Introduction
In 1904, Charles Spearman proposed the existence of a general factor that contributes to all cognitive activities (Spearman Reference Spearman1904, Reference Spearman1927). Spearman's general factor (g) was proposed to explain one of the strongest findings in the study of human intelligence – the universal positive correlations typically found between different cognitive tests. The best measures of g are generally tests of so-called fluid intelligence, involving novel problem-solving (Carroll, Reference Carroll1993). The cognitive functions reflected in g are still under active study. Positive g correlations for all manner of cognitive tasks, including tests of working memory, especially working memory for novel task rules (Duncan et al., in press), tests of processing speed (e.g. Nettelbeck, Reference Nettelbeck and Vernon1987), and many more, suggest that g reflects cognitive functions of importance in any form of organized behavior. Obvious candidates are the broad organizational functions of the frontal lobe, and indeed, performance in fluid intelligence tests is impaired after frontal lobe lesions, in particular lesions in lateral and dorsomedial frontal regions (Duncan et al. Reference Duncan, Burgess and Emslie1995; Woolgar et al. Reference Woolgar, Parr, Cusack, Thompson, Nimmo-Smith, Torralva, Roca, Antoun, Manes and Duncan2010). Similar regions are active in functional imaging studies of fluid intelligence test performance (Prabhakaran et al. Reference Prabhakaran, Smith, Desmond, Glover and Gabrieli1997; Esposito et al. Reference Esposito, Kirkby, Van Horn, Ellmore and Berman1999; Duncan et al. Reference Duncan, Seitz, Kolodny, Bor, Herzog, Ahmed, Newell and Emslie2000; Bishop et al. Reference Bishop, Fossella, Croucher and Duncan2008).
Many clinical and experimental tests are known to be sensitive to frontal impairment, even if they are also known to recruit other cognitive functions and brain areas. The Wisconsin Card Sorting Test (WCST) and Verbal Fluency, for example, are often used to measure frontal ‘executive’ impairment, even though both certainly also involve a variety of posterior cortical functions. Recent attention has also been given to tests of multitasking (e.g. Manly et al. Reference Manly, Hawkins, Evans, Woldt and Robertson2002) and theory of mind (e.g. Stone et al. Reference Stone, Baron-Cohen and Knight1998), although again, it is likely that individual tests have contributions from both frontal and posterior functions.
The importance of the frontal lobe in fluid intelligence and in a diversity of specific cognitive tests raises the question of how much a loss of fluid intelligence contributes to other frontal deficits. In a recent study (Roca et al. Reference Roca, Parr, Thomson, Woolgar, Torralva, Antoun, Manes and Duncan2010a ), we showed that, in a group of patients with frontal lesions, fluid intelligence (g) was a substantial contributor to many frontal deficits. For some classical ‘executive’ tasks, such as the WCST and Verbal Fluency, frontal deficits were entirely explained by individual scores of g. Once fluid intelligence was partialled out, there was no remaining difference between patients and normal controls. However, on a second set of frontal tasks, performance deficits remained even after fluid intelligence was statistically controlled. Such tasks were associated particularly with anterior frontal damage [Brodmann area (BA) 10] and included tests of theory of mind (Faux Pas) and multitasking (Hotel Task), among others.
Although Parkinson's disease (PD) is characterized by its motor symptoms, it is now widely accepted that cognitive changes can also be present, even during the early stages of the disease. Most frequently, cognitive deficits exhibited by PD patients resemble those produced after frontal-lobe damage, with particular difficulties on executive functioning (Foltynie et al. Reference Foltynie, Brayne, Robbins and Barker2004; Lewis et al. Reference Lewis, Foltynie, Blackwell, Robbins, Owen and Barker2005; Muslimovic et al. Reference Muslimovic, Post, Speelman and Schmand2005; Williams-Gray et al. Reference Williams-Gray, Foltynie, Brayne, Robbins and Barker2007), theory of mind (Saltzman et al. Reference Saltzman, Strauss, Hunter and Archibald2000; Mengelberg & Siegert, Reference Mengelberg and Siegert2003; Mimura et al. Reference Mimura, Oeda and Kawamura2006; Perón et al. Reference Péron, Vicente, Leray, Drapier, Drapier, Cohen, Biseul, Rouaud, Le Jeune, Sauleau and Vérin2009; Bodden et al. Reference Bodden, Dosel and Kalbe2010; Roca et al. Reference Roca, Torralva, Gleichgerrcht, Chade, Arévalo, Gershanik and Manes2010b ) and multitasking (Perfetti et al. Reference Perfetti, Varanese, Mercuri, Mancino, Saggino and Onofrj2010).
