Introduction
The perinatal period represents a time signified by much emotional turmoil for women, and studies suggest that 10–15% of women experience moderate to high levels of depressive symptoms during this period (O'Hara and Swain, Reference O'Hara and Swain1996; Eberhard-Gran et al., Reference Eberhard-Gran, Tambs, Opjordsmoen, Skrondal and Eskild2004; Gavin et al., Reference Gavin, Gaynes, Lohr, Meltzer-Brody, Gartlehner and Swinson2005). Factors associated with increased risk of perinatal depression include a history of depression, stressful life events, young age and other demographic variables (Robertson et al., Reference Robertson, Grace, Wallington and Stewart2004; Vesga-López et al., Reference Vesga-López, Blanco and Keyes2008; Lancaster et al., Reference Lancaster, Gold, Flynn, Yoo, Marcus and Davis2010; Silverman et al., Reference Silverman, Reichenberg, Savitz, Cnattingius, Lichtenstein and Hultman2017). A majority of studies emphasize the postpartum period as a particularly vulnerable time during which the woman is at increased risk for mental disorders (Munk-Olsen et al., Reference Munk-Olsen, Laursen, Pedersen, Mors and Mortensen2006; Vesga-Lopez et al., Reference Vesga-López, Blanco and Keyes2008; O'Hara and McCabe, Reference O'Hara and McCabe2013). More recent studies, however, highlight how women seem to be at equal risk of developing depression during pregnancy (Melville et al., Reference Melville, Gavin, Guo, Fan and Katon2010), and approximately 50% of depressive episodes after birth, start during pregnancy (American Psychiatric Association, 2013). This underscores the importance of early intervention, as the chronicity of depressive symptoms is of particular concern for the consequences of depression (Sohr-Preston and Scaramella, Reference Sohr-Preston and Scaramella2006). Perinatal depression carries a high personal cost for both the woman and her family (Lovestone and Kumar, Reference Lovestone and Kumar1993; Lovejoy et al., Reference Lovejoy, Graczyk, O'Hare and Neuman2000; Goodman et al., Reference Goodman, Rouse, Connell, Broth, Hall and Heyward2011) while all too often escaping detection and treatment (Bågedahl-Strindlund and Monsen Børjesson, Reference Bågedahl-Strindlund and Monsen Børjesson1998).
Although effective psychological treatments for perinatal depression exist (Stuart-Parrigon, and Stuart, Reference Stuart-Parrigon and Stuart2014; Stephens, Reference Stephens, Ford, Paudyal and Smith2016), there are several complicating factors that impede women's ability to receive appropriate treatment, such as limited access to treatment (Payne and Myhr, Reference Payne and Myhr2010; WHO, 2015), difficulties in attending therapy during usual business hours, struggles with geographical location (Overland et al., Reference Overland, Glozier, Krokstad and Mykletun2007), transportation and childcare (Goodman, Reference Goodman2009). Additionally, many women are reluctant to seek treatment due to a perceived stigma (Beck, Reference Beck2001; O'Mahen and Flynn, Reference O'Mahen and Flynn2008; Goodman, Reference Goodman2009). Some women even fear that their children will be ‘taken away’ if health providers discover that they suffer from perinatal depression (Dennis and Chung-Lee, Reference Dennis and Chung-Lee2006). Furthermore, many new mothers are unfamiliar with what constitute depressive symptoms, and do not recognize that they suffered from perinatal depression until it was over. Consequently, many people suffer ‘in silence’. The many barriers associated with face-to-face treatments clearly underscore a need for innovative approaches to public health prevention and promotion.
Internet-delivered interventions represent an innovative approach that circumvents many of the obstacles encountered in the dissemination of face-to-face treatment (Kohn et al., Reference Kohn, Saxena, Levav and Saraceno2004). Internet interventions allow anonymity, which reduces a sense of stigma and patients’ fear of seeking help (Dennis and Chung-Lee, Reference Dennis and Chung-Lee2006). In turn, such interventions can more readily reach the many people that suffer ‘in silence’. Indeed, many women with perinatal depression express an interest in internet interventions and report that they would use the internet to learn coping strategies for depressive symptoms (Maloni et al., Reference Maloni, Przeworski and Damato2013). Because of the scalability, internet-based interventions are likely cost-effective, which makes them especially advantageous for disorders characterized by a combination of high prevalence and low treatment-seeking rates.
