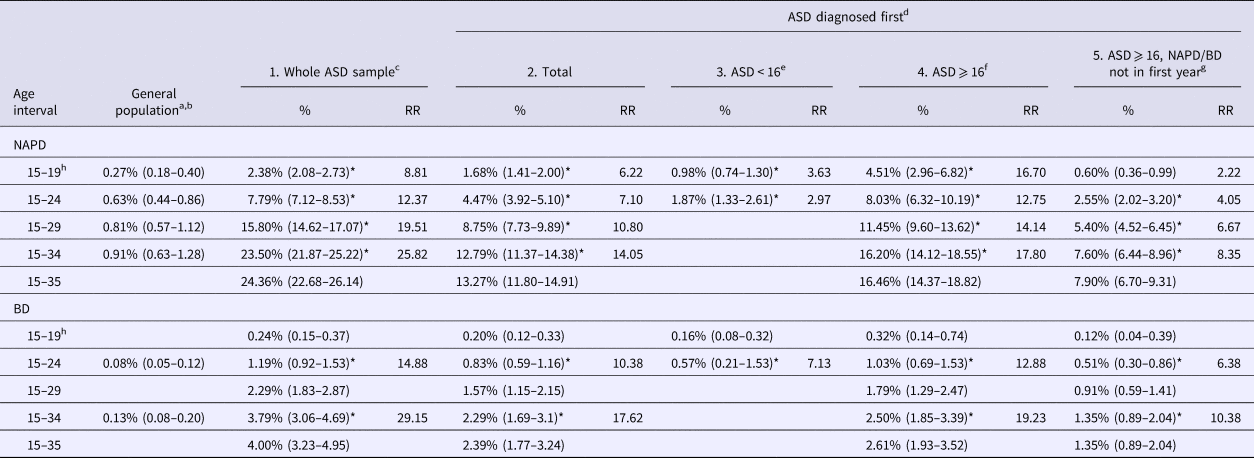
CIs, confidence intervals; NAPD, non-affective psychotic disorder; BD, bipolar disorder; ASD, autism spectrum disorder; RR, relative risk.
a, b Retrieved from Selten et al. (2001; NAPD) and Kroon et al. (2013; BD).
c No restrictions with regard to age or sequence in time of diagnosis (ASD before or after NAPD/BD).
d Restricted to ASD cases still at risk of a first diagnosis of NAPD or BD at the time of the ASD diagnosis.
e Restricted to cases diagnosed with ASD before age 16.
f Restricted to cases diagnosed with ASD after (or at) age 16.
g Restricted to cases diagnosed with ASD after (or at) age 16, in which an NAPD or BD diagnosis was not made within the first year after the ASD diagnosis.
h Individuals from the general population were included from age 15 onward. ASD cases were included from age 16 onward.
* 95% CIs not overlapping with (known) general population estimates.
The results of the first analysis, which did not take into account the age at ASD diagnosis or sequence of diagnoses, showed that an estimated 24.36% (95% CI 22.68–26.14) of individuals with ASD were diagnosed with NAPD and an estimated 4.00% (95% CI 3.23–4.95) were diagnosed with BD before 36 years of age.
For the second analysis, we excluded 464 of 845 individuals with NAPD (54.91%) and 55 of 116 individuals with BD (47.41%), as these diagnoses had been made before or simultaneously with an ASD diagnosis. Of the remaining individuals with ASD, 13.27% (95% CI 11.80–14.91) were diagnosed with NAPD and 2.39% (95% CI 1.77–3.24) were diagnosed with BD before 36 years of age.
In the third analysis, we restricted to individuals diagnosed with ASD before 16 years of age. These individuals could not be followed up until 36 years of age because the PCRs were not operational long enough, but an estimated 1.87% (95% CI 1.33–2.61) were diagnosed with NAPD and 0.57% (95% CI 0.21–1.53) with BD before 25 years of age.
We restricted the fourth analysis to individuals diagnosed with ASD at or after 16 years of age. The results showed that an estimated 16.46% (95% CI 14.37–18.82) were diagnosed with NAPD and an estimated 2.61% (95% CI 1.93–3.52) with BD before 36 years of age. In total, 159 of 313 of individuals diagnosed with NAPD (50.80%) and 22 of 48 individuals diagnosed with BD (45.83%) had been diagnosed within 1 year of the ASD diagnosis. After exclusion of these individuals in the fifth analysis, an estimated 7.90% (95% CI 6.70–9.31) of individuals were diagnosed with NAPD and an estimated 1.35% (95% CI 0.89–2.04) were diagnosed with BD before 36 years of age.