Fluid intelligence loss has also been described in PD, most commonly as measured by Raven's Colored Progressive Matrices (RCPM; Pillon et al. Reference Pillon, Gouider-Khouja, Deweer, Vidailhet, Malapani, Dubois and Agid1995; Bostantjopoulou et al. Reference Bostantjopoulou, Kiosseoglou, Katsarou and Alevriadou2001; Basić et al. Reference Basić, Katić, Vranicć, Zarevski, Babić and Mahović-Lakusić2004; Nagano-Saito et al. Reference Nagano-Saito, Washimi, Arahata, Kachi, Lerch, Evans, Dagher and Ito2005). In PD patients, performance in RCPM has been shown to correlate positively with gray matter density within the dorsolateral prefrontal cortex (Nagano-Saito et al. Reference Nagano-Saito, Washimi, Arahata, Kachi, Lerch, Evans, Dagher and Ito2005).
Although both frontal deficits and fluid intelligence loss have been described in PD, to our knowledge no previous study has investigated the role of fluid intelligence in frontal deficits associated with this disease. To achieve this objective, we assessed a group of patients with PD using tasks sensitive to frontal dysfunction and with the RCPM as a test of fluid intelligence. In addition to comparing PD patients with a group of controls, we investigated how far frontal deficits in PD were explained by fluid intelligence loss.
Method
Participants
Thirty-two patients who met the UK Parkinson's Disease Society Brain Bank criteria, between Hoehn and Yahr stages I–III (Hoehn & Yahr, Reference Hoehn and Yahr1967), were recruited from the INECO Data Base in Buenos Aires, Argentina and from the Movement Disorders Clinic at the Institute of Neuroscience of the Favaloro Foundation. Mean (±s.d.) age for the patient population was 62.25 (±10.23) years. Information on disease history and drug therapy was obtained by neurologists specialized in studying PD (A.C., G.G.A., O.G.). Patients with different neurological diagnoses or presenting radiological structural brain abnormalities compatible with diagnoses other than PD were excluded from this study. Patients who scored under 24 on the Mini-Mental State Examination (Folstein et al. Reference Folstein, Folstein and McHugh1975) were also excluded to ensure a good level of overall cognitive functioning. Of the patients selected, 15 were under pharmacological treatment with either levodopa or a dopamine agonist with a mean levodopa equivalent daily dose of 318.56 (±268.48) mg. Among those patients, assessment was conducted during the ‘on’ state of the medication. Seventeen of the patients were not taking any medication for their motor symptoms. Performance between medicated and non-medicated patients was compared to ensure that the results were not influenced by medication intake. For cases in which significant differences between medicated and non-medicated patients were found, the levodopa equivalent daily dose (mg) was introduced as a covariable in subsequent analysis. Permission for the study was initially obtained from the local research ethics committee and all participants gave their signed informed consent prior to inclusion. The subjects' consent was obtained according to the Declaration of Helsinki.
Healthy control volunteers (n=22) were recruited through word of mouth and were matched to patients, taking into account the mean and range of age and level of education. Controls were recruited from the same geographical area as patients. Participants were included in the control group if they reported no history of neurological or psychiatric disorders, including traumatic brain injury or substance abuse.
Clinical and demographical data for all participants are shown in Table 1.
Table 1. Clinical and demographical data

PD, Parkinson's disease; WAT-BA, Word Accentuation Test – Buenos Aires; s.d., standard deviation.