Recent studies have demonstrated the acceptability and feasibility of internet interventions for perinatal depression (Danaher et al., Reference Danaher, Milgrom, Seeley, Stuart, Schembri, Tyler, Ericksen, Lester, Gemmill and Lewinsohn2012; Haga et al., Reference Haga, Drozd, Brendryen and Slinning2013; Salonen, Reference Salonen, Pridham, Brown and Kaunonen2014; Logsdon et al., Reference Logsdon, Bennett, Crutzen, Martin, Eckert, Robertson, Myers, Tomasulo, Gregg and Barone2014). Findings from two recent systematic reviews on web-based interventions for perinatal mental health are encouraging and suggest they may be effective in reducing perinatal depression (Ashford et al., Reference Ashford, Olander and Ayers2016; Lee et al., Reference Lee, Denison, Hor and Reynolds2016). However, there is still an overall need for high-quality randomized controlled trials (RCTs) to assess the effects of web-based interventions on perinatal depression (Lee et al., Reference Lee, Denison, Hor and Reynolds2016). Interestingly, the reviews address how remarkably few of the existing interventions are tailored specifically to pregnancy and the postpartum period, which is curious considering how perinatal women face changes, difficulties, and mental health issues that are quite specific to this period (Ashford et al., Reference Ashford, Olander and Ayers2016). Interventions that target perinatal specific themes may increase perceived relevance and acceptability, which, in turn, may lower attrition rates (O'Mahen et al., Reference O'Mahen, Fedock, Henshaw, Himle, Forman and Flynn2012). The current intervention, Mamma Mia, was tailored specifically to the perinatal phase and targets risk and protective factors for perinatal depressive symptoms such as attachment, couple satisfaction, social support, and subjective well-being. It was developed to address the high prevalence rates of perinatal depressive symptoms and the need for preventive interventions. Our study aims to contribute to the understanding of the effectiveness of fully automated web-based preventive interventions for perinatal depression.
Aim of the study
The objective of the current study was to test the effectiveness of a universal preventive intervention for perinatal depressive symptoms (Mamma Mia) on depressive symptoms from pregnancy to 6 months after birth. Our main hypothesis was that the Mamma Mia group would display lower levels of depressive symptoms compared with the control group as measured by the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., Reference Cox, Holden and Sagovsky1987). Second, we investigated whether the effect of Mamma Mia changed over time. Third, we examined if the effect of Mamma Mia on depressive symptoms was moderated by baseline depressive symptoms, an indication of previously experienced depression, and parity, and whether this moderation changed over time. Finally, we examined if the prevalence of mothers with at least a probable minor depression (i.e. EPDS-score ⩾10) differed between the intervention and control group.
Method
Study design and recruitment
This was a two-armed RCT registered at http://www.controlled-trials.com (ISRCTN91808706). Participants were randomly assigned (ratio: 1:1) to one of two conditions: the intervention group (i.e. usual perinatal care and the web-based program Mamma Mia) or control group [i.e. usual perinatal care (Norwegian Directorate of Health, 2005, 2013)]. The overall goal with the well-baby clinic that offers perinatal care is to promote physical and mental health and prevent injuries and disease, and ensure a continuity of care for its users (Norwegian Directorate of Health, 2004). According to guidelines for prenatal care, all women are offered eight consultations throughout the pregnancy, and midwives are to identify women in need of extended care and offer additional consultations (Norwegian Directorate of Health, 2005). During the first 6 months following birth, the woman and her child should be offered six consultations. Although the child's development is of primary concern during these consultations, the psychological well-being of the mother is also addressed. Pregnant women across Norway were invited to take part in the study between December 2013 and February 2015. Participants were recruited at well-baby clinics across the country during routine prenatal care and via hospitals in eastern Norway during regular ultrasound [i.e. gestational week (gw)18–20]. Eligible participants had to be pregnant (up until gw25), at least 18 years of age, able to read and write Norwegian, have access to the internet and have an electronic mailing account.