Overall, 623 of 845 NAPD diagnoses (73.72%) and 77 of 116 BD diagnoses (66.38%) were made before, simultaneously with, or within 1 year of the ASD diagnosis.
In general, the risks of NAPD and BD were higher in individuals with ASD than in the general population (with non-overlapping 95% CIs), irrespective of the restrictions that we applied to the analysis. These differences were not explained by sex, as both men and women with ASD had a higher risk of developing NAPD or BD than did men and women without ASD. As shown in online Supplementary Table S1, risk estimates for NAPD and BD among men diagnosed with ASD before 16 years of age were an exception to this. The risk estimates were larger than the risk estimates among men in the general population, but the 95% CIs were partially overlapping.
Risk factors for NAPD or BD
We then examined potential risk factors for NAPD. The proportional hazards assumption was violated for the variable sex (χ2 = 6.94, p = 0.008), but not for other variables (all p values >0.05). Inspection of the data showed that the risk of NAPD among men and women with ASD was similar between 16 and 25 years of age, but diverged between 26 and 35 years of age. For this reason, we added a term for the interaction between time (follow-up during ages 16–25 v. 26–35) and sex to the analysis.
We initially conducted univariable analyses and subsequently added all variables to a multivariable model (see Table 3). Univariable analyses showed that men with ASD had a higher risk of NAPD than women with ASD, but only during the follow-up between age 26 and age 35 years. This risk remained increased in the multivariable model. Univariable analyses also showed an increased risk of NAPD among migrants than among native Dutch individuals (p = 0.04). In the multivariable model, this relative risk was of borderline statistical significance (p = 0.07). We found no other significant univariable or multivariable results (all p values >0.05).
Table 3. Cox regression analyses examining potential risk factors for NAPD or BD among individuals with ASD registered in Dutch PCRs (n = 16 528)
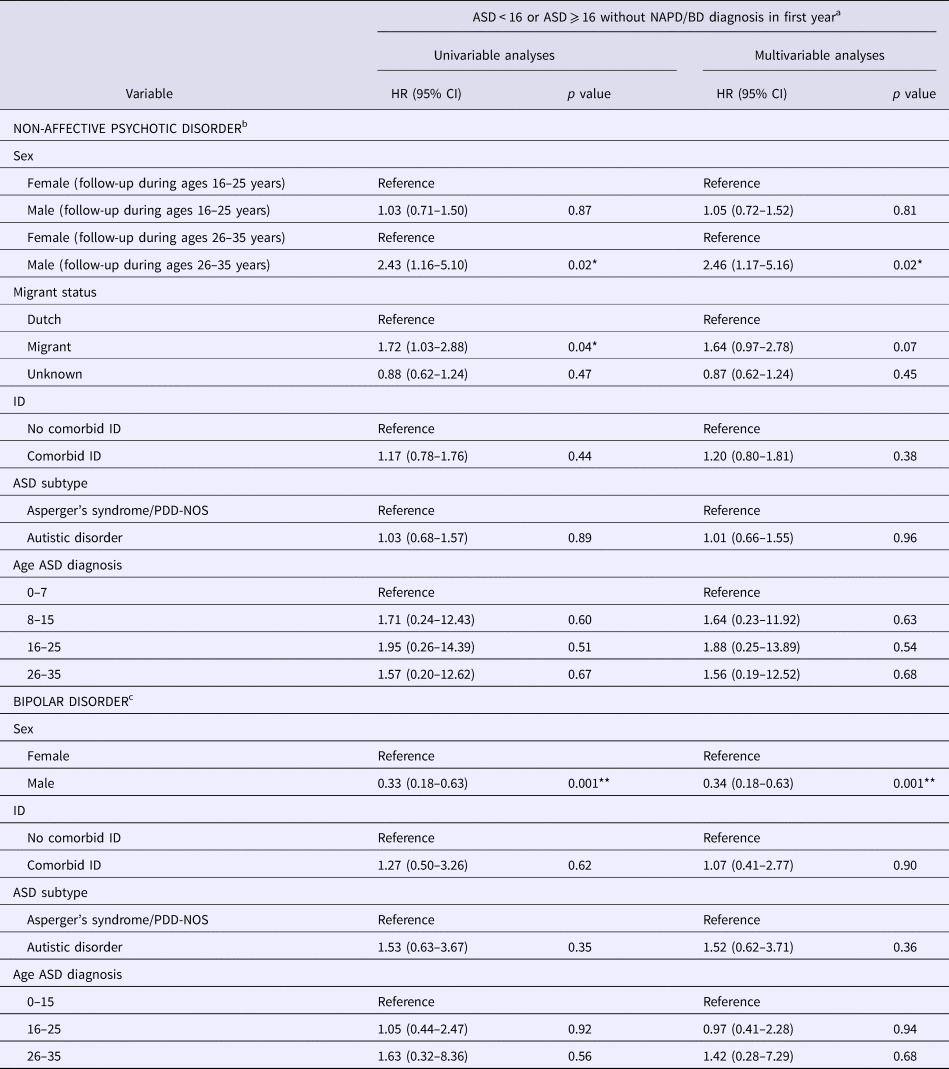
NAPD, non-affective psychotic disorder; BD, bipolar disorder; ASD, autism spectrum disorder; HR, hazard ratio; CI, confidence interval; ID, intellectual disability.