Procedure
All participants were initially assessed using a complete neuropsychological battery that included cognitive screening tests, tests of language, memory, praxis, attention and executive functions and pre-morbid IQ. Experimental tests were administered during a second session of assessment, including both theory of mind and multitasking tasks.
Neuropsychological testing
Word Accentuation Test – Buenos Aires (WAT-BA)
To estimate pre-morbid intelligence we used the WAT-BA (Burin et al. Reference Burin, Jorge, Arizaga and Paulsen2000). This test, similar to the National Adult Reading Test (Nelson & Willison, Reference Nelson and Willison1991), measures ability to read 44 irregularly stressed Spanish words. The score was the number of words stressed correctly.
RCPM
To assess fluid intelligence, we used the RCPM (Raven, Reference Raven1995), which is a multiple-choice test of novel problem-solving comprising 36 items. In each test item, the subject is asked to identify the missing item that completes a certain pattern. The test is organized in three sets of 12 items ranging in complexity (series A, Ab and B). The score was the total number of items solved correctly.
WCST (Nelson, Reference Nelson1976)
For the WCST, we used Nelson's modified version of the standard procedure. Cards varying on three basic features (color, shape and number of items) must be sorted according to each feature in turn. The participants' first sorting choice becomes the correct feature, and once a criterion of six consecutive correct sorts is achieved, the subject is told that the rules have changed, and cards must be sorted according to a new feature. After all three features have been used as sorting criteria, subjects must cycle through them again in the same order as they did before. Each time the feature is changed, the next must be discovered by trial and error. The score was the total number of categories achieved. Data were available for 31/32 patients.
Verbal Fluency (Benton & Hamsher, Reference Benton and Hamsher1976)
In verbal fluency tasks, the subject generates as many items as possible from a given category in a specific period of time. We used the standard Argentinean phonemic version (Butman et al. Reference Butman, Allegri, Harris and Drake2000), asking subjects to generate words beginning with the letter P in a 1-min block. The score was the total number of correct words generated.
Hotel Task (Manly et al. Reference Manly, Hawkins, Evans, Woldt and Robertson2002; Torralva et al. Reference Torralva, Roca, Gleichgerrcht, Bekinschtein and Manes2009)
The task comprised five primary activities related to running a hotel. Individual activities are described in more detail elsewhere (Torralva et al. Reference Torralva, Roca, Gleichgerrcht, Bekinschtein and Manes2009; Roca et al. Reference Roca, Parr, Thomson, Woolgar, Torralva, Antoun, Manes and Duncan2010a ). Subjects were told to execute at least some of all five activities during a 15-min period, so that, at the end of this period, they would be able to give an estimate of how long each would take to complete. It was explained that the time available was not enough to complete any of the tasks; the goal, instead, was to ensure that every task was attempted. Subjects were also asked to remember to open and close the hotel garage doors at particular times (open at 6 min, close at 12 min), using an electronic button. The score was time allocation: for each primary task we assumed an optimal allocation of 3 min, and measured the summed total deviation (in seconds) from this optimum. Total deviation was given a negative sign, so that high scores meant better performance. Data were available for 29/32 patients.
Faux Pas (Stone et al. Reference Stone, Baron-Cohen and Knight1998)
On each trial of this test, the subject was read a short, one-paragraph story. To reduce working memory load, a written version of the story was also placed in front of the subject. In 10 stories, there was a faux pas, involving one person unintentionally saying something hurtful or insulting to another. In the remaining 10 stories, there was no faux pas. After each story, the subject was asked whether something inappropriate was said and, if so, why it was inappropriate. If the answer was incorrect, an additional memory question was asked to check that basic facts of the story were retained; if they were not, the story was re-examined and all questions repeated. The score was 1 point for each faux pas identified correctly, or non-faux pas rejected correctly. Data were available for 31/32 patients.
Mind in the Eyes (Baron-Cohen et al. Reference Baron-Cohen, Jolliffe, Mortimore and Robertson1997)
This task consisted of 17 photographs of the eye region of different human faces. Participants were required to make a two-alternative forced choice that best described what the person was thinking or feeling (e.g. worried–calm). The score was the total number correct. Data were available for 31/32 patients.