The intervention – Mamma Mia
Participants in the intervention received Mamma Mia; a universal preventive intervention for perinatal depressive symptoms. It is a fully automated internet-based program available free of charge. The intervention comprises three phases. The first phase consists of 11 sessions beginning in the second trimester in gw 21–25 and ends in gw37. The second phase starts when the infant is 2–3-weeks-old, and lasts for 6 weeks, with three sessions per week. The final phase consists of 10 sessions over an 18-week period. In total, the intervention consists of 44 sessions over a period of 11.5 months. All sessions include themes specific to the perinatal period. Mamma Mia applies a tunneled design to guide the woman through the program in a step-by-step fashion in accordance with the psychological preparations of becoming a mother. The intervention is delivered by email and interactive websites, combining text, pictures, prerecorded audio files, and user input. Each session is designed to take about 10 min and must be completed before users can access the next session. This is done to ensure that relevant information has been reviewed and to create continuity and a narrative in the program (see Drozd et al., Reference Drozd, Haga, Brendryen and Slinning2015 for a comprehensive description of Mamma Mia). A recent study demonstrates the feasibility and acceptability of Mamma Mia (Haga et al., Reference Haga, Drozd, Brendryen and Slinning2013). For a demonstration of Mamma Mia, see: http://smarturl.it/psych_med.
Data collection and randomization
Data was gathered by means of internet-based surveys. First, participants reported on demographic information (incl. age, marital status, parity, education, and estimated due date). Based on the reported due date, it was calculated when participants were between gw21–25, and that was when they received their baseline questionnaire assessing depressive symptoms. This was done to ensure similarity in terms of when depressive symptoms were assessed. Upon completing the baseline assessment, an automated, unrestricted randomization procedure was carried out. Thus, each participant had an equal probability of being assigned to each of the two groups, resulting in 678 and 664 eligible participants being randomly assigned to the intervention and control group, respectively. Depressive symptoms were assessed twice during pregnancy; at baseline between gw21 and 25 and at gw37. After birth, depressive symptoms were assessed at 6 weeks, 3 and 6 months postpartum.
Depressive symptoms were measured by EPDS (Cox et al., Reference Cox, Holden and Sagovsky1987), which is a 10-item self-report instrument that assesses depressive symptoms during the last 7 days. It has been validated for use during pregnancy (Bunevicius et al., Reference Bunevicius, Kusminskas, Pop, Pedersen and Bunevicius2009; Bergink et al., Reference Bergink, Kooistra, Lambregtse-van den Berg, Wijnen, Bunevicius, van Baar and Pop2011), and postpartum (Eberhard-Gran et al., Reference Eberhard-Gran, Eskild, Tambs, Opjordsmoen and Samuelsen2001a). Items are rated on a 4-point scale from 0 to 3 to produce a summative score ranging from 0 to 30, with higher scores indicating elevated risk for perinatal depression. Cox et al. (Reference Cox, Holden and Sagovsky1987) recommend a cut-off score of EPDS ⩾10 to detect possible depression. The present study used the validated Norwegian translation of the EPDS (Eberhard-Gran et al., Reference Eberhard-Gran, Eskild, Tambs, Schei and Opjordsmoen2001b). Continuous EPDS-scores were used in main analyses, as recommended for the EPDS in population research (Green, Reference Green, Henshaw and Elliott2005), while a cut-off score of ⩾10 was used to assess group differences in prevalence rates of possible depression across time.