a Analyses were restricted to cases diagnosed with ASD before age 16 and cases diagnosed with ASD after (or at) age 16 who remained without NAPD and BD diagnoses for at least 1 year.
b 221 cases diagnosed with NAPD.
c 39 cases diagnosed with BD.
*p < 0.05.
**p < 0.001.
In the analysis of risk factors for BD, none of the variables violated the proportional hazards assumption (all p values >0.05). Univariable analyses showed that men with ASD had a lower risk of BD than did women with ASD. This finding remained significant in the multivariable analysis. No other variables were significantly related to risk of BD (all p values >0.05).
Discussion
The results of the current study show that men and women with ASD are at increased risk of developing NAPD or BD. Men had a relatively higher risk of developing NAPD and women a relatively higher risk of developing BD.
It is difficult to estimate the contribution of bias to the variability in risk reported in earlier studies. However, this study confirms and extends those earlier findings by showing that the increased risks persist after adjusting for potential diagnostic and selection bias. That is, when we restricted our analyses to individuals already diagnosed with ASD before age 16, approximately 1.87% were diagnosed with NAPD and 0.57% with BD by 25 years of age (Table 2, analysis 3). We obtained similar results when we restricted our analyses to individuals diagnosed with ASD after 16 years of age who were not diagnosed with NAPD or BD within a year of the ASD diagnosis (Table 2, analysis 5). Of these individuals, 2.55% were diagnosed with NAPD and 0.51% with BD by 25 years of age, which increased up to 7.90% and 1.35% by 36 years of age, respectively.
Strikingly, we found that 74% of NAPD diagnoses and 66% of BD diagnoses were made before, simultaneously with, or within 1 year of the ASD diagnosis. An earlier study that followed up children diagnosed with ASD also reported that a subsequent diagnosis of schizophrenia spectrum disorder was most often made within a year (Maibing et al., Reference Maibing, Pedersen, Benros, Mortensen, Dalsgaard and Nordentoft2015). How can these observations be explained? One possibility is that these individuals had had a milder form of autism since childhood that was only recognized by healthcare services when their mental health deteriorated. Alternatively, some clinicians may have forgotten that autism-like signs and symptoms are part of NAPD and assume too readily that these have been present since early childhood. This interpretation fits with the observation of Kendler (Reference Kendler2016) that some clinicians equate ‘schizophrenia’ with its DSM criteria only. It is less clear whether such confusion exists between ASD and BD, although signs such as irritability and being overly talkative appear in both disorders and may result in similar confusion.
The sex difference in the risk of developing NAPD or BD warrants further investigation, as previous studies show mixed results (Stahlberg et al., Reference Stahlberg, Soderstrom, Rastam and Gillberg2004; Billstedt et al., Reference Billstedt, Gillberg and Gillberg2005; Hofvander et al., Reference Hofvander, Delorme, Chaste, Nyden, Wentz, Stahlberg, Herbrecht, Stopin, Anckarsater, Gillberg, Rastam and Leboyer2009; Lugnegard et al., Reference Lugnegard, Hallerback and Gillberg2011; Croen et al., Reference Croen, Zerbo, Qian, Massolo, Rich, Sidney and Kripke2015). The same applies to the finding that the risk of NAPD was high among migrants with ASD. We found no relation between risk of NAPD or BD and DSM-IV subtypes of ASD or age at ASD diagnosis. Furthermore, we did not replicate the finding of Selten et al. (Reference Selten, Lundberg, Rai and Magnusson2015), who reported that individuals with ASD and ID had a lower risk of developing NAPD and BD than peers without such disability.