Results
The results are shown in Table 2. For all cognitive tasks, two-tailed t tests were used to compare patients and controls. As expected, the PD group was significantly impaired on all tests, including the RCPM [t(52)=−2.40, p=0.02], the classical executive tests [WCST: t(51)=−2.45, p<0.01; Verbal Fluency: t(52)=−2.78, p<0.01] and the tests of multitasking and theory of mind [Hotel: t(49)=−2.97, p<0.01; Mind in the Eyes: t(51)=−2.83, p<0.01; Faux Pas: t(51)=−2.35, p=0.02]. No significant differences were found between medicated and non-medicated patients on any of the aforementioned variables, except for the Faux Pas, on which medicated patients performed more poorly than non-medicated patients [t(29)=−2.46, p=0.02]. However, significant differences between patients and controls persisted after the levodopa equivalent daily dose (mg) was introduced as a covariable (p=0.03), suggesting that group differences were not related to medication intake.
Table 2. Patient and control scores, average within-group correlation with Raven Colored Progressive Matrices (RCPM), and significance of group differences for each task
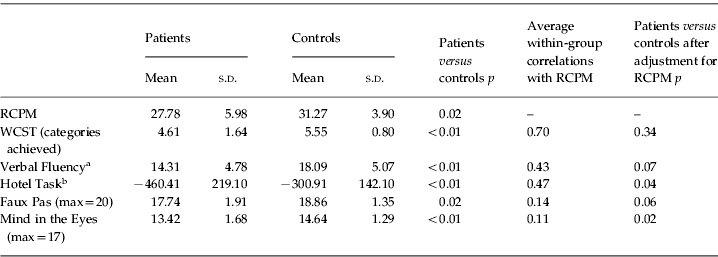
WCST, Wisconsin Card Sorting Test; s.d., standard deviation.
a Total number of words generated.
b Deviation from optimum time per task.
Scatterplots relating RCPM to the two classical frontal tests are shown in Fig. 1, revealing that higher scores in the RCPM were strongly associated with better performance on both the WCST and Verbal Fluency. Analysis of covariance (ANCOVA) was used to compare patients and controls, adjusting for the difference in RCPM; regression lines in Fig. 1 come from this ANCOVA model, reflecting the average within-group association of the two variables and constrained to have the same slope across groups. As calculated from the corresponding variance terms of the ANCOVA, average within-group correlations with RCPM were 0.70 for WCST and 0.43 for Verbal Fluency. The scatterplots suggest that, at least for the WCST, PD deficits were largely or entirely explained by fluid intelligence. The group effect was to shift the RCPM distribution downward, leaving its relationship to executive task performance largely unchanged. In line with this conclusion, for the WCST, the difference between patients and controls was far from significant once RCPM was introduce as a covariate (Table 2, p=0.34). For Verbal Fluency, ANCOVA showed a remaining but non-significant trend for a group difference (p=0.07).

Fig. 1. Scatterplots relating performance in (a) the Wisconsin Card Sorting Test (WCST) and (b) Verbal Fluency to Raven's Colored Progressive Matrices (RCPM) for patients with Parkinson's disease (PD) (circles) and controls (crosses). Regression lines (broken for PD and solid for controls) reflect the average within-group association of the two variables, as determined by ANCOVA, constrained to have the same slope across groups.
Scatterplots relating RCPM to the other frontal tests are shown in Fig. 2. For the Hotel Task, the results were somewhat similar to those observed in Verbal Fluency, with an average within-group correlation of 0.47. However, using ANCOVA to remove the influence of RCPM, the comparison between groups remained significant (Table 2, p<0.04). On the theory-of-mind tasks, the scores were barely related to RCPM, with average within-group correlations of 0.14 for Faux Pas and 0.11 for Mind in the Eyes. Using ANCOVA to remove the influence of RCPM, significant group differences for Mind in the Eyes persisted (Table 2, p<0.02), whereas for Faux Pas, the difference now fell just short of significance (p=0.06).