Statistical analysis
Baseline differences between groups were merely examined by descriptive information in line with recommendations in the CONSORT guidelines. Logistic regression analyses with group assignment as an independent variable were done at each measurement time to assess drop-out after baseline, unadjusted and adjusted for age, first language, parity, education, previous depression, and EPDS at baseline. A series of linear mixed effects (LME) models were estimated for the time development of EPDS-scores after randomization. Time was included as a categorical independent variable with four categories (gw37, 6 weeks, 3 and 6 months postpartum), and treatment assignment was included as a dichotomous independent variable (control or Mamma Mia). Mixed-effects models are commonly used for repeated measures data (Pinheiro and Bates, Reference Pinheiro and Bates2012) as they do not require balanced data and give valid results under the less restrictive missing at random assumption. LME models handle repeated measure data in a long format and are therefore especially advantageous in longitudinal studies with missing data, as all data available is used and no cases are deleted. All models included a random intercept. For fixed effects, the first model was by group and time only. The second model included a group by time interaction to test for differences in intervention effects during follow-up. The third model also included interactions of group by parity, previous depression and depressive symptoms at baseline, and the final fourth model included third-order interactions between the group, time, and these background characteristics. It should be noted that EPDS-scores at baseline were not included in the dependent variable because when randomization is successful, group differences at baseline are random.
In supplementary analyses of the prevalence of depression over time after randomization, we performed logistic regression analyses for repeated measurements by means of generalized estimating equations (GEE). The binary outcome was EPDS-scores <10 (coded as 0) and EPDS-scores ⩾10 (coded as 1). In addition, we performed separate logistic regressions at each time point, adjusted for a baseline from gw37 to 6 months postpartum. The significance level was set to 0.05, but in line with the recommendations of the American Statistical Association, this boundary was not used strictly (Wasserstein and Lazar, Reference Wasserstein and Lazar2016). All tests were two-tailed. SPSS version 23 (IBM SPSS, Armonk, NY, USA) was used for descriptive analyses. The R (The R Foundation for Statistical Computing, Vienna, Austria) package nlme was employed for the mixed effects analyses and the GEE analyses employed the R package gee.
Results
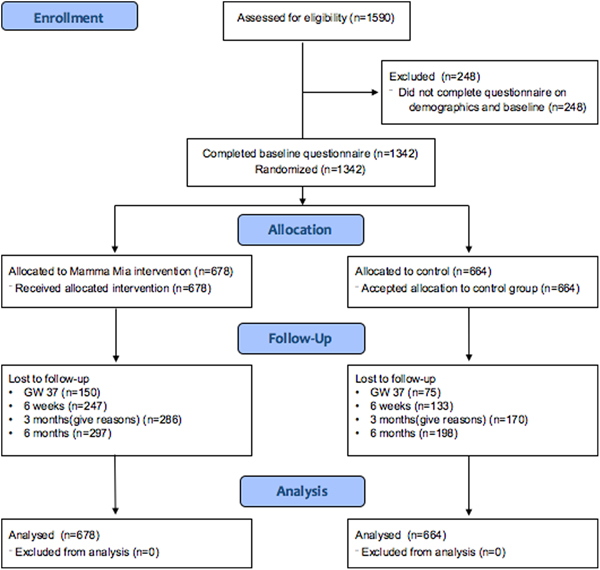
The flow of participants is illustrated in Fig. 1. In total, 678 participants were randomly assigned to the Mamma Mia group, and 644 were randomly assigned to the control group.
Fig. 1. Participant flowchart.
The number of respondents in the total sample was 1342 at baseline, 1117 (83.2%) at gw37, and 962 (71.7%), 886 (66.0%), 847 (63.1%) at 6 weeks, 3 and 6 months postpartum, respectively.
Demographics are presented in Table 1. Mean maternal age was comparable with that of the average age for all births in Norway, which is 30.8 years (Statistics Norway, 2017b). In terms of education and parity, more than half of the mothers reported having higher education (i.e. ⩾4–5 years in college or university) and being primipara. This may indicate that the level of education was substantially higher than the 8.7% of women with higher education in the general population (Statistics Norway, 2017a), although the level of education among pregnant women in Norway is unknown. Using mothers’ first language as a proxy for ethnicity, the proportion of mothers with non-Scandinavian language was 7.7%, while the proportion of births given by mothers born outside of Norway was 27% in 2014 (The Norwegian Insitute of Public Health, 2015). The skewness in ethnicity may, however, be explained by the implicit inclusion criterion; the ability to read and understand Norwegian to participate in Mamma Mia.