The current study has several strengths. To the best of our knowledge, it is the largest to study the risk of NAPD or BD in individuals with ASD. Furthermore, we conducted longitudinal analyses and took steps to avoid diagnostic and selection bias. However, the study also has a number of limitations. First, general population risk estimates were retrieved from previous studies, which collected data in partially non-overlapping periods and geographical areas. The study of Selten et al. (Reference Selten, Veen, Feller, Blom, Schols, Camoenie, Oolders, van der Velden, Hoek, Rivero, van der Graaf and Kahn2001) was conducted in The Hague, which is a large city in the Netherlands, whereas our data were partially collected in more rural, northern parts of the Netherlands. Since the risk of psychosis is reported to be higher in urban areas (e.g. van Os et al., Reference van Os, Hanssen, Bijl and Vollebergh2001), the relative risk of NAPD in individuals with ASD may have been underestimated. As Kroon et al. (Reference Kroon, Wohlfarth, Dieleman, Sutterland, Storosum, Denys, de Haan and Sturkenboom2013) excluded individuals with BD and co-morbid ID, the relative risk of BD among individuals with ASD may have been overestimated. A second limitation is that misdiagnosis cannot entirely be excluded as an explanation for some findings, as we did not have information about the diagnostic process. In this respect, the availability of extensive diagnostic information can be seen as a strength of some of the previous smaller studies (e.g. Stahlberg et al., Reference Stahlberg, Soderstrom, Rastam and Gillberg2004; Billstedt et al., Reference Billstedt, Gillberg and Gillberg2005; Lugnegard et al., Reference Lugnegard, Hallerback and Gillberg2011; Joshi et al., Reference Joshi, Wozniak, Petty, Martelon, Fried, Bolfek, Kotte, Stevens, Furtak, Bourgeois, Caruso, Caron and Biederman2013).
There is still much uncertainty about why individuals with ASD have an increased risk of NAPD and BD (Padgett et al., Reference Padgett, Miltsiou and Tiffin2010 Chisholm et al., Reference Chisholm, Lin, Abu-Akel and Wood2015). Understanding the mechanisms underlying the increased risk will be an important next step for the prevention and treatment of these disorders in individuals with ASD. Shared risk factors, such as genetic overlap between ASD, NAPD, and BD (Carroll and Owen, Reference Carroll and Owen2009; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013), may increase the risk of all three disorders. Individuals with ASD may also be exposed to environmental risk factors, such as an urban upbringing (Lauritsen et al., Reference Lauritsen, Astrup, Pedersen, Obel, Schendel, Schieve, Yeargin-Allsopp and Parner2014), or psychosocial factors, such as social exclusion or childhood trauma (Cappadocia et al., Reference Cappadocia, Weiss and Pepler2012; Kerns et al., Reference Kerns, Newschaffer and Berkowitz2015), that increase the risk of developing NAPD and/or BD (Read et al., Reference Read, van Os, Morrison and Ross2005; Laursen et al., Reference Laursen, Munk-Olsen, Nordentoft and Bo Mortensen2007; van Dam et al., Reference van Dam, van der Ven, Velthorst, Selten, Morgan and de Haan2012; Selten et al., Reference Selten, van der Ven, Rutten and Cantor-Graae2013; Cunningham et al., Reference Cunningham, Hoy and Shannon2016). Additionally, it may be worthwhile to consider the possibility that there are different risk factors for distinct, DSM-independent, autism subtypes (Chisholm et al., Reference Chisholm, Lin, Abu-Akel and Wood2015).
In conclusion, the current study showed that individuals with ASD are at increased risk of developing NAPD or BD. These results are likely not the result of diagnostic or selection bias. Future research should examine the mechanisms underlying these increased risks.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003483.
Author ORCIDs
R. Schalbroeck https://orcid.org/0000-0002-9855-5797.
Acknowledgements
We are thankful to Janneke Giele for her assistance with the PCR data, and to Hjalmar Bos for his help with data preparation and literature search. Furthermore, we wish to thank Jojanneke Kroon for her substantial help in providing data about the incidence of BD in the general population.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.