Fig. 2. Scatterplots relating performance in (a) Hotel, (b) Faux Pas and (c) Mind in the Eyes to Raven's Colored Progressive Matrices (RCPM) for patients with Parkinson's disease (PD) (circles) and controls (crosses). Regression lines (broken for PD and solid for controls) reflect the average within-group association of the two variables, as determined by ANCOVA, constrained to have the same slope across groups.
Discussion
In this study, we aimed to investigate the role of fluid intelligence in different frontal deficits shown in a group of patients with PD. In line with previous studies (Pillon et al. Reference Pillon, Gouider-Khouja, Deweer, Vidailhet, Malapani, Dubois and Agid1995; Bostantjopoulou et al. Reference Bostantjopoulou, Kiosseoglou, Katsarou and Alevriadou2001; Basić et al. Reference Basić, Katić, Vranicć, Zarevski, Babić and Mahović-Lakusić2004; Nagano-Saito et al. Reference Nagano-Saito, Washimi, Arahata, Kachi, Lerch, Evans, Dagher and Ito2005), we found a loss of fluid intelligence in PD patients relative to control subjects. In the present study, this was found even in the absence of significant differences between the groups on pre-morbid IQ. We then asked what other cognitive deficits remained after statistical control for this fluid intelligence deficit.
For the classical executive tasks, WCST and Verbal Fluency, deficits in PD patients were no longer present once g was introduced as a covariate. However, for other tasks, including multitasking and theory-of-mind tests, performance deficits remained once fluid intelligence was partialled out.
These results are largely compatible with data from patients with focal frontal lesions. In a recent study (Roca et al. Reference Roca, Parr, Thomson, Woolgar, Torralva, Antoun, Manes and Duncan2010a ) we showed that for the WCST and Verbal Fluency deficits of frontal patients were entirely explained by their fluid intelligence loss. The present study extends those results to patients with PD: even if original differences emerged when the PD group was compared with a group of control subjects, such differences became non-significant when fluid intelligence was introduced as a covariate.
Our data also replicate previous reports of multitasking and theory-of=mind deficits in patients with PD. PD patients showed deficits in their ability to infer other people's thoughts and feelings (theory of mind) and in their ability to hold in mind a higher-order goal while performing other subgoals (Hotel Task). Unlike the findings for the WCST and Verbal Fluency, performance deficits on the Hotel Task and Mind in the Eyes remained significant even after fluid intelligence was statistically corrected. Again the results resemble those obtained previously in patients with focal frontal lesions (Roca et al. Reference Roca, Parr, Thomson, Woolgar, Torralva, Antoun, Manes and Duncan2010a ). In that study also, we found that deficits in multitasking and theory of mind were not fully explained by fluid intelligence, with some evidence of link to lesions in the anterior frontal cortex (BA 10). In the Roca et al. (Reference Roca, Parr, Thomson, Woolgar, Torralva, Antoun, Manes and Duncan2010a ) study, the theory-of-mind test that showed these results was the Faux Pas rather than Mind in the Eyes. In the present data, by contrast, the Faux Pas deficit fell just short of significance once fluid intelligence was controlled. Nevertheless, our findings confirm that deficits on multitasking and theory of mind shown by PD patients cannot be fully explained by their loss of fluid intelligence.