Table 1. Participant characteristics

Table 2 shows levels of depressive symptoms in the Mamma Mia and control group over time. It also shows the percentage of participants who scored ⩾10 on the EPDS, which is an indication of possible depression. As can be seen, levels of depressive symptoms were highest at baseline in both groups, and the greatest reduction in symptoms occurred from baseline (gw21–25) to gw37. Noticeably, the proportion of women scoring above the cut-off at baseline was just over 23% in both groups. This is a higher proportion than what recent studies have found in Norway (Eberhard-Gran et al., Reference Eberhard-Gran, Eskild, Tams and Samuelson2002; Dørheim et al., Reference Dørheim, Bondevik, Eberhard-Gran and Bjorvatn2009; Glavin et al., Reference Glavin, Smith, Sorum and Ellefsen2010; Haga et al., Reference Haga, Ulleberg, Slinning, Kraft, Steen and Staff2012, Reference Haga, Lisøy, Drozd, Valla and Slinning2017), indicating that perhaps women with higher levels of depressive symptoms than the rest of the perinatal population have signed up for the study. This is not surprising considering that the participants were self-selected, and the study revolved around an intervention for perinatal depression.
Table 2. Means, standard deviations, and number of women scoring above the cut-off for EPDS over time (N = 1 342)

Drop-out and missing data
At all measurement times, drop-out was greater in the intervention group [odds ratio (OR) = 0.43–0.55, all ps <0.001 both in adjusted and unadjusted analysis]. There was little difference when comparing the adjusted and unadjusted analyses. For the whole sample, later dropout was predicted by a higher score on baseline EPDS (all ps ⩽0.047) and lower level of education (all ps ⩽0.004).
Adherence to the intervention
Adherence to the intervention refers to the completion of sessions. A total of 678 participants received the Mamma Mia intervention. Of these, 226 (33%) completed all 44 sessions. As many as 345 (51%) completed 36 or more sessions (>80% of the intervention), and merely 41 (6%) participants did not initiate using the program. The completion rate is high considering that Mamma Mia is a comprehensive, unguided, and fully automated prevention program that is delivered at a time that is already quite hectic.
Symptom reduction
Our main hypothesis was that the level of depressive symptoms would differ between treatment and control group. The first model, by treatment assignment and time, showed a significant effect of Mamma Mia on depressive symptoms [F (1) = 7.03, p = 0.008] (see Fig. 2).

Fig. 2. Mamma Mia and control group trajectories of depressive symptoms. Numbers at baseline are means, while numbers during follow-up are model-based estimates.
Effect on depressive symptoms over time
In the second model, there were no significant differences in the rate of change between the Mamma Mia and control group over time [F (3) = 1.02, p = 0.384 for the time by group interaction]. This means that the intervention group had lower levels of depressive symptoms compared with the control group, but both groups seemed to follow a similar trajectory in depressive symptoms. As seen in Table 3, participants in the Mamma Mia group had significantly lower symptoms than the control group at gw37 and 6 weeks postpartum. Group differences were not statistically significant at 3 and 6 months postpartum.
Table 3. Contrasts between the Mamma Mia and control group at different time points (N = 1117)

Testing moderating variables – over time
In the third model, only EPDS-scores at baseline moderated the effect of group (p = 0.051). The group difference was somewhat more pronounced for higher values of baseline EPDS (data not shown). All other interactions were clearly non-significant (all ps ⩾0.317). In the final model, there was no indication of any third-order interactions (all ps ⩾0.133).
In sum, results from the mixed effects models indicate that participants in the Mamma Mia group scored lower on depressive symptoms than participants in the control group on EPDS during follow-up (gw37–6 months). The differences between the groups were significant at gw37 and 6 weeks. There were indications that the effect of Mamma Mia was moderated by EPDS score at baseline; indicating that a higher initial EPDS-score yielded a greater effect of Mamma Mia.
Prevalence of possible depression
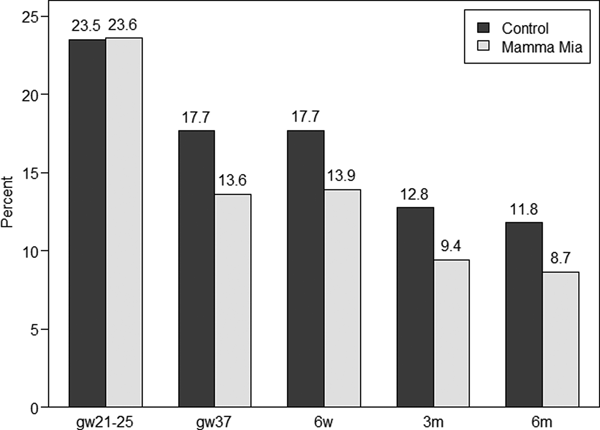
Finally, we examined if the prevalence of women with a possible minor depression (i.e. EPDS-score ⩾10) differed between the intervention and control group. Figure 3 below presents a histogram showing the percentage of women with EPDS-scores ⩾10 across time in the Mamma Mia and control group, respectively. These results indicate a 21.5–26.6% reduction in the prevalence of possible depression in the Mamma Mia group compared with the control group across time. The prevalence of women who scored ⩾10 on the EPDS was lower in the Mamma Mia group from gw37 and onwards (GEE adjusted for time and baseline, OR 0.75, 95% CI 0.58–0.96, p = 0.024). This difference was fairly stable over time (ORs between 0.71 and 0.78, see Table 4).

Fig. 3. Percentages of women with EPDS-scores ⩾10 in the Mamma Mia and control group across time.
Table 4. Results from separate logistic regression analyses comparing Mamma Mia to controls at each time point using EPDS ⩾10 as an outcome

Estimates from gw37 to 6 months are adjusted for baseline EPDS.
Discussion
The focus of this study was to evaluate the effectiveness of an internet-based program (‘Mamma Mia’) on depressive symptoms from pregnancy to 6 months postpartum. Results indicated that the reduction in mean depressive symptoms in the intervention group compared with the control group was small but significant. In fact, participants who received Mamma Mia displayed less depressive symptoms than participants in the control group on all measurements occasions after baseline. This difference was statistically significant at gw37 and 6 weeks postpartum. Although group differences were no longer statistically significant at 3 and 6 months postpartum, participants in the intervention group still had lower levels of depressive symptoms than the control group. As symptom severity is at its highest towards the end of pregnancy and early postpartum period (Cox et al., Reference Cox, Murray, Chapman, Cox, Murray and Chapman1993; Eberhard-Gran et al., Reference Eberhard-Gran, Tambs, Opjordsmoen, Skrondal and Eskild2003; Gaynes et al., Reference Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, Brody and Miller2005; Munk-Olsen et al., Reference Munk-Olsen, Laursen, Pedersen, Mors and Mortensen2006), and typically remits over time (Heron et al., Reference Heron, O'Connor, Evans, Golding and Glover2004), it is not surprising that Mamma Mia was most effective during the third trimester and early postpartum period. Importantly, as the end of pregnancy (Eberhard-Gran et al., Reference Eberhard-Gran, Tambs, Opjordsmoen, Skrondal and Eskild2004) and first weeks after birth are particularly vulnerable time periods, it is reassuring that Mamma Mia is most effective at precisely those times.
Although Mamma Mia is a universal intervention, analyses were undertaken to assess whether Mamma Mia was more effective given certain background characteristics. Only EPDS-scores at baseline emerged as a moderator, indicating that a higher initial EPDS score yielded a greater effect of Mamma Mia. This finding is clinically meaningful as people with higher levels of distress have greater potential for improvement (Dennis, Reference Dennis2005).