Both lesion and neuroimaging studies have previously linked multitasking and theory of mind to the prefrontal cortex. For theory of mind, lesion studies have indicated the particular importance of the orbitofrontal cortex (e.g. Stone et al. Reference Stone, Baron-Cohen and Knight1998; Rowe et al. Reference Rowe, Bullock, Polkey and Morris2001; Stuss et al. Reference Stuss, Gallup and Alexander2001), whereas neuroimaging studies indicate the parallel importance of other regions including the anterior cingulate cortex, the superior temporal sulcus, the temporal poles and the amygdala (Baron-Cohen et al. Reference Baron-Cohen, Ring, Wheelwright, Bullmore, Brammer and Simmons1999; Gallagher & Frith, Reference Gallagher and Frith2003; Frith & Frith, Reference Frith and Frith2006). Multitasking and planning deficits have also been described in patients with frontal cortex damage (e.g. Hebb & Penfield, Reference Hebb and Penfield1940; Shallice & Burgess, Reference Shallice and Burgess1991; Goldstein et al. Reference Goldstein, Bernard, Fenwick, Burgess and McNeil1993), and the particular importance of the anterior prefrontal cortex has been suggested by both lesion and neuroimaging studies (e.g. Burgess et al. Reference Burgess, Dumonheil and Gilbert2007; Gilbert et al. Reference Gilbert, Williamson, Dumontheil, Simons, Frith and Burgess2007; Dreher et al. Reference Dreher, Koechlin, Tierney and Grafman2008; Badre & D'Esposito, Reference Badre and D'Esposito2009;Roca et al. Reference Roca, Torralva, Gleichgerrcht, Woolgar, Thompson, Duncan and Manes2011). In PD, the impairment in these functions has been explained by the progressive deterioration of frontostriatal circuits that occurs during the course of the disease (Bodden et al. Reference Bodden, Dosel and Kalbe2010; Roca et al. Reference Roca, Torralva, Gleichgerrcht, Chade, Arévalo, Gershanik and Manes2010b ).
Although here we have discriminated two groups of tests, distinguished by whether frontal deficits are entirely explained by fluid intelligence, a more realistic possibility may be a continuum. For the WCST, we found the strongest overlap with fluid intelligence, with the patient–control difference far from significance (p=0.34) once fluid intelligence was controlled. The results are very similar to those we obtained previously for patients with focal lesions (p=0.36). For Verbal Fluency the evidence of overlap was less, with a marginal difference (p=0.07) remaining after fluid intelligence was controlled. Again this resembles our previous results (p=0.07). For multitasking and theory of mind, overlap with fluid intelligence may be weaker, although especially for multitasking, some correlation certainly exists. For some tests, accordingly, fluid intelligence accounts for the major part of frontal deficit, whereas for others, probably with a somewhat different anatomical substrate, it does not.
Our data have strong implications for the use and interpretation of executive tests such as the WCST and Verbal Fluency in patients with PD. Although several reports have highlighted the sensitivity of such tests in the detection of cognitive dysfunction in PD (e.g. Green et al. Reference Green, McDonald, Vitek, Evatt, Freeman, Haber, Bakay, Triche, Sirockman and DeLong2002; Azuma et al. Reference Azuma, Cruz, Bayles, Tomoeda and Montgomery2003; Ong et al. Reference Ong, Seel, Carne, Brown, Pegg and Jehle2005; Muslimovic et al. 2006; Williams-Gray et al. Reference Williams-Gray, Foltynie, Brayne, Robbins and Barker2007), our results reveal that the deficits detected by such tasks may not be related to their particular cognitive content and that, instead, they might solely reflect a general cognitive loss. In our view, neuropsychological assessment in PD should include both fluid intelligence tests and specifics test of multitasking and theory of mind. Further studies should investigate the contribution of fluid intelligence to other executive tests used in PD.
Our data also have powerful implications for the understanding of the relationship between fluid intelligence and frontal functions. The previously reported results in patients with focal lesions now extend to PD: whereas some frontal deficits are entirely explained by fluid intelligence, others are not. Very possibly, this dissociation reflects dependence on somewhat different frontal regions, with fluid intelligence dependent in particular on lateral and dorsomedial regions (Bishop et al. Reference Bishop, Fossella, Croucher and Duncan2008; Woolgar et al. Reference Woolgar, Parr, Cusack, Thompson, Nimmo-Smith, Torralva, Roca, Antoun, Manes and Duncan2010), whereas more of the anterior frontal cortex is crucial for multitasking and theory of mind. Further studies should investigate such relationships in other clinical populations with frontal involvement.
Acknowledgments
This work was supported by Fundación INECO, and the Medical Research Council (MRC) intramural programme MC_US_A060_0001.
Declaration of Interest
None.