The clinical implications of Mamma Mia for the individual and the population
Although the effect on the mean level of EPDS-scores was modest, is important to consider the clinical implications of Mamma Mia for the individual. Interesting research has investigated whether ‘a touch of depression matters’ (Brenner, Reference Brenner1985; Rose, Reference Rose2008). What Brenner (1985) found was that the likelihood of needing health care support increased with every increase on a scale measuring depressive symptoms, regardless of whether the person was over or below the cut-off score. This finding implies that even the mildest subclinical degree of depression is problematic for the individual and associated with impaired functioning, suggesting that even a minor reduction in depressive symptoms over time will matter for a new mother and her baby. Because all levels of symptoms matter to the people involved, preventive medicine ought to target the whole spectrum of illness and health. What is more, ‘the mild symptom can be the father of the severe’ (Rose, Reference Rose2008).
In a population, the mean level of symptoms is related to the prevalence of a disorder (Anderson et al., Reference Anderson, Huppert and Rose1993; Whittington and Huppert, Reference Whittington and Huppert1996). Universal interventions that can reduce mean symptoms will thus, in turn, reduce the prevalence of a disorder. Rose (Reference Rose2008) underscored this argument by using an analogy of how the ‘visible part of the iceberg (prevalence) is a function of its total mass (the population average)’ (Rose, Reference Rose2008). In the current study, analyses supported this analogy by showing that a small mean sample reduction in depression was also accompanied by a reduced proportion of women with an EPDS-score ⩾10 in the Mamma Mia group. Indeed, EPDS-score of ⩾10 prevalence rates were reduced between 21.5 and 26.6% in the Mamma Mia group compared with the controls, across time. These are promising results indicating that Mamma Mia may prevent at least minor to moderate depression. Due to the substantial barriers to care once women become ill, Mamma Mia holds a considerable potential for clinical effectiveness.
Study limitations
Current drop-out rates fall within the typical range reported on internet-based interventions (Christensen et al., Reference Christensen, Griffiths and Farrer2009), but is nevertheless a limitation in this study. The drop-out was greater in the intervention group than the control group and missing data analysis showed that participants with less formal education and those with higher EPDS-scores at baseline were more likely to drop out. Therefore, EPDS baseline score and educational level were used as covariates in the regression models as a deliberate effort to preclude systematic differences between drop-outs and completers. Nevertheless, both from a methodological and a prevention standpoint, it is unfortunate that participants with more depressive symptomatology were more likely to drop out. Participant drop-out may also have affected the power to detect differences, particularly at later measurement occasions because drop-out became more pronounced over time.
While we used continuous EPDS-scores in our main analyses, an EPDS-score ⩾10 was used as an indicator of possible depression. However, while the EPDS-scores are indicative of depressive symptomatology, scores do not confirm or refute the presence of depression (Cox and Holden, Reference Cox and Holden2003). Cases were not confirmed with clinical assessment. The mean EPDS-scores in both groups at baseline, as well as the proportion of women with an EPDS-score ⩾10, were higher than found in recent Norwegian studies (Dørheim, Reference Dørheim, Bondevik, Eberhard-Gran and Bjorvatn2009; Glavin et al., Reference Glavin, Smith, Sorum and Ellefsen2010; Haga et al., Reference Haga, Ulleberg, Slinning, Kraft, Steen and Staff2012; Haga et al., Reference Haga, Lisøy, Drozd, Valla and Slinning2017). This is likely due to the self-selection of participants and that women with more depressive symptomatology may have been attracted to an intervention that targets depression. Thus, a limitation of the present study is the potential for sampling bias from not achieving a full inclusion of the intended population.
Finally, as in any effectiveness trial, a mix of good and poor responders will have been recruited, so the average therapeutic response may have been mitigated.
Future recommendations and studies
Research demonstrates how few of the existing interventions are tailored to the perinatal period, which is surprising as tailored interventions are likely perceived as more relevant and acceptable, and, in turn, associated with lower attrition rates. Moreover, perinatal care that is tailored to the woman's needs has been found to reduce the prevalence rate of perinatal depression (MacArthur et al., Reference MacArthur, Winter, Bick, Knowles, Lilford, Henderson, Lancashire, Braunholtz and Gee2002). Mamma Mia is a comprehensive intervention that addresses a wide range of themes that preoccupy perinatal women, ranging from attachment, breastfeeding, regulatory capacity in infants to partner relationship, all of which comprise important health determinants for perinatal depression. A key challenge with such a comprehensive program, however, is the ‘black box’ phenomenon. That is, it is not clear which components are effective for whom and what minimum dosage of the intervention is required to achieve the effect. Moreover, the intervention might be too comprehensive for those most at risk. Future studies should attempt to link the use of intervention components and their effects and determine their dose-response relationships.
Although the current study took place in Norway, the content of Mamma Mia was largely developed based on international studies addressing risk and protective factors for perinatal depression. Moreover, the national clinical guidelines for perinatal care in Norway are in line with World Health Organization recommendations (2015) and practices are comparable with those in Britain (National Institute for Health and Excellence, 2014) and the other Scandinavian countries (Danish Health Authority, 2013; The Swedish Association of Midwives, 2016). Thus, there are reasons to assume that the current results are generalizable to other Western cultures. This should be addressed in future studies.
Recent research shows that internet-based programs in combination with guidance from health personnel for treating perinatal depression may produce effects equivalent to those of face-to-face interventions (Cuijpers et al., Reference Cuijpers, Donker, van Straten, Li and Andersson2010). Therefore, guidelines for the clinical use of Mamma Mia (i.e. with added guidance) and implementation in well-baby clinics have been developed (Drozd et al., Reference Drozd, Haga, Slinning and Langrial2017), designed to fit with existing national guidelines for pre- and postnatal care for well-baby clinics (Norwegian Directorate of Health, 2005, 2013). With a few case-finding questions health personnel could identify women at risk of depression who should be offered the guided program, while the unguided version of Mamma Mia, as tested in this study, may continue to be disseminated as universal prevention. A future study should, however, investigate whether adding guidance by well-baby personnel enhances the effects Mamma Mia. However, until then, conditions are in place for primary care to make the unguided internet-based program available as universal prevention to perinatal women.
Conclusion
Due to the prevalence, substantial risks, problems with detection of perinatal depression, as well as the substantial barriers to care once women become ill, an effective universal intervention holds substantial potential for clinical effectiveness. The present study demonstrated promising effects of Mamma Mia, and it is readily available for perinatal women at no cost. Primary health services are in a position to relay information about preventive programs for perinatal depression, and Mamma Mia has the potential to aid health care professionals in preventing depressive symptoms in the perinatal population.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The participants were regularly screened for depressive symptoms, and those scoring above a given threshold were provided feedback about where they could receive support and help (beyond what the program could offer). Power analyses were conducted so as not to include (and burden) any more participants than necessary. The trial was approved by the Regional Ethics Committee, Norway, South East (project number: 2012/1716).
Acknowledgements
First and foremost, we would like to thank all the women who took part in this comprehensive study at the same time as they were having a baby. We would also like to thank the hospitals and well-baby clinics who helped immensely with recruitment. Mamma Mia was developed in Norway and is owned by The Norwegian Women's Public Health Association (N.K.S.) who offers the program free of charge to women (see https://tinyurl.com/MammaMia-NKS). Mamma Mia is currently only offered in Norwegian language. The program was developed by Changetech AS in collaboration with the Department for Infant Mental Health at RBUP (Regional Centre for Child and Adolescent Mental Health, Eastern and Southern Norway). The project was supported by funding provided by the Research Council of Norway (#213737).
Conflict of interest
Mamma Mia was developed by Changetech in collaboration with the Regional Centre for Child and Adolescent Mental Health (RBUP) for The Norwegian Women's Public Health Association (N.K.S.).